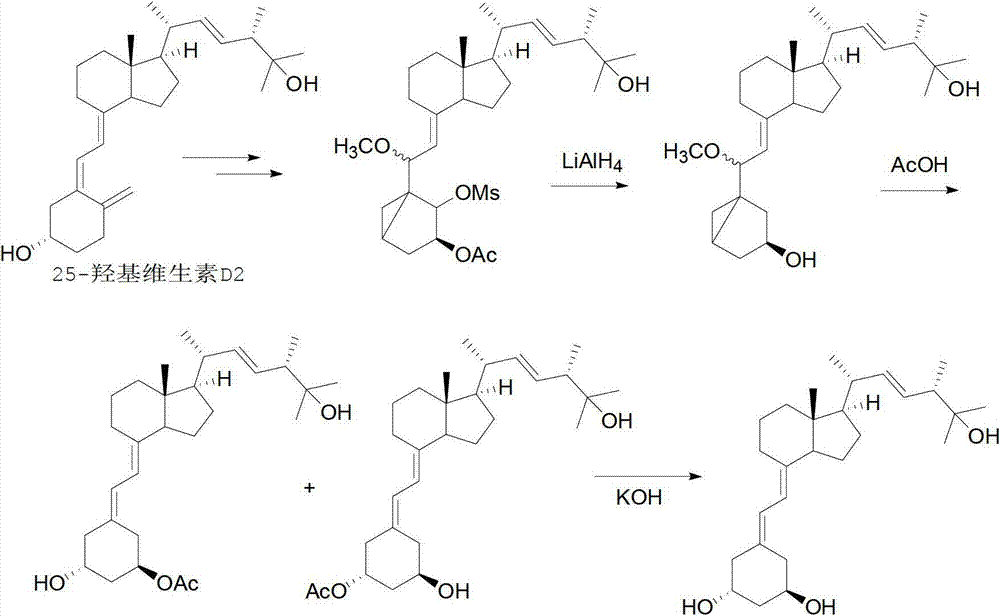
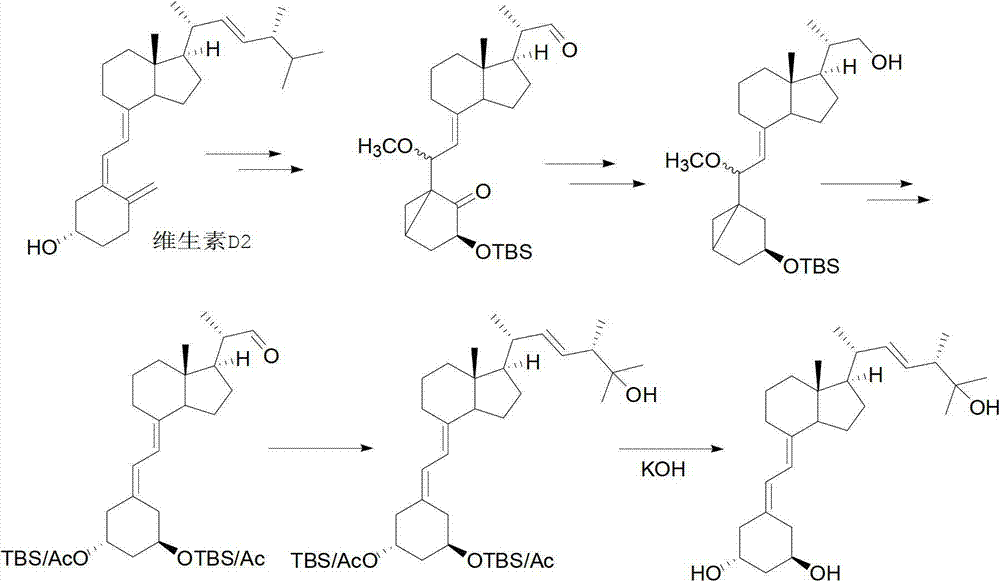
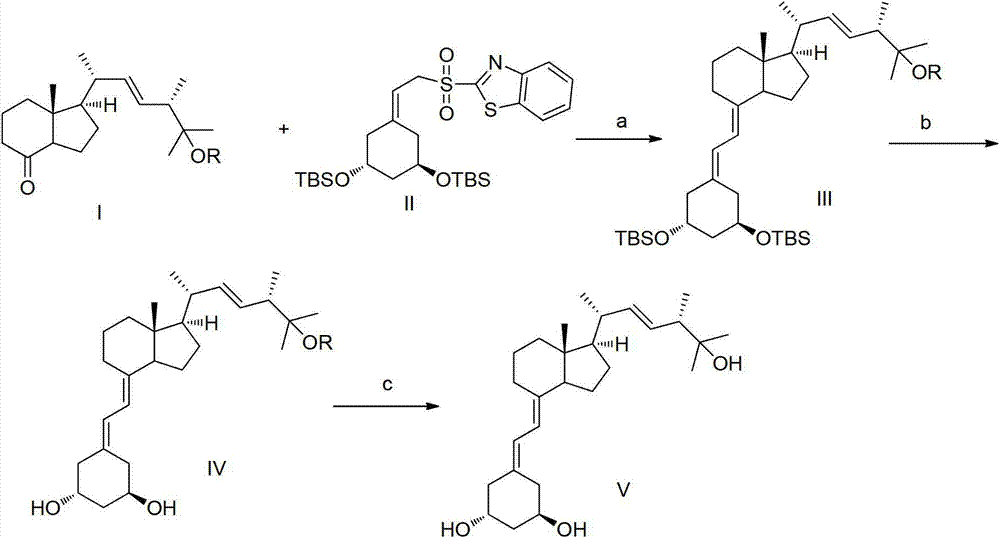
Method for synthesizing paricalcitol
A technology for paricalcitol and a synthesis method, which is applied in the directions of organic chemistry, bulk chemical production, etc., can solve problems such as being unsuitable for industrialized production of paricalcitol, difficult to identify and purify, and complex intermediates, etc. Structural identification and purification, easy large-scale preparation, simple operation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] 1.1 Preparation of compound IIIa
[0026]
[0027] Under nitrogen protection, compound II (0.51 g) was dissolved in 4 ml of anhydrous tetrahydrofuran, the temperature was lowered to -65 ° C, 1 ml of bistrimethylsilylamide lithium (1M) was added dropwise to the above solution, and stirred for 10 Minutes, then dissolve compound Ia (0.3g) in 2 ml of tetrahydrofuran, add dropwise to the above reaction system, keep the internal temperature at -57°C for 3 hours, after the reaction, warm up to room temperature, add ethyl acetate and chloride Ammonium solution, the organic layer was separated, dried over sodium sulfate and evaporated to dryness, and purified by column chromatography to obtain 0.45 g of compound IIIa with a yield of 74%.
[0028] 1 H NMR (CCl 3 ,400MHz):δ=-0.04(s,6H),0.00(s,6H),0.60(s,3H),0.83(s,18H),0.95(m,6H),1.10(s,3H),1.14 (s,3H),1.21(m,3H),1.54(m,3H),1.67(m,2H),1.90(m,7H),2.15(m,4H),2.38(m,1H),3.34( s,3H),4.00(m,2H),4.68(s,2H),5.25(m,2H),6.03(d,J=12H...
Embodiment 2
[0066] 2.1 Preparation of compound IVa
[0067]
