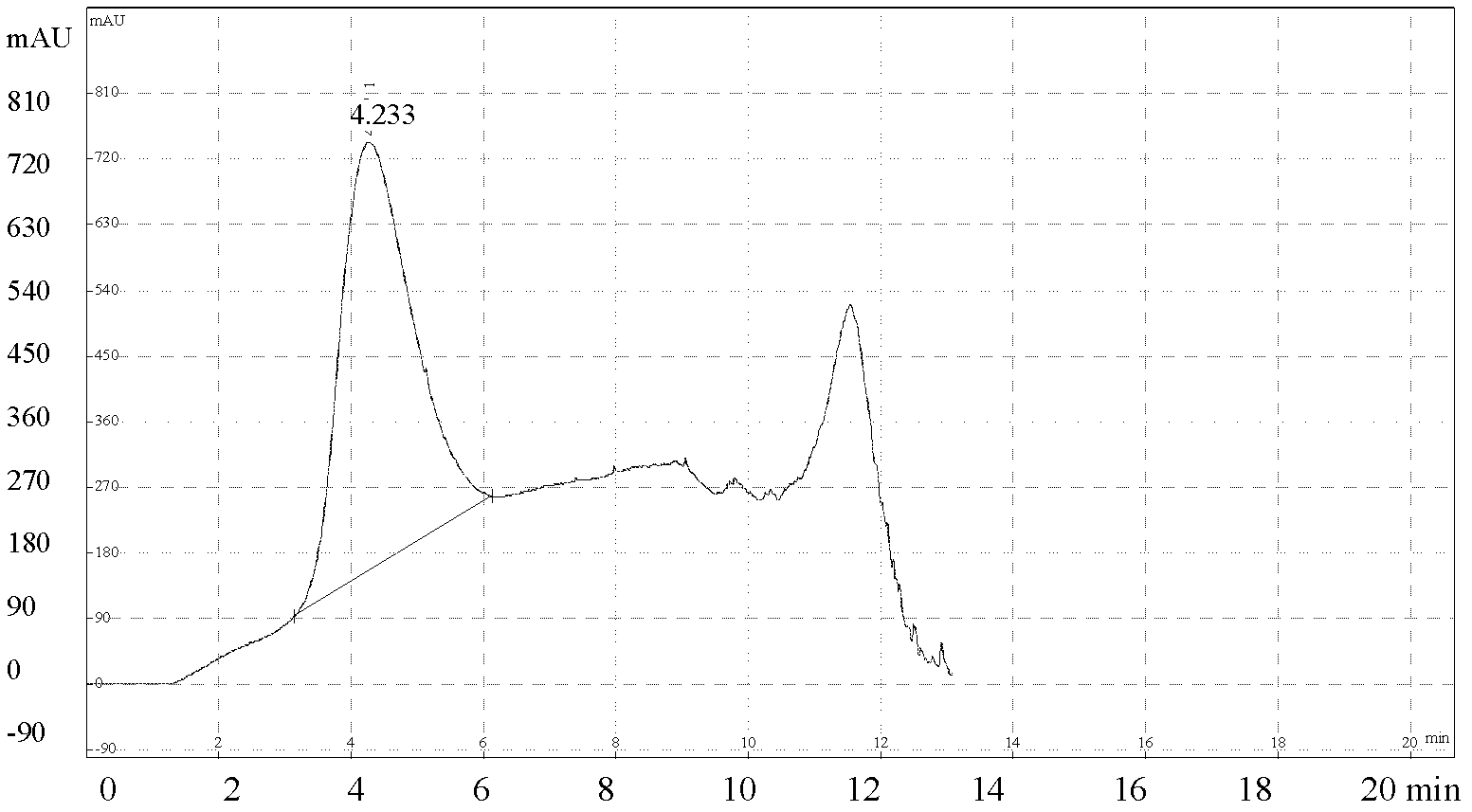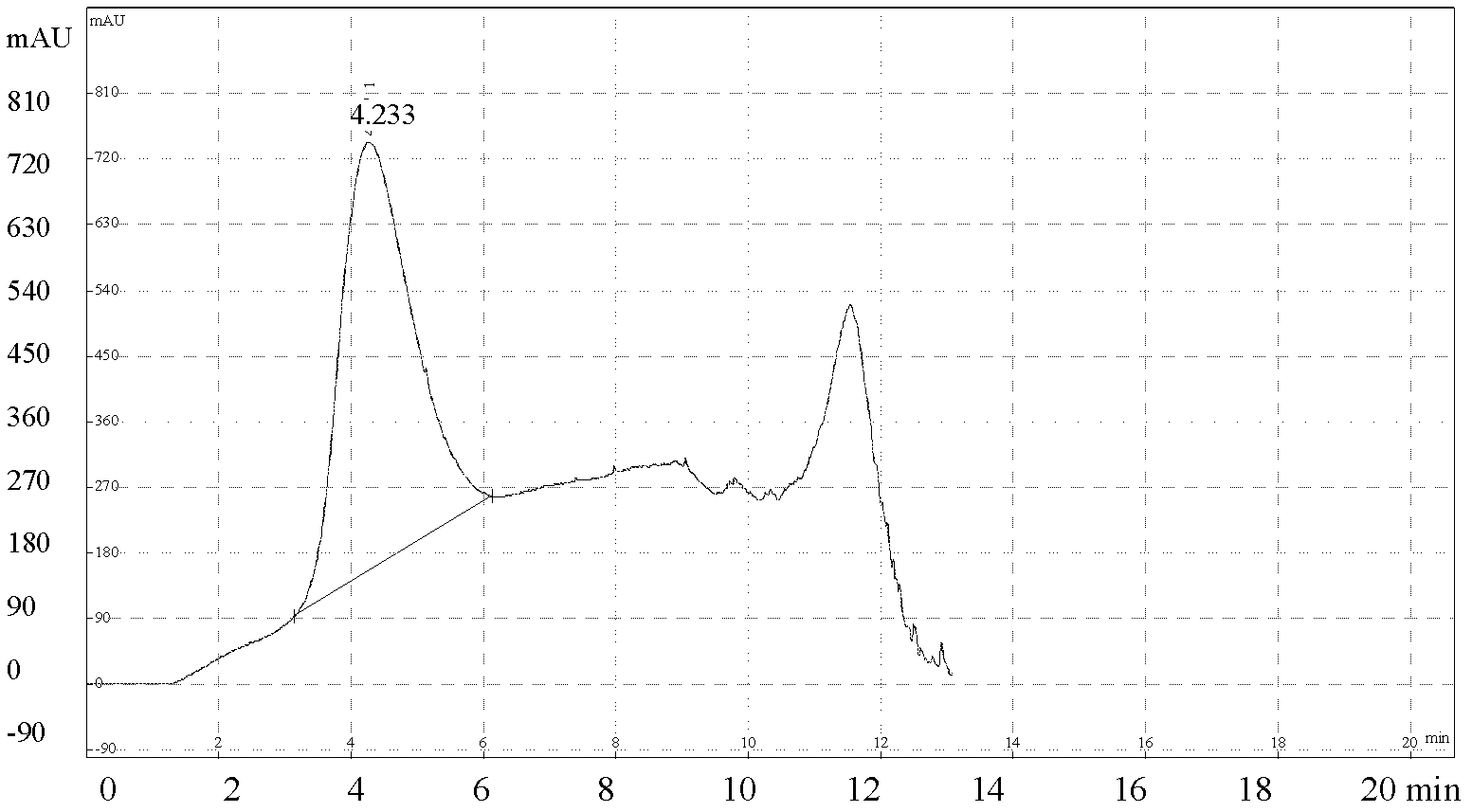Patents
Literature
37 results about "Protamine sulfate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Protamine sulfate is a medication that is used to reverse the effects of heparin. It is specifically used in heparin overdose, in low molecular weight heparin overdose, and to reverse the effects of heparin during delivery and heart surgery. It is given by injection into a vein. The onset of effects is typically within five minutes.
Method for quantification of 146S content in foot-and-mouth disease antigen by using liquid chromatography detection system
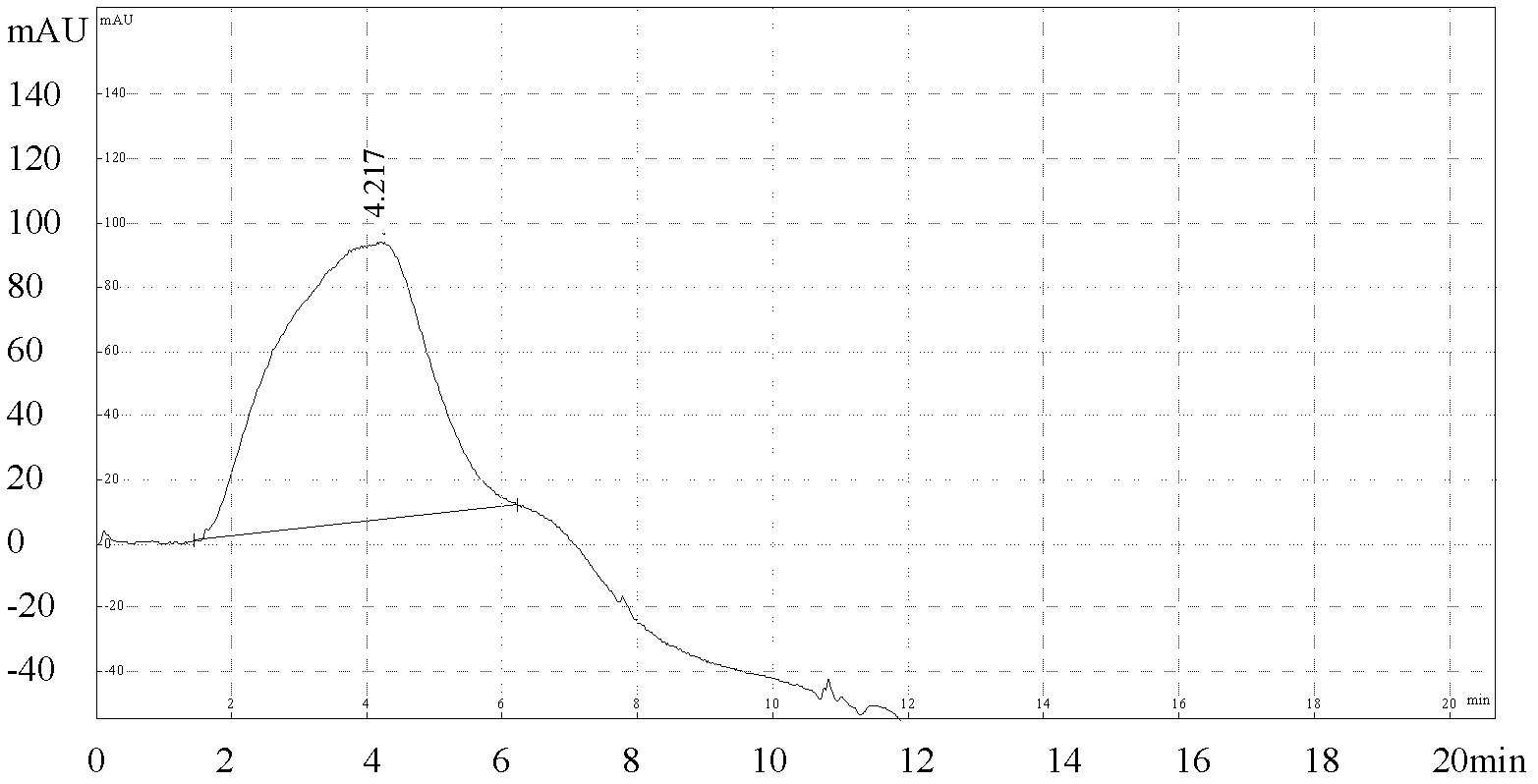
The present invention discloses a method for quantification of 146s content in foot-and-mouth disease antigen by using a liquid chromatography detection system. The method comprises: adopting protamine sulfate to purify virus, adopting PEG6000 to concentrate the virus, carrying out sucrose density gradient ultracentrifugation on the concentrated virus, adopting a liquid chromatography detection system to detect an absorption peak of the centrifugated sample at a wavelength of 259 nm, and calculating 146S content according to the following formula: C=FRS / 76Wb. The method can be used for determination of the 146S content in various types of foot-and-mouth disease antigens. With quantification of the 146S content in the foot-and-mouth disease antigen, foot-and-mouth disease vaccine preparation can be guided, vaccine efficacy can be indirectly evaluated, and major guiding significances are provided for enhancing quality of the foot-and-mouth disease vaccine in our country.
