Pharmaceutical composition containing paricalcitol
A technology for paricalcitol and a composition is applied in the field of soft capsule pharmaceutical compositions containing paricalcitol and the preparation thereof, and can solve the problems of poor disintegration uniformity, poor dissolution, and soft capsules of paricalcitol. Problems such as long disintegration time of capsules
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
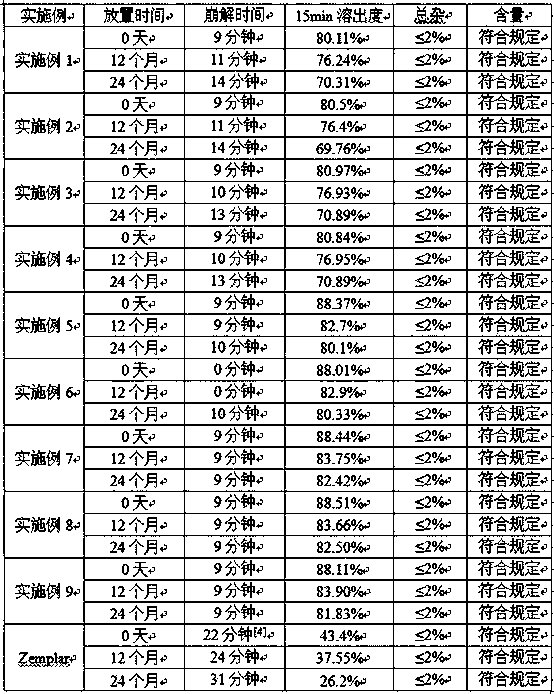
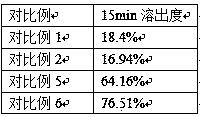
Examples
Embodiment 1
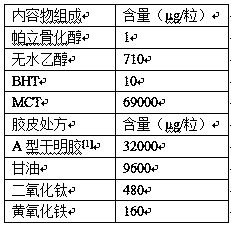
[0090] Follow the prescription below:
[0091]
[0092] [1] Type A gelatin was purchased from Rousselot 200 AH type gelatin.
[0093] Preparation process: Add BHT and Paricalcitol into absolute ethanol, stir to dissolve, add medium-chain triglycerides in equal amounts and stir to mix evenly.
[0094] Grind and disperse titanium dioxide and yellow iron oxide with some water to obtain a pigment suspension; after heating up to 60-70°C, add gelatin and pigment suspension in sequence, wash the adhered materials in the container with remaining water and put them into the solution; Keep the temperature of the glue solution at 60-70°C, stir and vacuumize to remove air bubbles; measure the viscosity of the glue solution, it should be 8000-30000mPa·s, and keep the glue solution at 50-60°C after removing air bubbles for later use.
[0095] Adjust the temperature of the plastic box, the temperature of the spray body, and the thickness of the rubber in the range of 0.6-0.9mm, and press...
Embodiment 2
[0097] Follow the prescription below:
[0098]
[0099] [1] Type A gelatin was purchased from Rousselot type 200FG gelatin.
[0100] The preparation method is the same as in Example 1. Finally dry to the target rubber moisture of 19%.
Embodiment 3
[0102] Follow the prescription below:
[0103]
[0104] [1] Type A gelatin was purchased from Rousselot 180PS type gelatin.
[0105] The preparation method is the same as in Example 1. Finally dry until the target rubber moisture is 9%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com