Compound preparation and preparation method thereof
A technology of compound preparations and active ingredients, applied in the field of medicine, can solve the problem of undetermined exact relationship, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Example 1 Compound Tamsulosin Dutasteride Capsules
[0018] prescription:
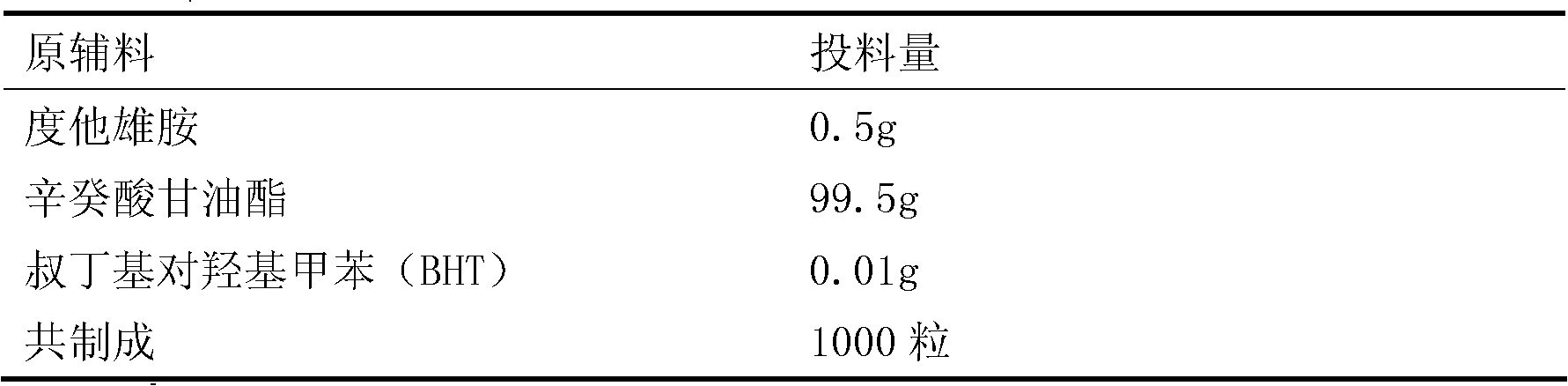
[0019] Dutasteride fraction:
[0020]
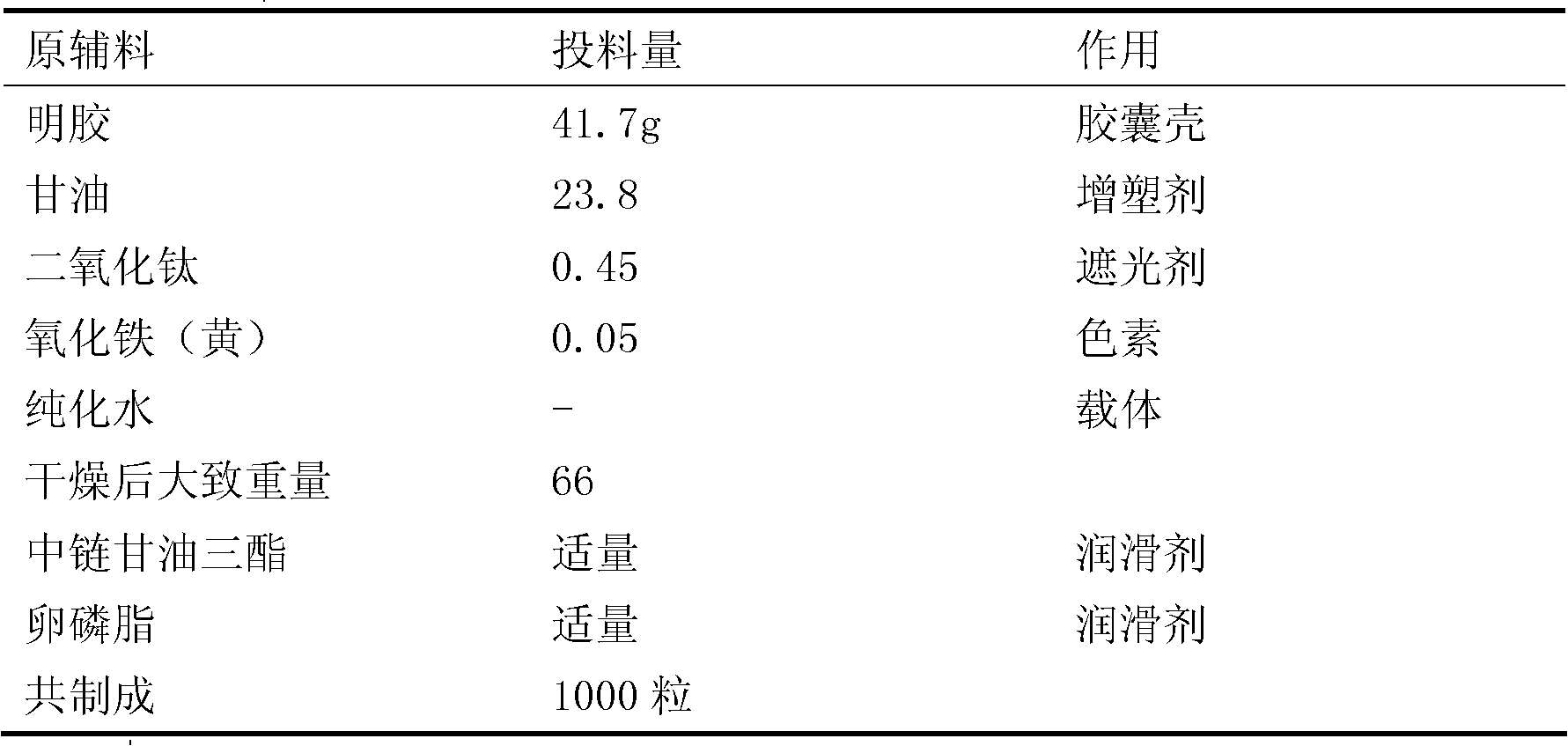
[0021] Capsule shell part:
[0022]
[0023] Process:
[0024] 1. Get gelatin, glycerin, titanium dioxide, iron oxide (yellow) purified water, heat on a water bath to melt, and stir evenly, with medium-chain triglycerides and medium-chain triglycerides containing lecithin (0.3%) as Lubricant, for the preparation of soft capsule shells.