Owner:内蒙古必威安泰生物科技有限公司
Mass-production method of hydrophobic vaccine
InactiveCN1966076AEasy to zoom inSmall pressure dropAntiviralsAntibody medical ingredientsUltrafiltrationFixed bed
The invention relates to a method for producing batch hydrophobia vaccine, wherein it comprises that: (1), in the biological reactor with fixed bed mixing system and Fibra-Cel disks carrier, using cell increment cultivate liquid to cultivate Vero cell; (2), when the Vero cell grows to some density, using cell hold cultivate liquid, seeding hydrophobia vaccine, and affecting cell; (3), increasing virus; (4), obtaining virus continuously; (5), ultra-filter concentrating and inactivating via beta-propanolide; (6), using protamine sulfate or DNA enzyme treatment to remove Vero cell DNA; (7), using Sepharose 4 Fast Flow as stuff to process chromatography purification; (8), adding human blood albumin and sugar as protector to be made into liquid agent; or adding human blood albumin, sugar, and gelatin, as protector and shaping agent to be made into dried agent. The inventive method can produce virus continuously in small biological reactor, to realize batch production.
Owner:广州市嘉合生物技术有限公司
Macrophage targeting carrier system and preparation method thereof
InactiveCN104771764AStrong targetingIncrease intakeGenetic material ingredientsOther foreign material introduction processesGene deliverySynthesis methods
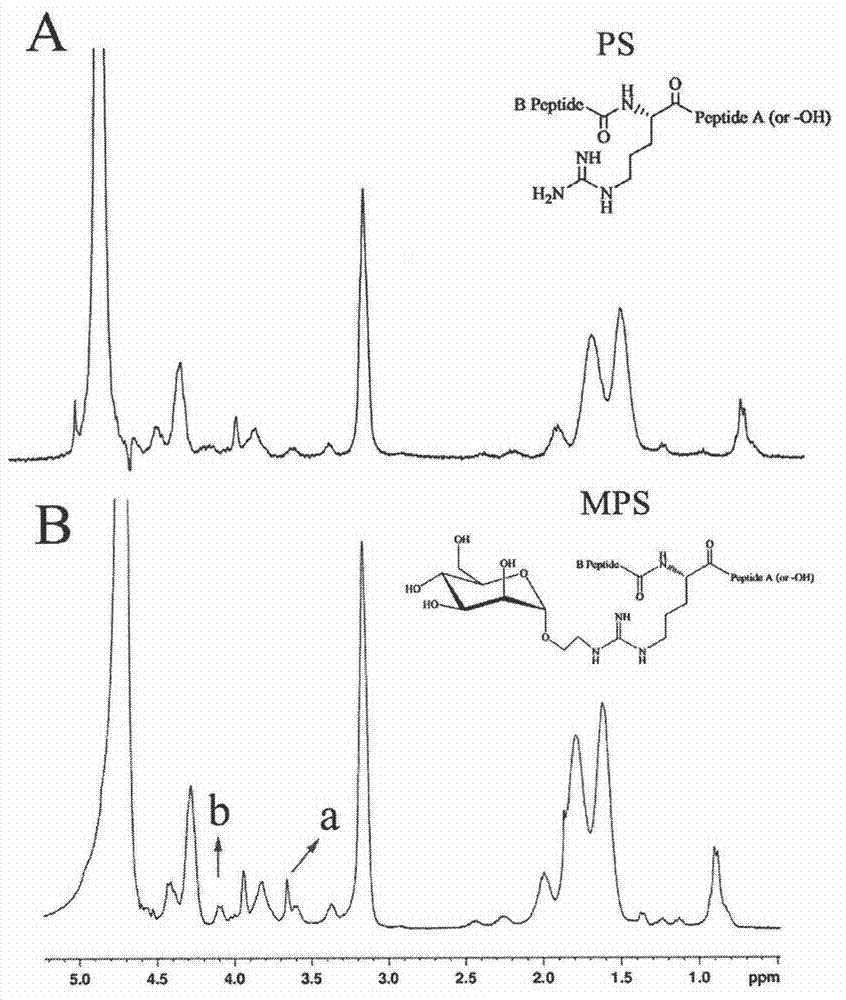
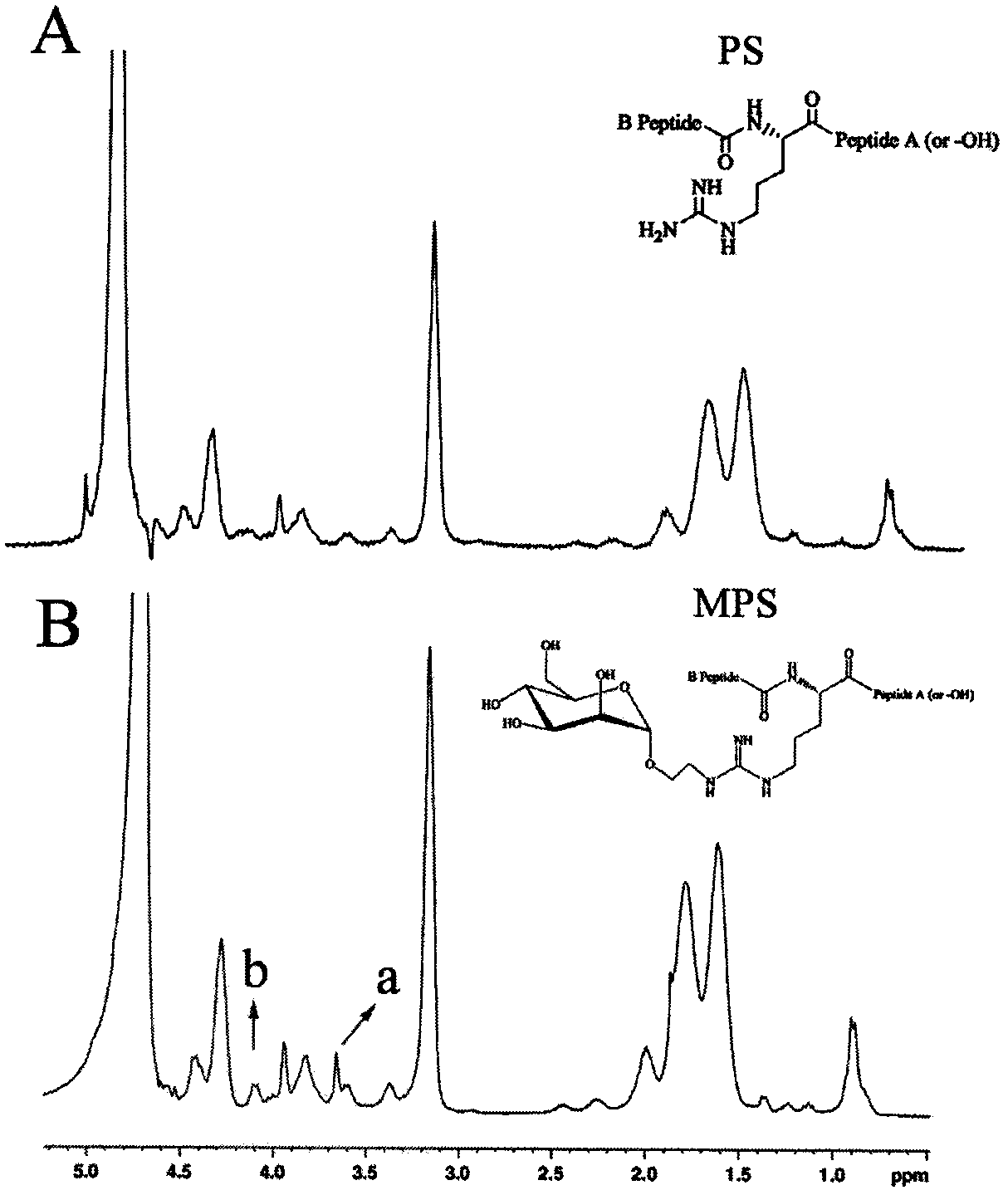
The invention belongs to the field of pharmacy, and discloses a synthesis method of a macrophage targeting carrier system and a preparation method of a gene delivery system. The macrophage targeting carrier system is characterized in that a macrophage targeting carrier is mannosylation protamine; electropositive mannosylation protamine loads electronegative nucleic acid to form a positively charged nano particle; and protamine modified by carubinose is prepared from formyl methyl mannopyranoside and protamine sulfate through a reductive amination reaction. Compared with non-viral gene carrier protamine, mannosylation protamine has a nuclear localization function and a macrophage targeting property, and can improve the gene transfection mediation efficiency of protamine in macrophage. The preparation method is simple, and mature in technology, and has good application prospects.
Owner:CHINA PHARM UNIV
Foot-and-mouth disease purification vaccine, preparation method and applications thereof
ActiveCN102988970AGood immune effectStable in natureAntiviralsRecovery/purificationAdjuvantFiltration
The present invention discloses a foot-and-mouth disease purification vaccine, a preparation method and applications thereof. The preparation method comprises: adopting protamine sulfate precipitation to remove most of hybridproteins and nucleic acids of host cells; condensing a virus liquid through a concentration system to be adopted as a chromatography sample; and selecting a polyacrylamide dextran gel as a chromatography medium to carry out purification elution, collecting a first elution peak, carrying out filtration sterilization inactivation, adding an adjuvant to carry out emulsification, and carrying out sub-packaging to obtain the foot-and-mouth disease purification vaccine. The preparation method has characteristics of economy, efficiency, low cost, simple and reasonable process, and effective removal of impurities in the host cells and the culture medium, wherein virus recovery rate is more than 85%, protein and nucleic acid removal rate is more than 90%, and the foot-and-mouth disease purification vaccine prepared by the preparation method has characteristics of safety, good immunity and stable property.
