Method for separation determination of related substances in bulk drugs and preparations of paricalcitol through HPLC method
A technology of paricalcitol and related substances, applied in the field of analytical chemistry, which can solve the problems of low product specifications, reduced retention time of main peaks, interference detection, etc., and achieve good separation, accurate results, and strong specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] The determination of embodiment 1 chromatographic conditions
[0046]Based on the limitations of the determination of related substances in Paricalcitol Soft Capsules by reversed-phase liquid chromatography, as described in the background of the present invention, after repeated screening, a normal-phase chromatography system was finally selected, and a gradient elution method was adopted for Paricalcitol Soft Capsules. Related substances inspection of riscalcidol soft capsules to improve detection sensitivity and reduce peak width expansion caused by large-volume injection; increase column temperature to 45°C to speed up peak elution time of oily matrix; choose to use silica gel column for this product The separation effect of impurity peaks and the peak shape of the main peak were ideal; at the same time, in order to improve the detection ability of impurities, the injection volume was selected as 100 μl. The results of the specificity study showed that the peak of th...
Embodiment 2
[0052] Impurity A is an impurity that may be introduced during the synthesis of the Paricalcitol bulk drug, and its structure is as follows:
[0053]
[0054] Weigh about 20 mg of the paricalcitol reference substance, put it in a 10ml measuring bottle, add absolute ethanol to dissolve and dilute to the mark, and use it as the paricalcitol reference substance stock solution.