[0025] 2. Take caprylic capric acid glyceride and heat it to 40°C on a water bath, and fill the container with nitrogen for the next step. Add BHT and dutasteride to dissolve completely and become a homogeneous transparent liquid. Cool to 25°C-33°C. Filter and set aside.
[0026] 3. Prepare drug-loaded soft capsules with the above-mentioned capsule material and medicinal solution. About 66mg in each capsule shell, its content weighs about 100mg. Obtain drug-loaded dutasteride soft capsules.
[0027] Tamsulos...
Embodiment 2
[0034] Example 2 Compound Tamsulosin Dutasteride Capsules
[0035] prescription:
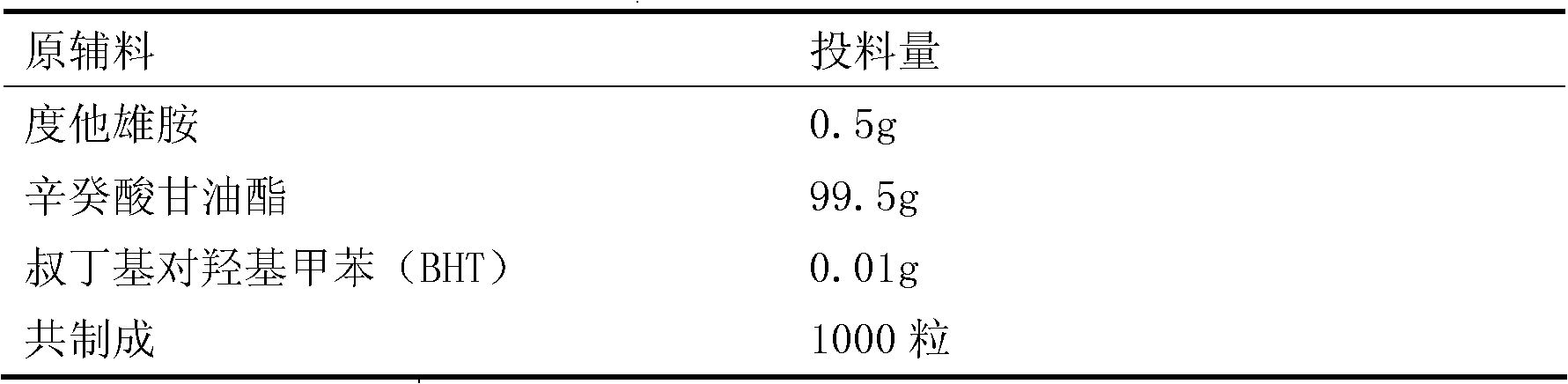
[0036] Dutasteride fraction:
[0037]
[0038] Capsule shell part:
[0039]
[0040] Process:
[0041] 1. Get gelatin, glycerin, titanium dioxide, iron oxide (yellow) purified water, heat on a water bath to melt, and stir evenly, with medium-chain triglycerides and medium-chain triglycerides containing lecithin (0.3%) as Lubricant, for the preparation of soft capsule shells.
[0042] 2. Take caprylic capric acid glyceride and heat it to 40°C on a water bath, and fill the container with nitrogen for the next step. Add BHT and dutasteride to dissolve completely and become a homogeneous transparent liquid. Cool to 25°C-33°C. Filter and set aside.
[0043] 3. Prepare drug-loaded soft capsules with the above-mentioned capsule material and medicinal solution. About 66mg in each capsule shell, its content weighs about 100mg. Obtain drug-loaded dutasteride soft capsules.
[0044] Tamsulos...
Embodiment 3
[0051] Example 3 Compound Tamsulosin Dutasteride Capsules
[0052] prescription:
[0053] Dutasteride fraction:
[0054]
[0055] Capsule shell part:
[0056]
[0057] Process:
[0058] 1. Get gelatin, glycerin, titanium dioxide, iron oxide (yellow) purified water, heat on a water bath to melt, and stir evenly, with medium-chain triglycerides and medium-chain triglycerides containing lecithin (0.3%) as Lubricant, for the preparation of soft capsule shells.
[0059] 2. Take caprylic capric acid glyceride and heat it to 40°C on a water bath, and fill the container with nitrogen for the next step. Add BHT and dutasteride to dissolve completely and become a homogeneous transparent liquid. Cool to 25°C-33°C. Filter and set aside.
[0060] 3. Prepare drug-loaded soft capsules with the above-mentioned capsule material and medicinal solution. About 66mg in each capsule shell, its content weighs about 100mg. Obtain drug-loaded dutasteride soft capsules.
[0061] Tamsulosin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com