Owner:内蒙古必威安泰生物科技有限公司
Broad spectrum biological disinfection air filtering material and preparation method thereof
InactiveCN101293107AStable in natureAvoid pollutionDeodrantsFiltration separationAir filtrationProtamine sulfate
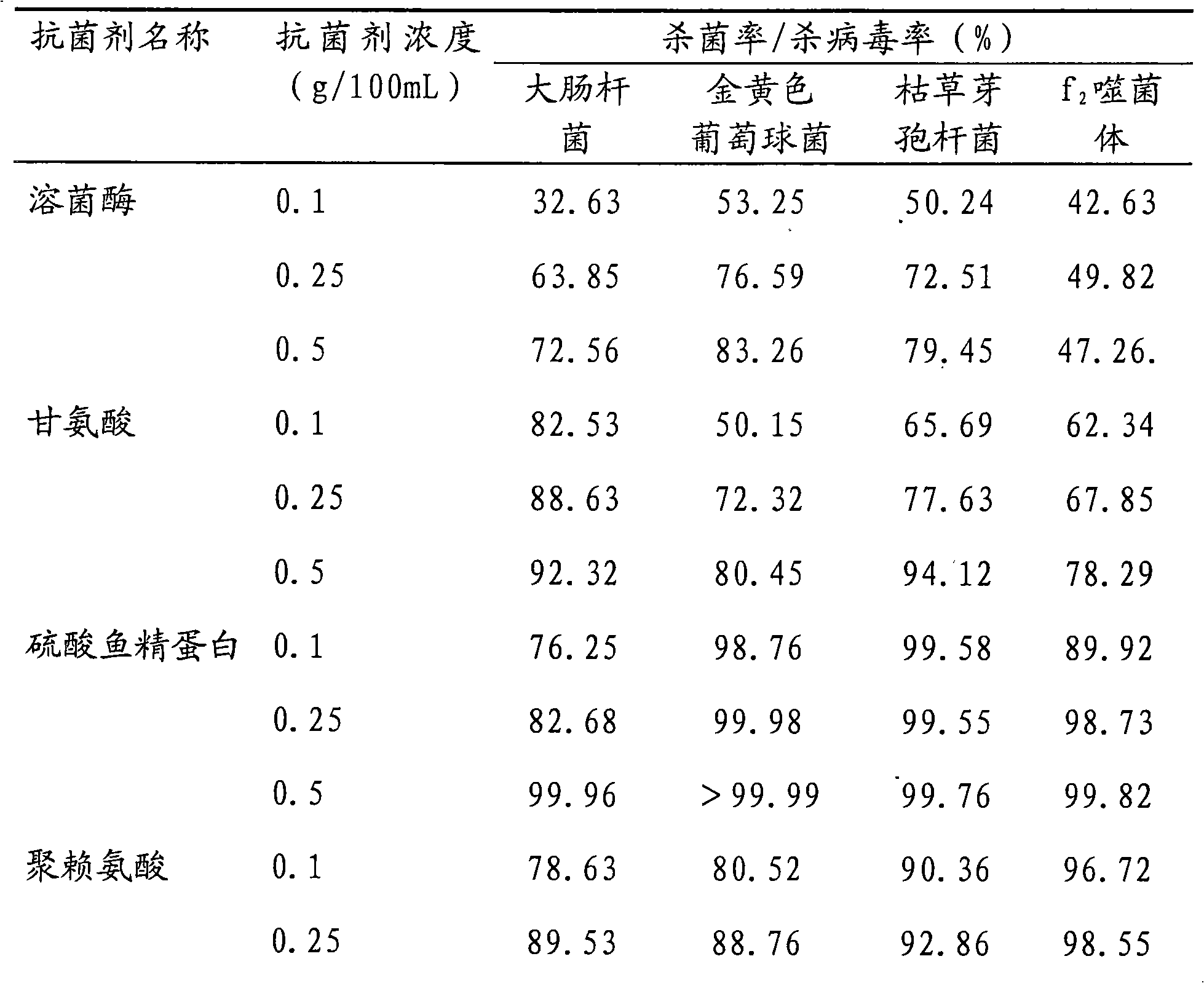
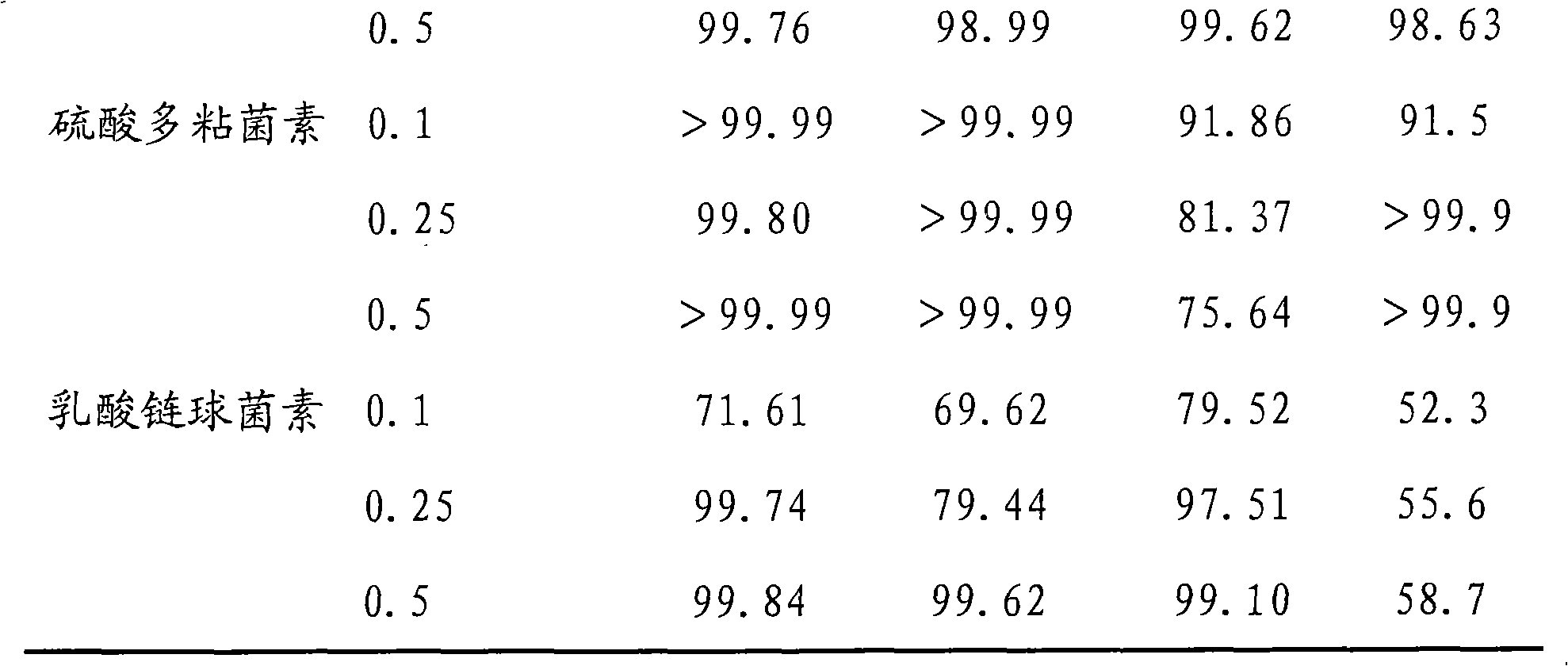
The invention discloses a broad-spectrum biological bactericidal air filtration material and a preparation method thereof. The invention is not only applicable to the general families and business departments with the need of air purification, but is also applicable to the semiconductor, pharmaceutical, hospitals and other environments with higher requirements of cleanliness. The broad-spectrum biological bactericidal air filtration material of the invention comprises at least one biological anti-bacterial agent and glass fiber filter paper, and the biological anti-bacterial agent is lysozyme, protamine sulfate, poly-lysine, nisin, polymyxin sulfate, glycine or natamycin. The preparation method of the invention is that the air filtration material is prepared by silanization, hydroformylation, condensation reaction and removal of the physically absorbed anti-bacterial agent. The biological bactericidal air filtration material prepared by the invention has low consumption of the anti-bacterial agent and no influence on the resistance of the filtration material; the sterilization rate is high, most of the killing rate of bacterial, spores and viruses is more than 99 percent, and the fungal growth is inhibited.
Owner:SANITARY EQUIP INST ACAD OF MILITARY MEDICAL SCI PLA
Double timephase neo-insulin zinc injection (30%) and its prepn process
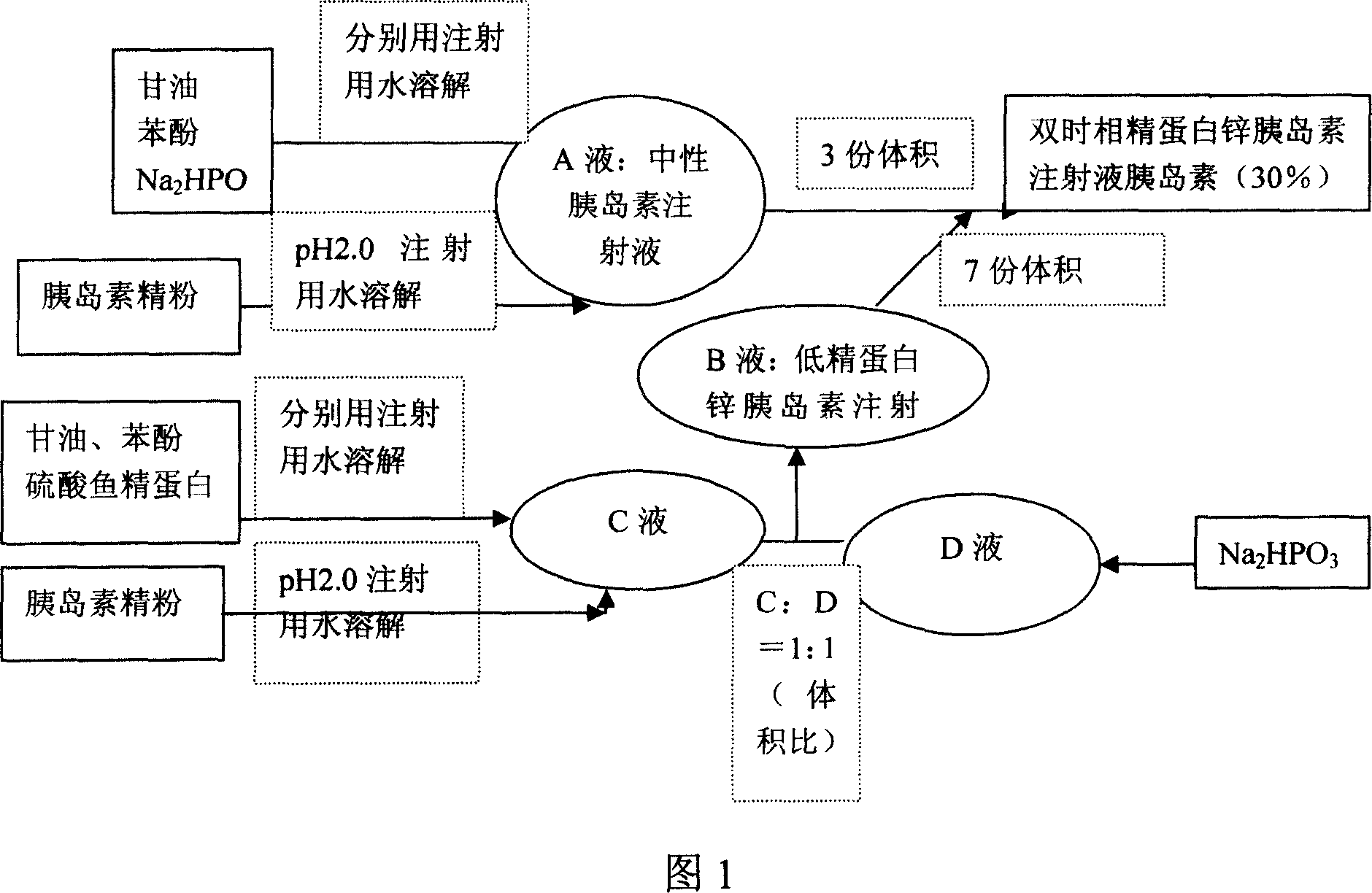
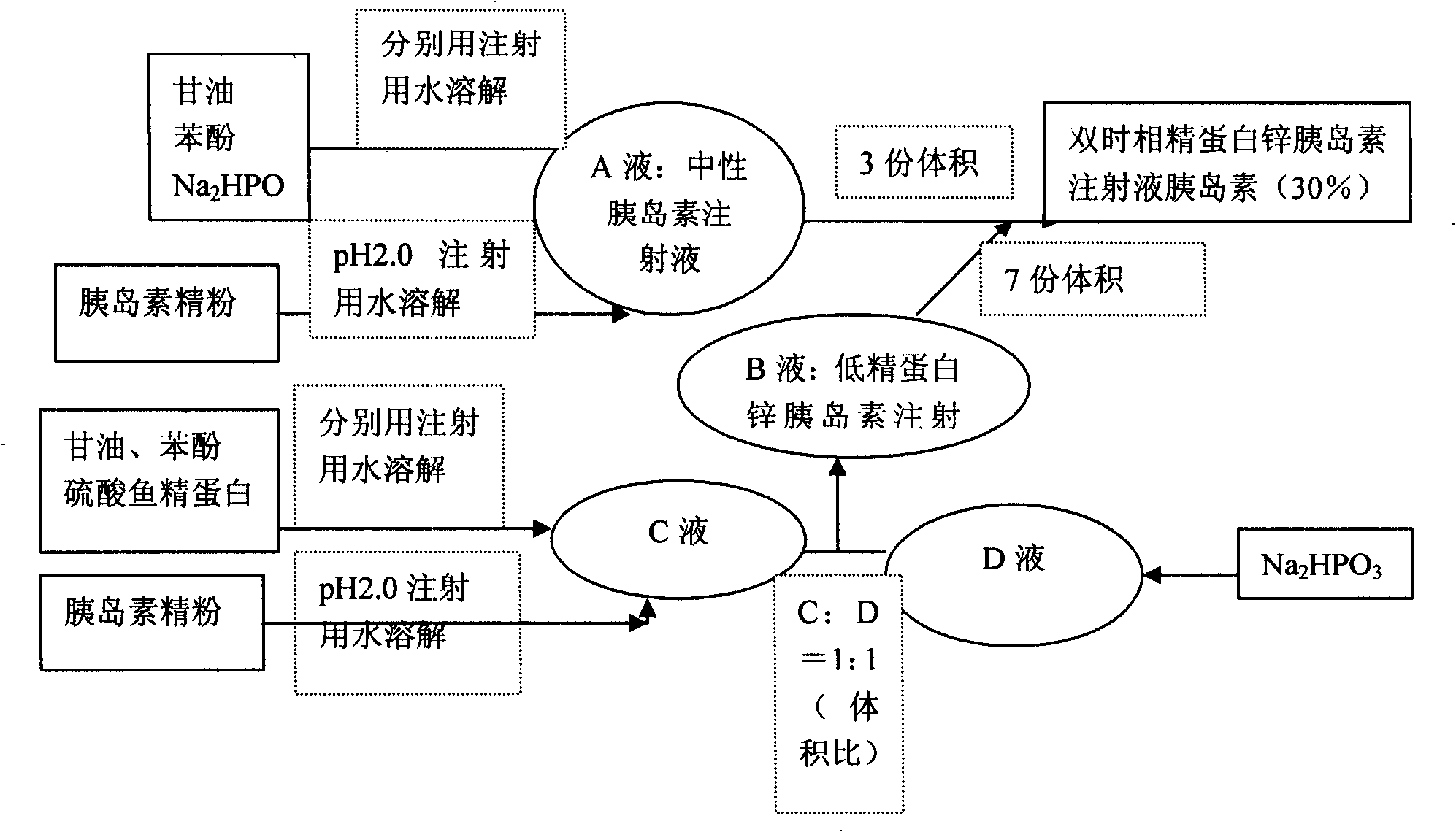
ActiveCN1931360AReduce the number of injectionsRelieve painPeptide/protein ingredientsMetabolism disorderDouble-timeInsulin injection
The present invention is double time phase protamine zinc insulin injection (30 %) and its preparation process, and belongs to the field of medicine preparation technology. The double time phase protamine zinc insulin injection (30 %) consists of insulin 100000 IU, isosmotic agent 35-45 g, preservative 5.5-7 g, pH regulator 12-12.5 g and fish protamine sulfate 0.21-0.42 g, and has pH value of 6.9-7.8. Its preparation process includes the steps of preparing neutral insulin injection, preparing low protamine zinc insulin injection, and mixing these two kinds of injection. The double time phase protamine zinc insulin injection (30 %) has two forms of insulin, including one in solution for quick acting and the other in precipitate with combined fish protamine sulfate as the middle acting phase. It has less required injection times.