[0055] Weigh about 10 mg of the impurity A reference substance (content 95.5%), put it in a 100ml measuring bottle, add absolute ethanol to dissolve and dilute to the mark, shake well, and use it as the impurity A reference substance stock solution.
[0056] Measure the appropriate amount of Paricalcitol reference substance stock solution and impurity A reference substance stock solution respectively, add n-hexane-isopropanol (volume ratio 90:10) to dilute, and prepare to contain about 10.0% of Paricalcitol in every 1ml. μg and impurity A 0.1μg mixed solution, as the system suitability solution.
[00...
Embodiment 3
[0061] The mensuration of embodiment 3 paricalcitol soft capsule
[0062] Get the contents of 10 paricalcitol soft capsules and mix them as the test solution. At the same time, a blank matrix (that is, a blank matrix without the main drug paricalcitol) was taken as a control solution.
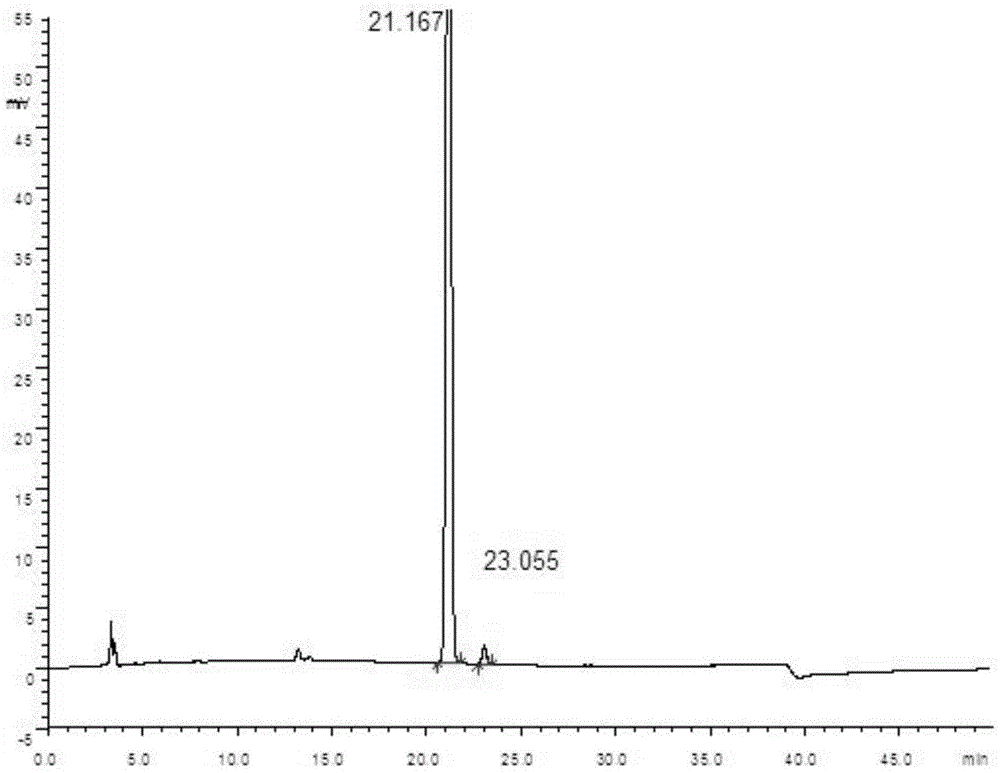
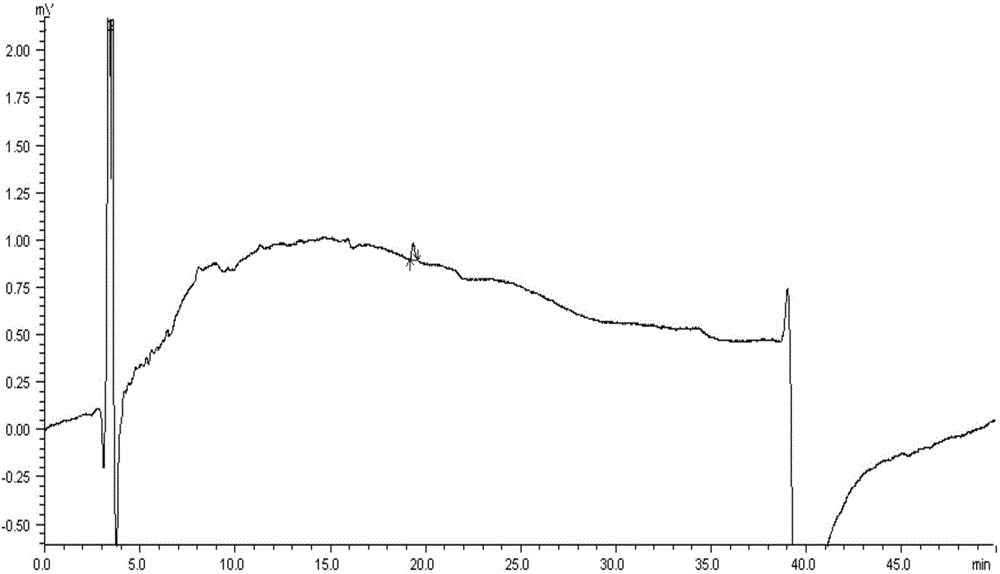
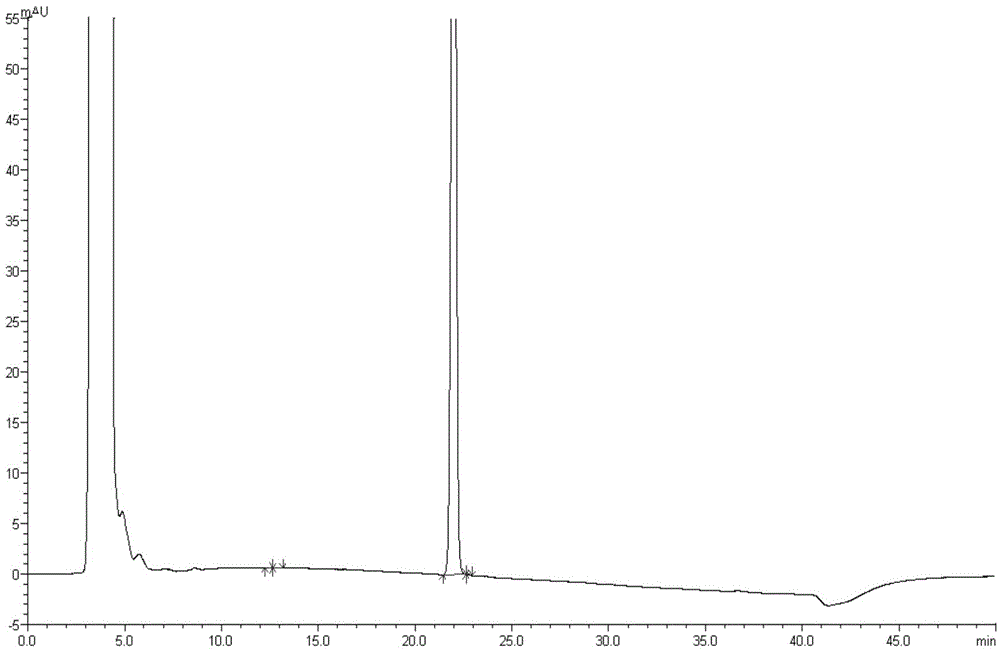
[0063] Accurately measure 50 μl each of the test solution and the reference solution respectively, inject it into a liquid chromatograph, measure according to the optimum chromatographic conditions determined in Example 1, and record the chromatogram. The chromatogram of paricalcitol soft capsule need testing solution detection is as follows image 3 as shown, Figure 4 It is the detection chromatogram of the blank matrix control solution. Depend on Figure 4 It can be seen that the blank matrix of Paricalcitol Soft Capsules in the normal phase chromatographic system, due to poor solubility, basically no peaks, and does not interfere with the detection of the main peak of Paricalcitol and i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com