[0068] Compound IIIa (0.40 g) obtained in Example 1 and 7 ml of tetrahydrofuran were added at room temperature, and tetrabutylammonium fluoride trihydrate (0.73 g) was heated to 60° C. for 8 h. After the reaction was completed, ethyl acetate was added. It was washed with saturated brine, dried over anhydrous sodium sulfate, filtered, and concentrated to obtain a crude product, which was purified by column chromatography to obtain 0.23 g of IVa with a yield of 88%.
[0069] 1 H NMR (CCl 3 ,400MHz): δ=0.65(s,3H),1.02(m,6H),1.13(s,3H),1.17(s,3H),1.25-1.54(m,3H),1.86-2.10(m,14H ),2.15-2.23(m,3H),3.37(s,3H),4.07(m,2H),4.72(s,2H),5.30(m,2H),6.13(d,J=11.2Hz,1H) ,6.47(d,J=11.2Hz,1H).
[0070] MS (EI): m / e=460.
[0071] 2.2 Preparation of compound IVa
[0072]
[0073]Compound IIIa (0.40 g) obtained in Example 1, 3 ml of tetrahydrofuran, and 5 ml of methanol were added at room temperature, and potassium fluoride (0.14 g) wa...
Embodiment 3
[0112] 3.1 Preparation of Compound V, i.e. Paricalcitol
[0113]
[0114] Compound IVa (0.20 g) prepared in Example 2 was dissolved in 3 ml of methanol, p-toluenesulfonic acid (83 mg) was added, and reacted at room temperature 25° C. for 8 h. After the reaction was completed, triethylamine was added to adjust the pH to 7~8, ethyl acetate was added to extract, washed with saturated brine, dried over anhydrous sodium sulfate, filtered, concentrated and purified by column chromatography to obtain compound V (0.14g, yield 75%) .
[0115] 1 H NMR (CCl 3,400MHz): δ=0.65(s,3H),1.01(m,6H),1.11(s,3H),1.14(s,3H),1.26-1.30(m,3H),1.60-2.15(m,17H ), 4.03-4.10(m, 2H), 5.28-5.40(m, 2H), 6.11(d, J=11.6Hz, 1H), 6.44(d, J=11.6Hz, 1H).
[0116] MS (EI): m / e=416.
[0117] 3.2 Preparation of Compound V, i.e. Paricalcitol
[0118]
[0119] Compound IVa (0.20 g) prepared in Example 2 was dissolved in 5 ml of methanol, concentrated hydrochloric acid (50 mg) was added, and reacted at room ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com