Owner:JIANGSU WANBANG BIOPHARMLS
Insulin-protamine zinc injection and method for preparing the same
InactiveCN101219209AImprove stabilityReduce the number of injectionsPeptide/protein ingredientsMetabolism disorderInsulin injectionProtamine sulfate
The invention pertains to a pharmaceutical preparation field, and in particular relates to a zinc protamine insulin ampoule injection and a preparation method thereof. The zinc protamine insulin ampoule injection comprises insulin, isotonizing agent, antiseptic agent, pH regulator, protamine sulfate and bi-distilled water for injection, wherein, the bi-distilled water for injection contains divalent zinc salt and the concentration of zinc ion is 0.01-0.04g per100000IU of insulin. The injection of the invention has the advantages that minim zinc ion is added into the insulin to form a stable polymer, which stabilizes the insulin and has a prolonged curative effect; the invention has simple, scientific and reasonable preparation and easy manipulation. The insulin can exist in two patterns: firstly, in liquor with quick effect; secondly, combining with protamine sulfate and in precipitation with action characters of both two insulin preparations, which reduces injection time and pain caused by rejection. Animal insulin is used for the injection, thus reducing the cost.
Owner:JIANGSU WANBANG BIOPHARMLS
Self-assembly nano adjuvant and preparation method of nano vaccine formed by self-assembly nano adjuvant and application
InactiveCN108714213AEfficient packagingSolve the problem of easy degradation in the body and low bioavailabilitySsRNA viruses positive-senseViral antigen ingredientsAdjuvantMedicine
The invention relates to a self-assembly nano adjuvant and a preparation method of a nano vaccine formed by the self-assembly nano adjuvant and application. The nano adjuvant comprises self-assembly materials of protamine sulfates and carboxymethyl glucan, the self-assembly materials and CpG oligodeoxynucleotides self-assembly form the nano adjuvant, and the nano vaccine is formed by the nano adjuvant and the virus antigen. The nano adjuvant increases the bioavailability of the CpG oligodeoxynucleotides, degradation in the body is avoided, and the B type CpG is added the function of A type CpG. The nano vaccine not only can induce the humoral immune response of TH1 type, but also induce relatively high cellular immune response. The unvaccinated experiments in mice body proves that the nanovaccine has good protection function, which provides help in the application of nano vaccine in the future.
Owner:BEIJING UNIV OF TECH
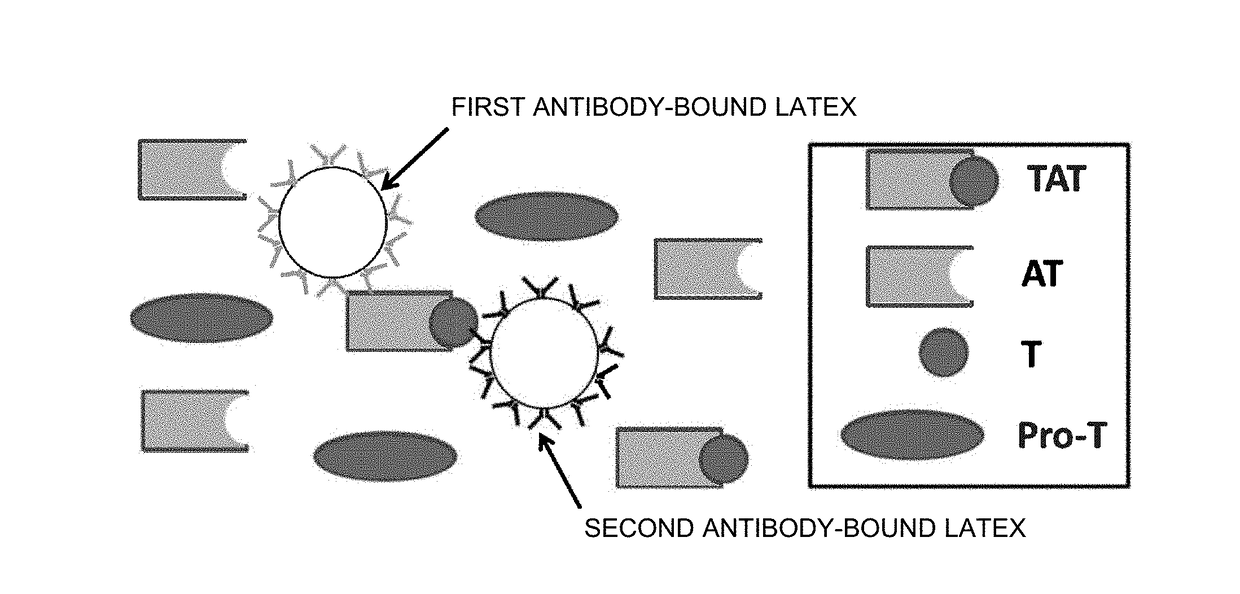
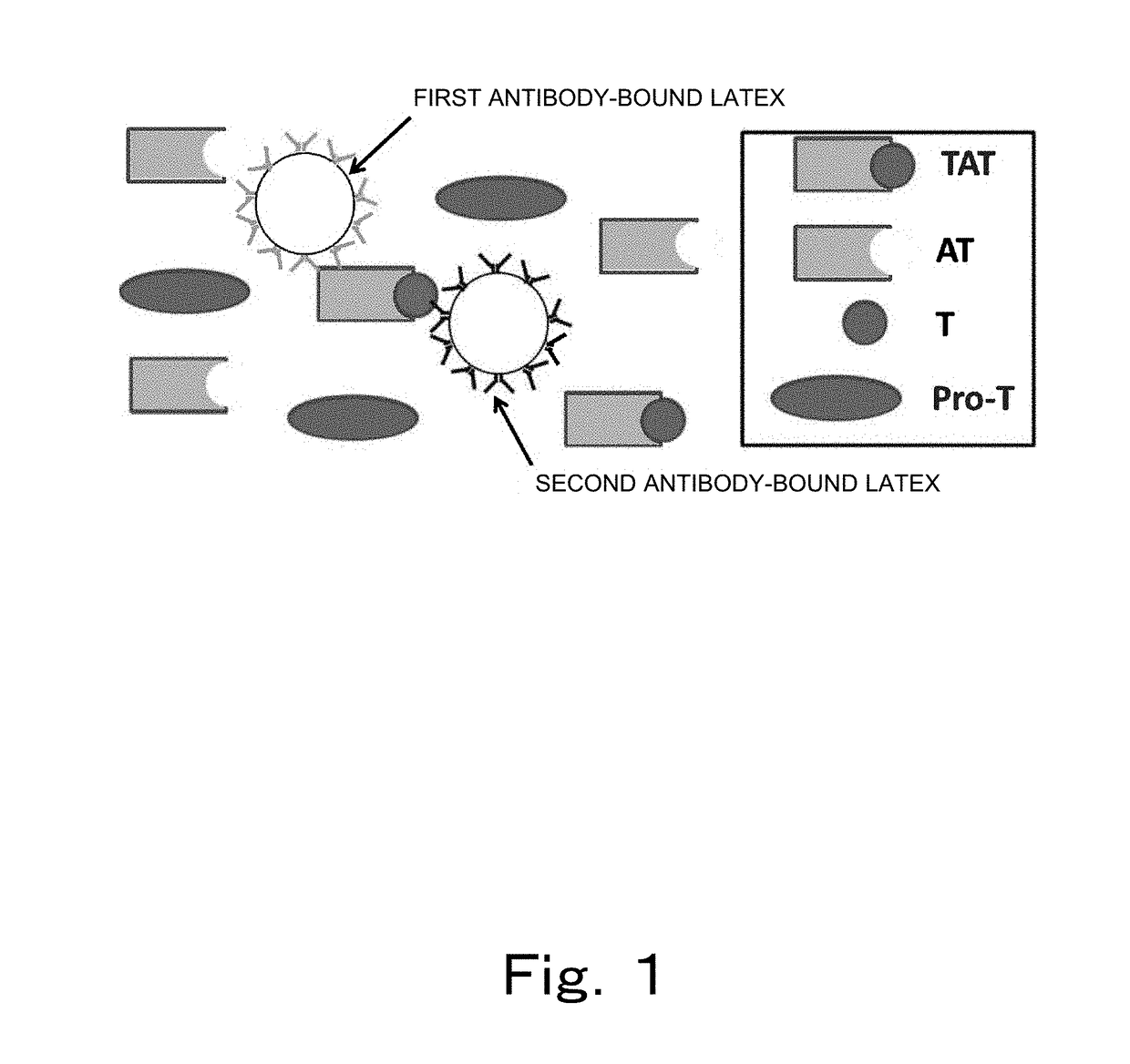
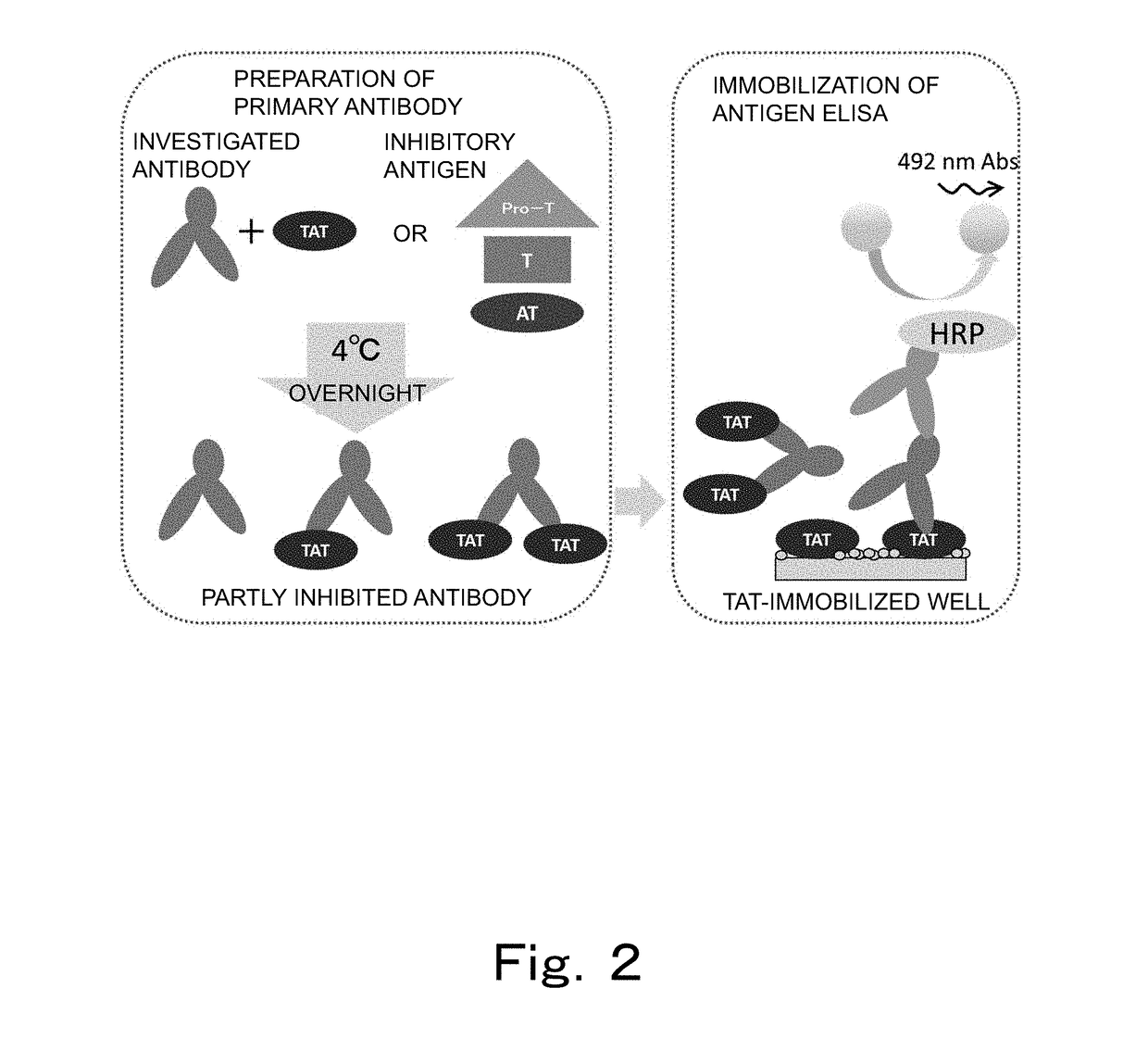
Reagent and method for measuring thrombin-antithrombin complex
ActiveUS20180238871A1Small amountAccurate measurementDisease diagnosisBiological testingDextranAmmonium chloride mixture
Owner:MITSUBISHI CHEM MEDIENCE
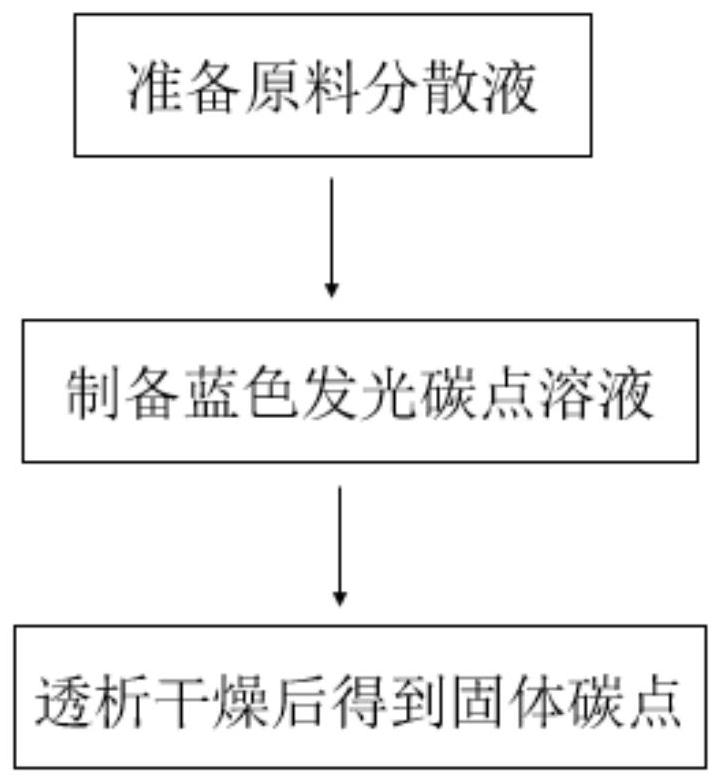
Blue light-emitting antibacterial carbon dots as well as preparation method and application thereof
ActiveCN111944524AGood water solubilityStable photochemical propertiesAntibacterial agentsCarbon active ingredientsCytotoxicityProtamine sulfate
The invention discloses a blue light-emitting antibacterial carbon dot and a preparation method and application thereof. The preparation method comprises the steps that a carbon source is subjected toa heating reaction to prepare the carbon dot, wherein the carbon source comprises protamine sulfate. In the method, the protamine sulfate is specifically selected as a carbon source to be heated to prepare the carbon dots, the blue light-emitting carbon dots capable of imaging and inhibiting bacteria at the same time can be synthesized through a one-step method, and the preparation method is simple. In addition, the blue light-emitting carbon dot is good in water solubility, stable in photochemical property, low in cytotoxicity and good in blood compatibility. Therefore, the antibacterial carbon dot has a wide application prospect in the field of dual functions of bacterial therapy and imaging.
Owner:SOUTH CENTRAL UNIVERSITY FOR NATIONALITIES
Stable antiheparin lipase detection kit
InactiveCN107782680AReduced stabilityImprove stabilityColor/spectral properties measurementsGlycerolProtamine sulfate
