Synthesis technology of dutasteride
A synthesis process, the technology of dutasteride, is applied in the field of chemical synthesis of drugs, can solve problems such as being unfavorable to large-scale production, complicated post-processing, long reaction time, etc., and achieve high-yield, high-purity, green, large-scale clean production, Easy purification and good product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
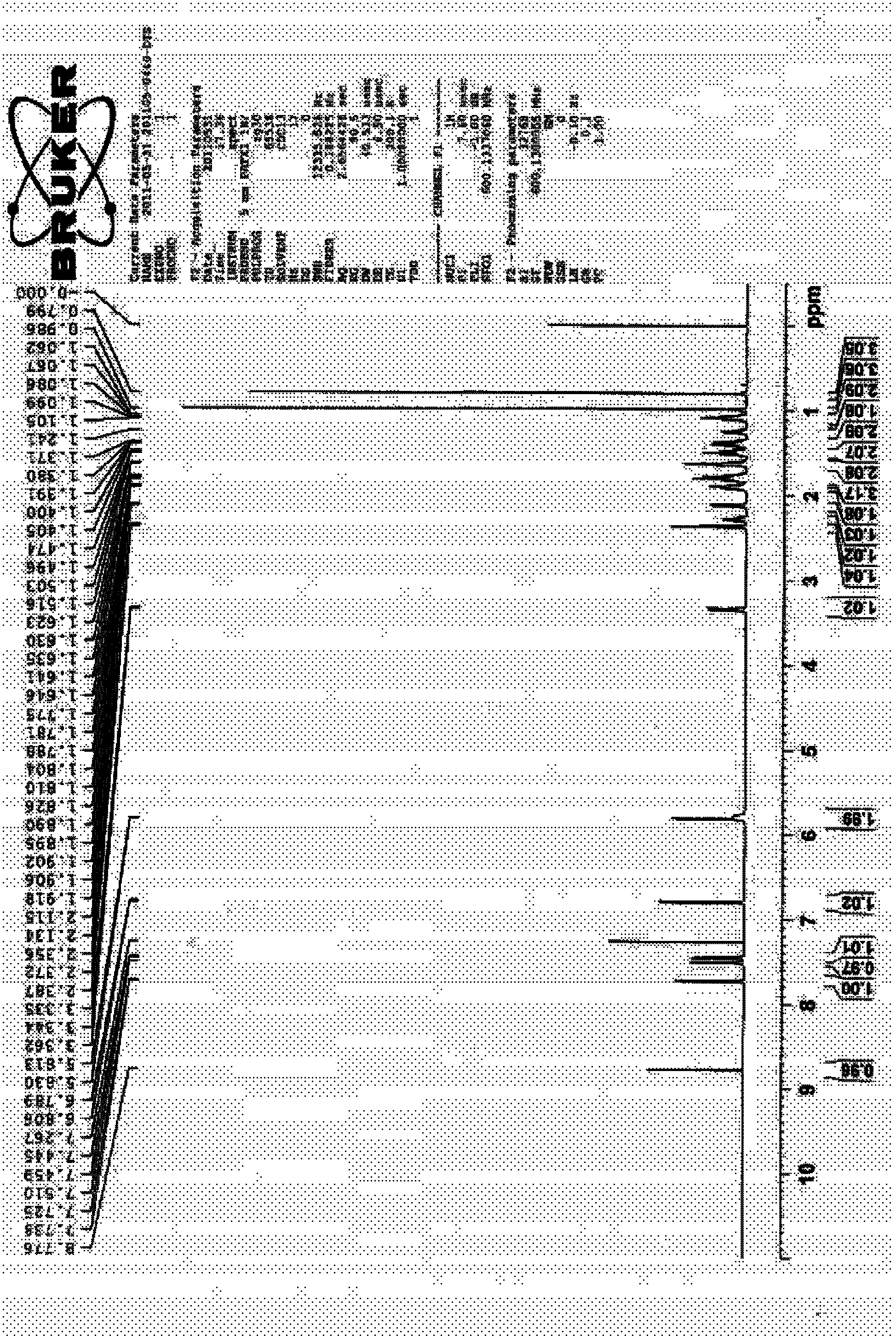
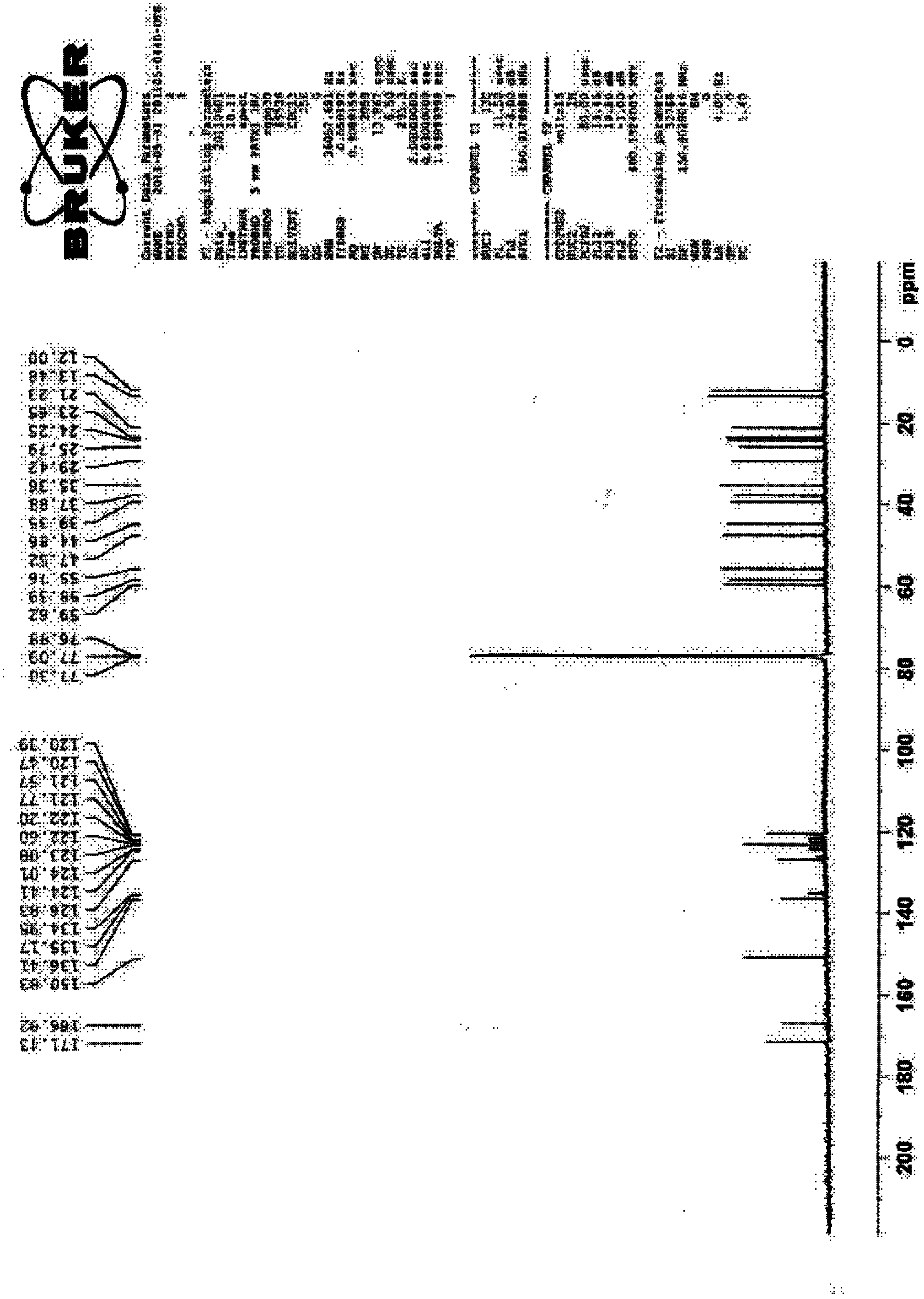
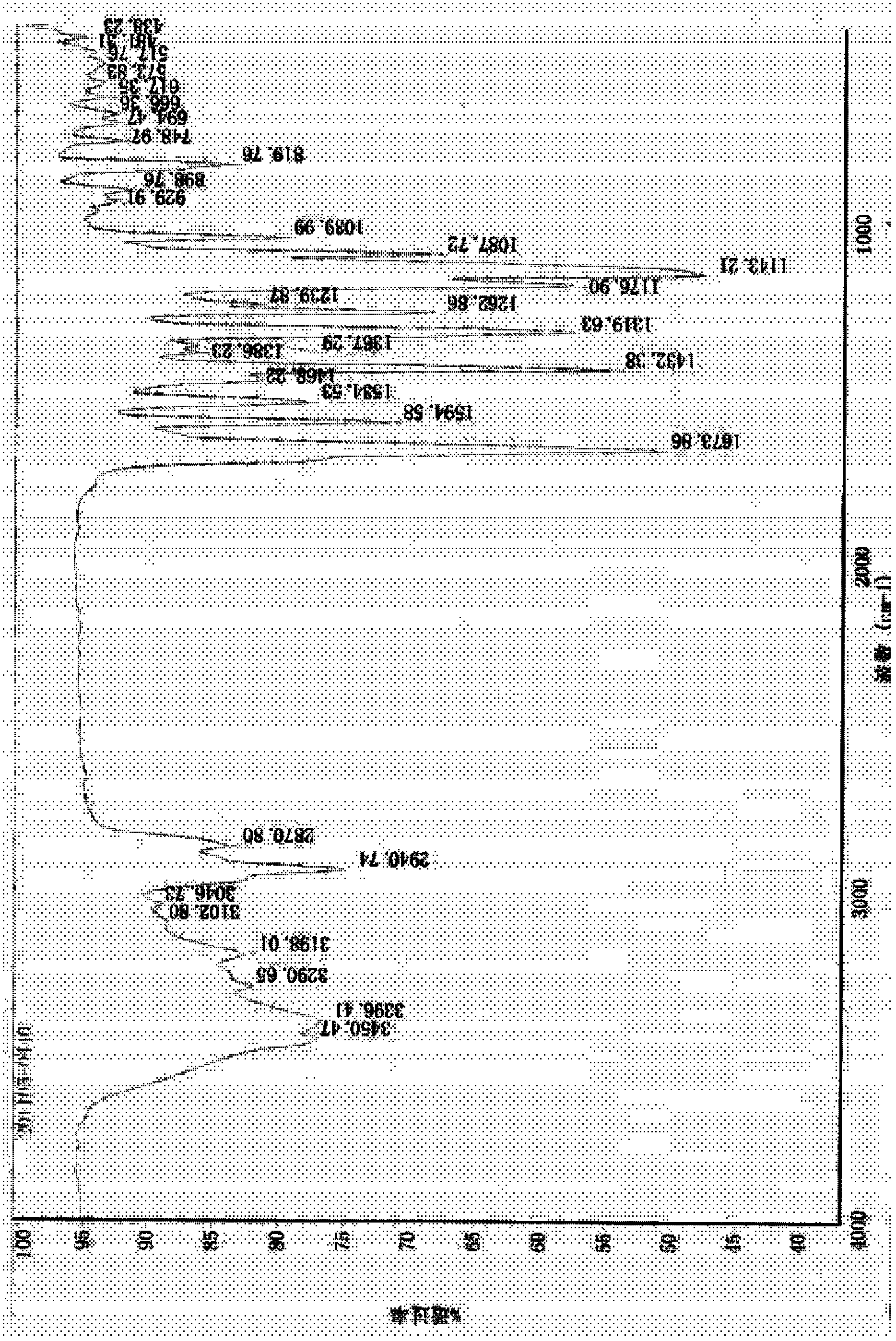
Image
Examples
Embodiment 2
[0056] 1.3.2.1 Synthesis of I and II (salt formation reaction, dehydration reaction)
[0057] In a clean 1000ml reaction bottle equipped with distillation concentration and tail gas absorption device, put 600ml of ammonia water with a concentration of 20%, put 50.00g of DT4 under stirring, slowly raise the temperature