The invention discloses an enzymatic colorimetric lipase detection kit. The invention prepares a stable antiheparin lipase detection kit. The stable antiheparin lipase detection kit is a liquid-type double-reagent diagnostic detection kit mainly comprising a reagent R1 and a reagent R2, wherein chloroform, glucan and protamine sulfate are introduced into the reagent R1 on the basis of the conventional formula, glucan and glycerol are introduced into the reagent R2, and qualitative change of the reagent properties are achieved through the newly introduced substances; after introduction of a newformula, the lipase detection kit can eliminate interference on a result in the presence of heparin and bilirubin, and through introduction of a glucan series and the glycerol, the stability of the reagents is greatly enhanced. According to the stable antiheparin lipase detection kit provided by the invention, the stability of the reagents is enhanced, the anti-interference ability of the reagents is greatly improved, and raw materials related in the reagents are conventional raw materials, so that a condition is created for mass collocation and production of the reagents.
Owner:BIOBASE BIODUSTRY (SHANDONG) CO LTD
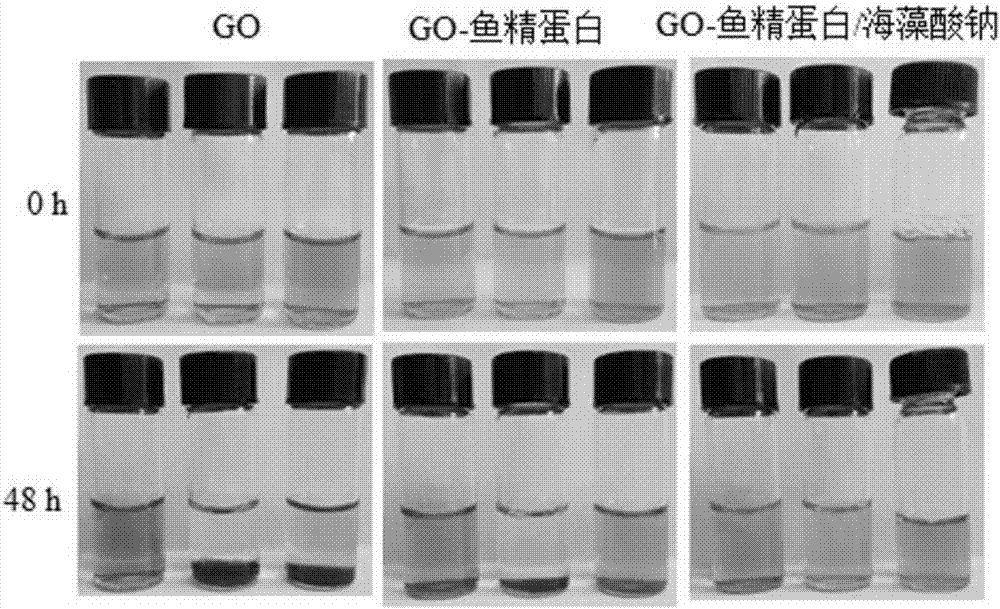
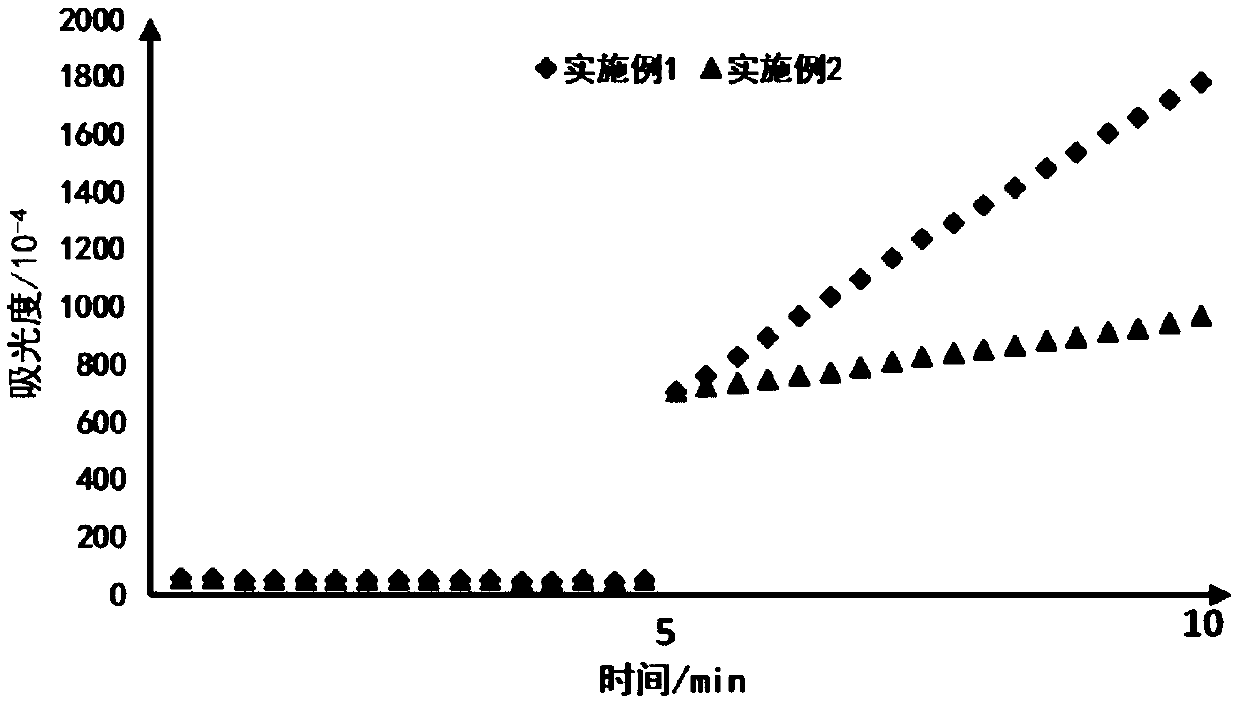
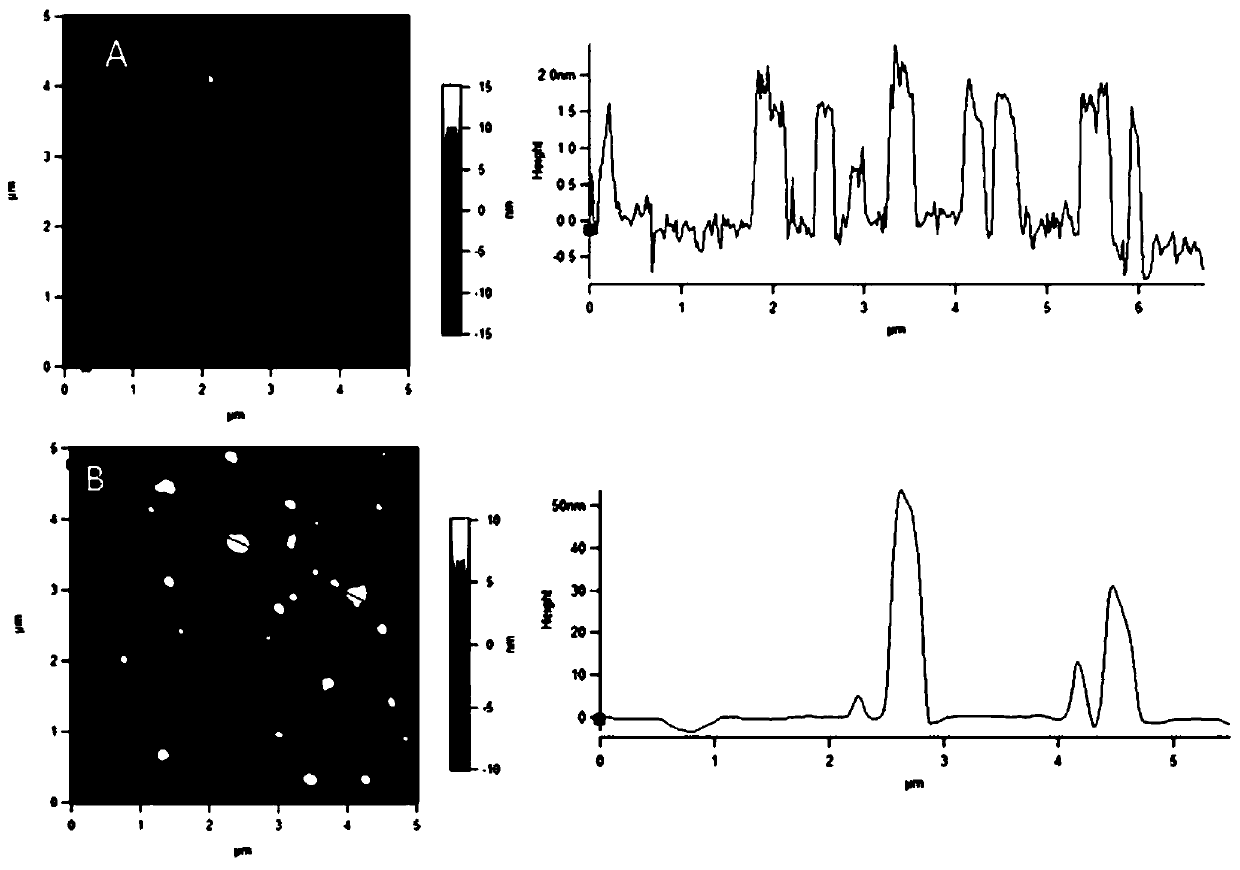
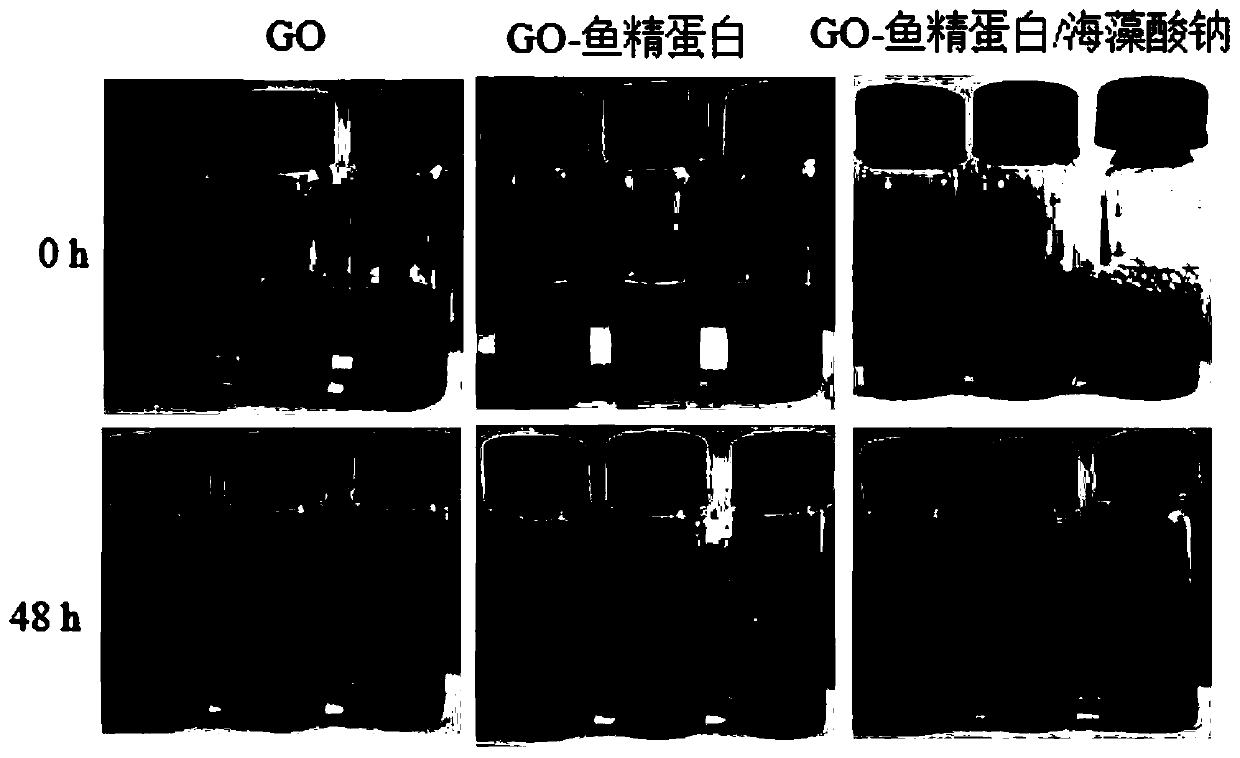
Preparation method and application of graphene oxide-protamine/sodium alginate compound
ActiveCN107281494AUniform particle size distributionHigh drug loadingEnergy modified materialsInorganic non-active ingredientsPolyelectrolyteProtamine sulfate
The invention discloses a preparation method and application of a graphene oxide-protamine / sodium alginate compound and belongs to the technical field of material synthesis and biological medicines. According to the preparation method disclosed by the invention, small-size graphene oxide (GO) capable of loading a drug is used as an inner core and a shell layer is formed by protamine sulfate and sodium alginate in sequence by adopting a layer-by-layer self-assembly technology through non-covalent adsorption. Layer-by-layer self-assembled nano-carrier water has good dispersion performance and stability. The natural protamine sulfate and the sodium alginate are used as polyelectrolyte and the small-size graphene oxide is used as a core; all the materials have good biocompatibility; an anti-tumor drug is loaded in the core and has a great drug loading amount and good stability; the drug has long-period release performance and the toxic side effect of pure utilization of the drug is reduced.
Owner:山东省循证医学研究院有限公司
Method for refining protamine sulfate
InactiveCN104530210ASmall molecular weightRetains resistance to heparinPeptide preparation methodsFermentationThermolysinHydrolysate
The invention discloses a method for refining protamine sulfate. According to the technical scheme adopted by the invention, the method comprises the following steps: performing enzymolysis on the protamine sulfate, performing quick protein liquid-phase separation to purify enzymatic hydrolysate, desalting a protamine high-salt solution to obtain high-purity protamine sulfate. A polypeptide mixture with average molecular weight of 1100Da, obtained by performing enzymolysis on the protamine with thermolysin has the capacity of neutralizing heparin; immune response caused after injection is remarkably lowered, and the toxic and side effects are relieved.
Owner:QINGDAO KANGYUAN PHARMA
Foot-and-mouth disease purification vaccine, preparation method and applications thereof
ActiveCN102988970BGood immune effectStable in natureAntiviralsRecovery/purificationAdjuvantFiltration
The present invention discloses a foot-and-mouth disease purification vaccine, a preparation method and applications thereof. The preparation method comprises: adopting protamine sulfate precipitation to remove most of hybridproteins and nucleic acids of host cells; condensing a virus liquid through a concentration system to be adopted as a chromatography sample; and selecting a polyacrylamide dextran gel as a chromatography medium to carry out purification elution, collecting a first elution peak, carrying out filtration sterilization inactivation, adding an adjuvant to carry out emulsification, and carrying out sub-packaging to obtain the foot-and-mouth disease purification vaccine. The preparation method has characteristics of economy, efficiency, low cost, simple and reasonable process, and effective removal of impurities in the host cells and the culture medium, wherein virus recovery rate is more than 85%, protein and nucleic acid removal rate is more than 90%, and the foot-and-mouth disease purification vaccine prepared by the preparation method has characteristics of safety, good immunity and stable property.
Owner:内蒙古必威安泰生物科技有限公司
Method for quantification of 146S content in foot-and-mouth disease antigen by using liquid chromatography detection system
The present invention discloses a method for quantification of 146s content in foot-and-mouth disease antigen by using a liquid chromatography detection system. The method comprises: adopting protamine sulfate to purify virus, adopting PEG6000 to concentrate the virus, carrying out sucrose density gradient ultracentrifugation on the concentrated virus, adopting a liquid chromatography detection system to detect an absorption peak of the centrifugated sample at a wavelength of 259 nm, and calculating 146S content according to the following formula: C=FRS / 76Wb. The method can be used for determination of the 146S content in various types of foot-and-mouth disease antigens. With quantification of the 146S content in the foot-and-mouth disease antigen, foot-and-mouth disease vaccine preparation can be guided, vaccine efficacy can be indirectly evaluated, and major guiding significances are provided for enhancing quality of the foot-and-mouth disease vaccine in our country.
Owner:内蒙古必威安泰生物科技有限公司
Nanometer grain preparation method of bionic SiO2 fixing Beta-glucuronide enzyme
InactiveCN101182511AEasy to fixHigh vitality maintenance rateOn/in inorganic carrierSilica nanoparticlesProtamine sulfate
Owner:TIANJIN UNIV
Healthy natural preservative for thick chili sauce products and production process thereof
InactiveCN105767843AExtended shelf lifeLow costFood preservationNatural extract food ingredientsFood additiveProtamine sulfate
The present invention provides a healthy natural preservative for thick chili sauce products and a production process thereof, and relates to the technical field of food additives. The preservative is made from the following raw materials: protamine sulfate, chitosan, tea polyphenol extract, spice extract, Chinese medicinal herb extract, trans-Cinnamic acid, agar oligosaccharides, sour agents, ethanol and water. The produced preservative is healthy and pure natural, is a compound natural preservative combining plant and animal sources, is completely harmless to the human body, can effectively prolong the shelf life of the thick chili sauce products, is capable of environmental protection and sterilization, and has a strong antibacterial ability.
Owner:HEXIAN COUNTY JILONGSHAN CONDIMENT
Preparation method of magnetic graphene oxide-protamine/sodium carboxymethyl cellulose composite and application
InactiveCN108014092AGood dispersionImprove stabilityOrganic active ingredientsEnergy modified materialsNano compositesGraphene derivatives
The invention discloses a preparation method of a magnetic graphene oxide-protamine / sodium carboxymethyl cellulose composite material, and belongs to the technical field of material synthesis and biological medicine. The method specifically comprises the steps that magnetic graphene oxide prepared after a graphene derivative loads ferroferric oxide serves as a matrix, a layer-by-layer self-assembly technology is adopted, and the matrix is sequentially coated with protamine and sodium carboxymethyl cellulose to form the composite. According to the method, the layer-by-layer self-assembly composite material has superparamagnetism and good dispersion performance and stability in water; natural protamine sulfate and sodium carboxymethyl cellulose are utilized as polyelectrolyte, nano-sized magnetic graphene oxide serves as a core, and all the materials have good biocompatibility; the material has certain magnetism, antineoplastic drugs are loaded on the cores of carriers, drug loading is stable, the drug loading quantity is large, and the drugs can have a targeted and sustained release function and better exert the therapeutic effect.