to 80°C, and keep warm until the reaction solution changes from cloudy to clear gradually , continue to heat up and concentrate until a large amount of precipitation and thicken, cool to below 60°C, add 500ml of xylene, change the distillation and concentration device to a reflux water separation device, heat up to reflux, divide water until the distillate is clear, continue to heat up and concentrate to a large amount Precipitates and thickens. Cool down to room temperature, filter, wash the reaction bottle with 100ml xylene in stages, then wash the filter cake, filter dry, and vacuum-dry at 60-80°C to constant weight to obtain 49.55g of white II crystals with a...
Embodiment 3
[0061] 1.3.3.1 Synthesis of I and II (salt formation reaction, dehydration reaction)
[0062] In a clean 1000ml reaction bottle equipped with distillation concentration and tail gas absorption device, put 600ml of ammonia water with a concentration of 20%, put 50.00g of DT4 under stirring, slowly raise the temperature to 80°C, and keep warm until the reaction solution changes from cloudy to clear gradually , then continue to heat up and concentrate until a large amount of precipitation and thicken, cool to below 60°C, add 500ml of xylene, change the distillation and concentration device to a reflux water separation device, heat up to reflux, divide water until the distillate is clear, and then continue to heat up and concentrate To a large amount of precipitation and thick. Cool down to room temperature, filter, wash the reaction flask with 100ml of xylene in stages, wash the filter cake, filter to dryness, and vacuum-dry at 60°C to constant weight to obtain 49.46g of white II...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mp | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com