Owner:JIANGSU UNIV
A macrophage targeting carrier system and its preparation
InactiveCN104771764BStrong targetingIncrease intakeGenetic material ingredientsOther foreign material introduction processesGene deliverySynthesis methods
The invention belongs to the field of pharmacy, and discloses a synthesis method of a macrophage targeting carrier system and a preparation method of a gene delivery system. The macrophage targeting carrier system is characterized in that a macrophage targeting carrier is mannosylation protamine; electropositive mannosylation protamine loads electronegative nucleic acid to form a positively charged nano particle; and protamine modified by carubinose is prepared from formyl methyl mannopyranoside and protamine sulfate through a reductive amination reaction. Compared with non-viral gene carrier protamine, mannosylation protamine has a nuclear localization function and a macrophage targeting property, and can improve the gene transfection mediation efficiency of protamine in macrophage. The preparation method is simple, and mature in technology, and has good application prospects.
Owner:CHINA PHARM UNIV
Preparation method of protamine sulfate
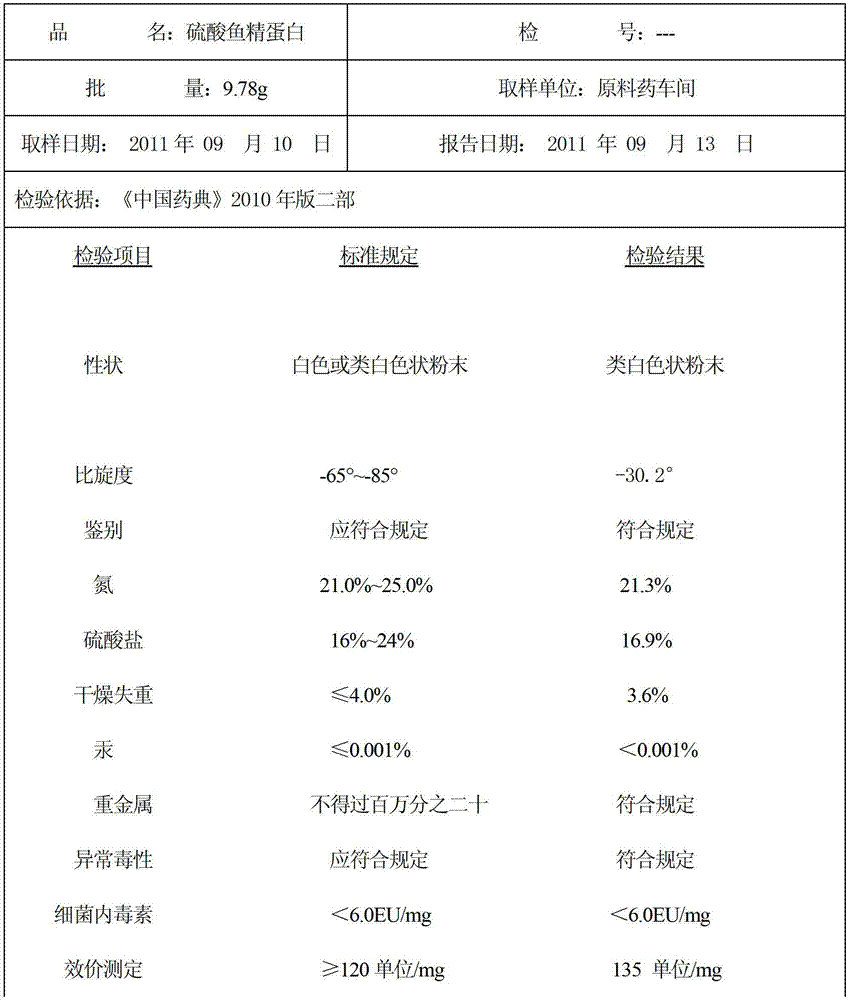
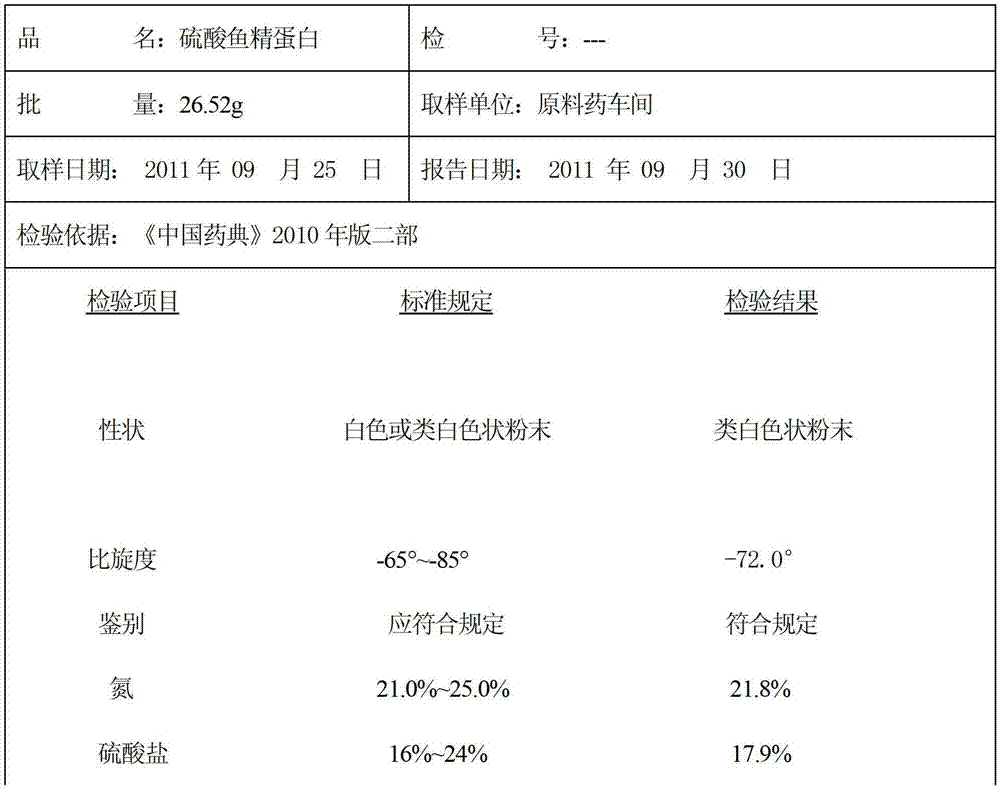
ActiveCN102796189BSimple processLow costPeptide preparation methodsAnimals/human peptidesWarm waterSpecific rotation
The invention discloses a preparation method of protamine sulfate. According to Chinese Pharmacopoeia 2010 Edition, the quality standard of protamine sulfate is raised, and the requirement of specific rotation (-65 degrees to -85 degrees) index is increased. In sperm from fish, protamine usually exists in the form of nucleic acid-protein. By using a traditional production technology, a finished product of protamine sulfate contains nucleic acid impurity, thus the specific rotation only reaches -30 degrees that can not satisfy the quality requirement of protamine sulfate in the new pharmacopoeia. According to the invention, by extracting with 3% sulfuric acid, extracting with warm water of 50-79 DEG C, conducting alcohol deposition, conducting low temperature treatment, and using salmon milt as a raw material, the protamine sulfate is prepared, and the specific rotation reaches -72 degrees.
Owner:QINGDAO KANGYUAN PHARMA
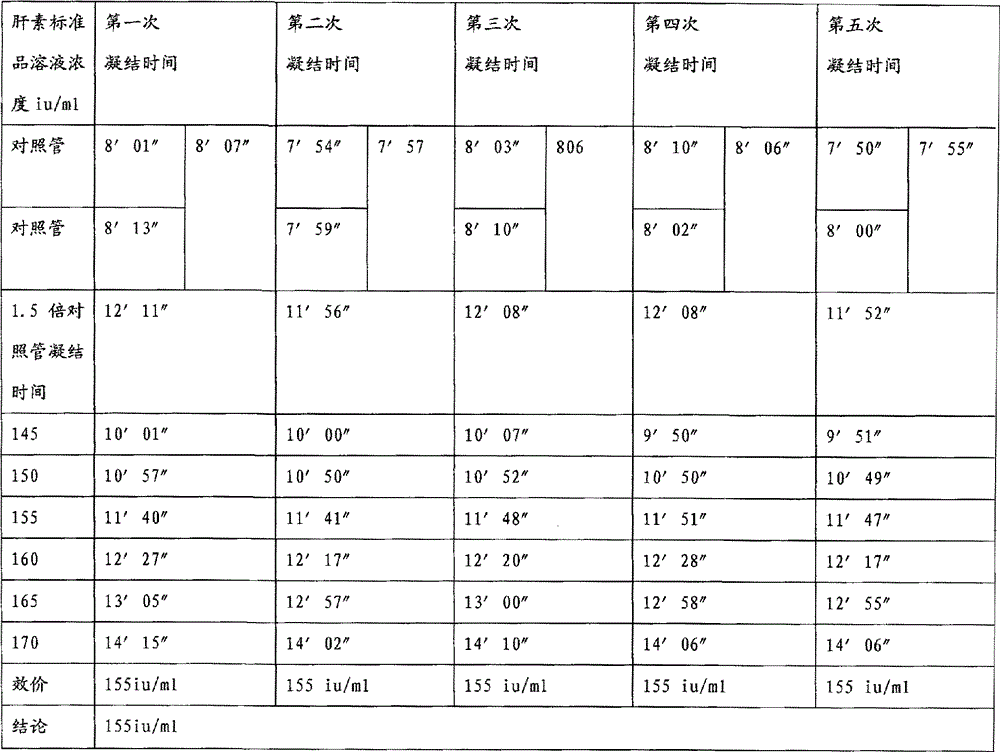
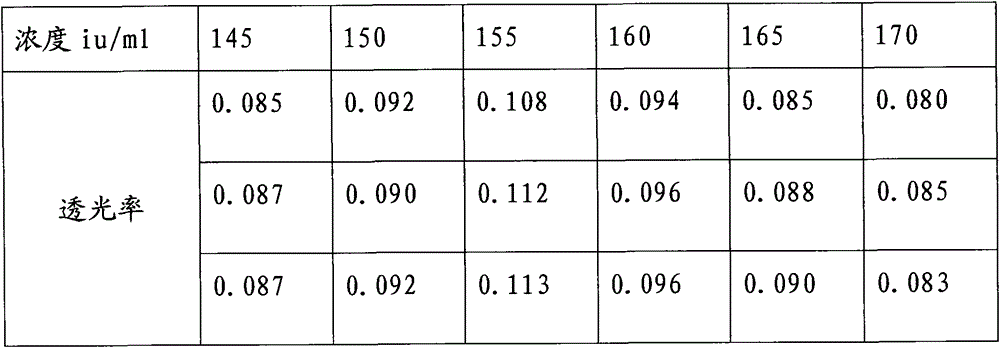
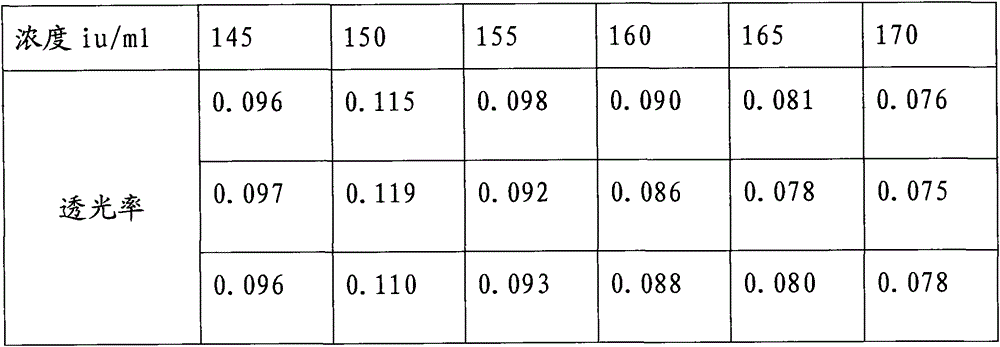
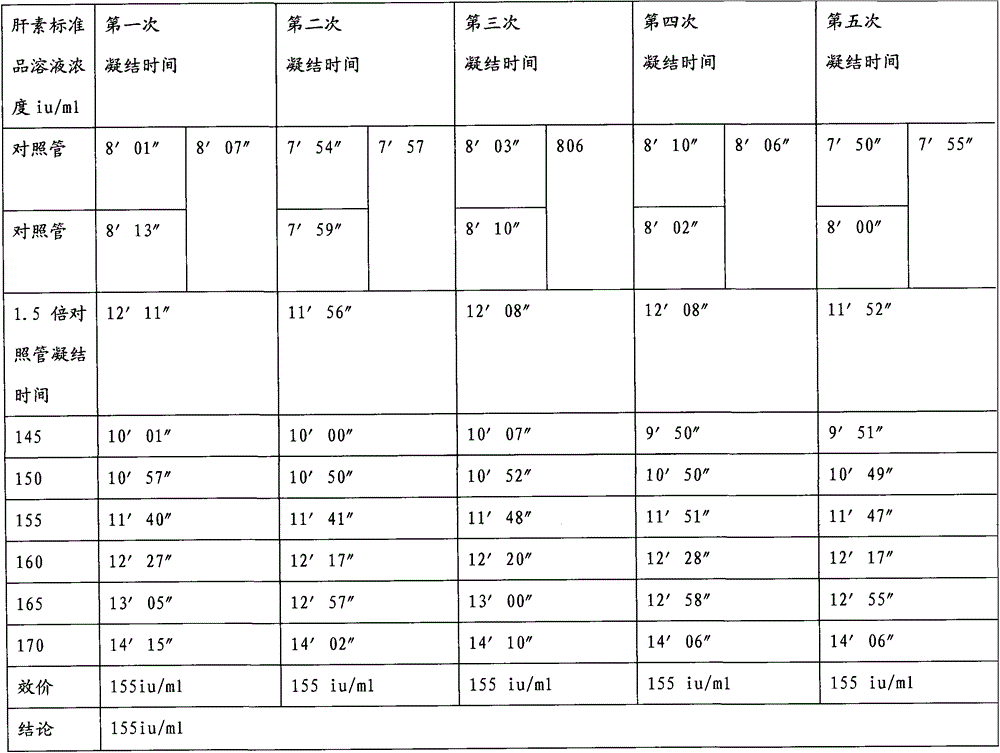
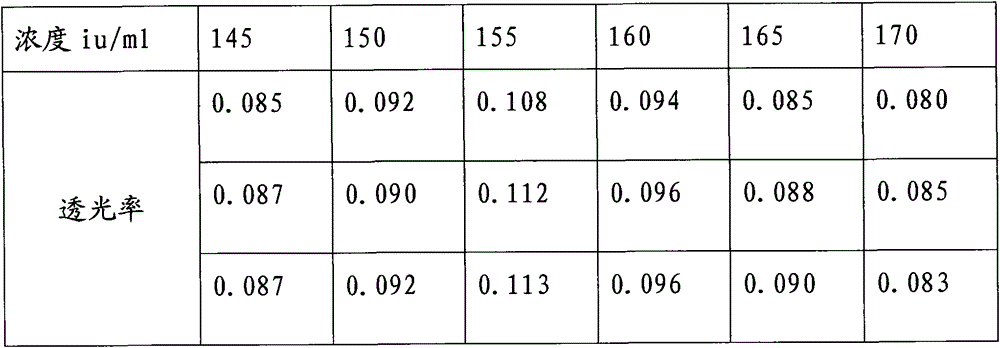
A kind of protamine sulfate potency determination method
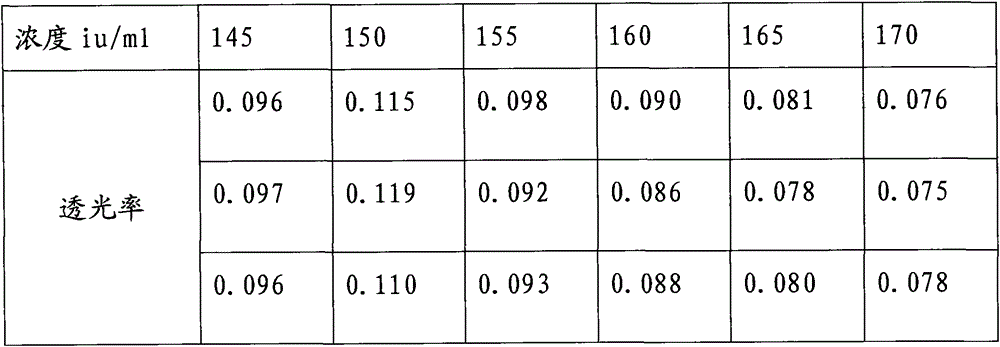
ActiveCN103604779BReduce experiment costEasy to operateTransmissivity measurementsMedicineTransmittance
"Chinese pharmacopoeia" (2010 edition) regulates that protamine sulfate titer measurement should adopt bioassay methods. A titer of a sample is measured by comparing the clotting times of fresh rabbit bloods, pig plasmas, or rabbit plasmas which are individually added with a heparin standard substance (S) and the sample (T). The market price of rabbit plasma is high, one dose of rabbit plasma costs 10 yuan, and contains 0.5ml of rabbit plasma; and at least 60 doses are needed to measure a protamine sulfate sample. An ultraviolet spectrophotometer is adopted, the solution light-transmittance is measured under a light with a wavelength of 550 nm, then the protamine sulfate titer is measured, in the operation rabbit plasma is not needed, thus the cost is reduced, and moreover the operation is more convenient.
Owner:QINGDAO JIULONG BIO PHARMA
A preparation method of insulin lispro protamine sulfate preparation and the prepared insulin lispro protamine sulfate preparation
The invention provides a preparation method of an insulin lispro-protamine sulfate preparation. The insulin lispro-protamine sulfate preparation contains the following ingredients in concentration: 3.47mg / ml of insulin lispro, 0.095mg / ml to 0.28mg / ml of protamine sulfate, 0.025mg / ml to 0.05mg / ml of zinc, 0.5mg / ml to 1.0mg / ml of phenol, 1.5mg / ml to 2.5mg / ml of m-cresol, 16mg / ml of glycerine and 2mg / ml to 4mg / ml of disodium hydrogen phosphate. The preparation method comprises the steps of carrying out solution mixing on an acidic solution of insulin lispro and an alkaline solution of protamine sulfate, then, carrying out crystallization, adding an m-cresol and glycerine solution into completely-crystallized crystalline liquid, and carrying out uniform mixing, thereby obtaining the insulin lispro-protamine sulfate preparation. According to the preparation method, the consumed time is short, and the obtained product is stable in quality.
Owner:TONGHUA DONGBAO PHARMA
Reagent used for measuring pancreatic lipase in serum or plasma, and preparation method and application thereof
InactiveCN109580515AReduce interferenceImprove featuresMaterial analysis by observing effect on chemical indicatorColor/spectral properties measurementsHepatic lipaseProtamine sulfate
The invention relates to the technical field of biochemical detection, in particular to a reagent used for measuring pancreatic lipase in serum or plasma, and a preparation method and application thereof. The invention provides pancreatic lipase measurement reagent which comprises sodium dodecyl sulfate, protamine sulfate and sphingomyelin. Since the sodium dodecyl sulfate, the protamine sulfate and the sphingomyelin are added into the pancreatic lipase measurement reagent, the interference function of hepatic lipase, lipoprotein lipase and cholesterol esterase in the serum for pancreatic lipase measurement can be obviously lowered, nonspecific lipolysis reaction in the serum or the plasma in a reaction process of the pancreatic lipase measurement can be effectively inhibited, meanwhile, the catalytic activity of the pancreatic lipase can guarantee to be effectively performed, pancreatic lipase measurement specificity and accuracy is improved, and the false positive rate of pancreaticlipase detection is effectively lowered.
Owner:BIOSINO BIO TECH & SCI
A kind of preparation method and application of graphene oxide-protamine/sodium alginate composite
ActiveCN107281494BUniform particle size distributionHigh drug loadingEnergy modified materialsInorganic non-active ingredientsPolyelectrolyteProtamine sulfate
The invention discloses a preparation method and application of a graphene oxide-protamine / sodium alginate compound and belongs to the technical field of material synthesis and biological medicines. According to the preparation method disclosed by the invention, small-size graphene oxide (GO) capable of loading a drug is used as an inner core and a shell layer is formed by protamine sulfate and sodium alginate in sequence by adopting a layer-by-layer self-assembly technology through non-covalent adsorption. Layer-by-layer self-assembled nano-carrier water has good dispersion performance and stability. The natural protamine sulfate and the sodium alginate are used as polyelectrolyte and the small-size graphene oxide is used as a core; all the materials have good biocompatibility; an anti-tumor drug is loaded in the core and has a great drug loading amount and good stability; the drug has long-period release performance and the toxic side effect of pure utilization of the drug is reduced.
Owner:山东省循证医学研究院有限公司
Bionic nanometer grain preparation method of sio2 fixed beta-glucuronide enzyme
InactiveCN101182511BEasy to fixHigh vitality maintenance rateOn/in inorganic carrierFreeze-dryingProtamine sulfate
The invention discloses a preparation method of Beta- glucuronidase nanometer particle which is immobilized by bionic silicon dioxide. The process of the method comprises that the protamine sulfate isdissolved in the trishydroxymethylaminomethane-hydrochloride buffer or the deionized water for the preparation of protamine sulfate solution; the Beta- glucuronidase is dissolved in the trishydroxymethylaminomethane-hydrochloride buffer or the deionized water for the preparation of Beta- glucuronidase solution; the protamine sulfate solution is mixed with the Beta- glucuronidase solution to obtain the mixed solution; the sodium silicate is dissolved in the deionized water or the saline water and the pH value is adjusted to obtain the sodium silicate solution; the mixed solution is added intothe sodium silicate solution; through standing, separation, removal of the supernatant, washing and freeze drying, the Beta- glucuronidase nanometer particle which is immobilized by bionic silicon dioxide is obtained. The invention has the advantages that the preparation process is simple; the immobilized Beta- glucuronidase has a high activity maintenance rate and good stability for repeat use.
Owner:TIANJIN UNIV
Oral pH responsive intestinal targeting vector as well as preparation method and applications thereof
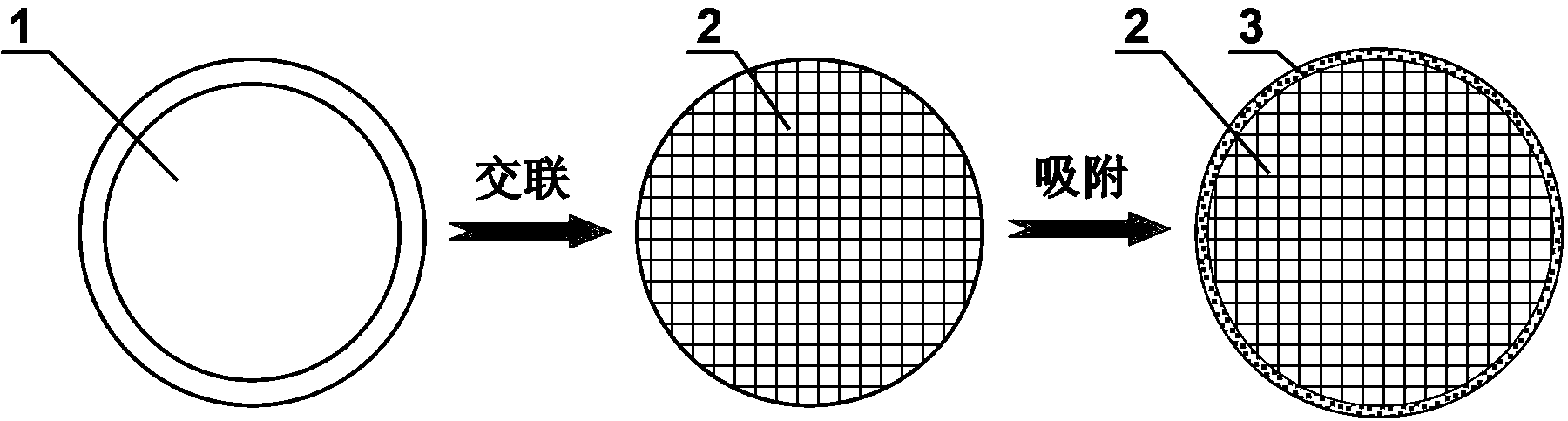
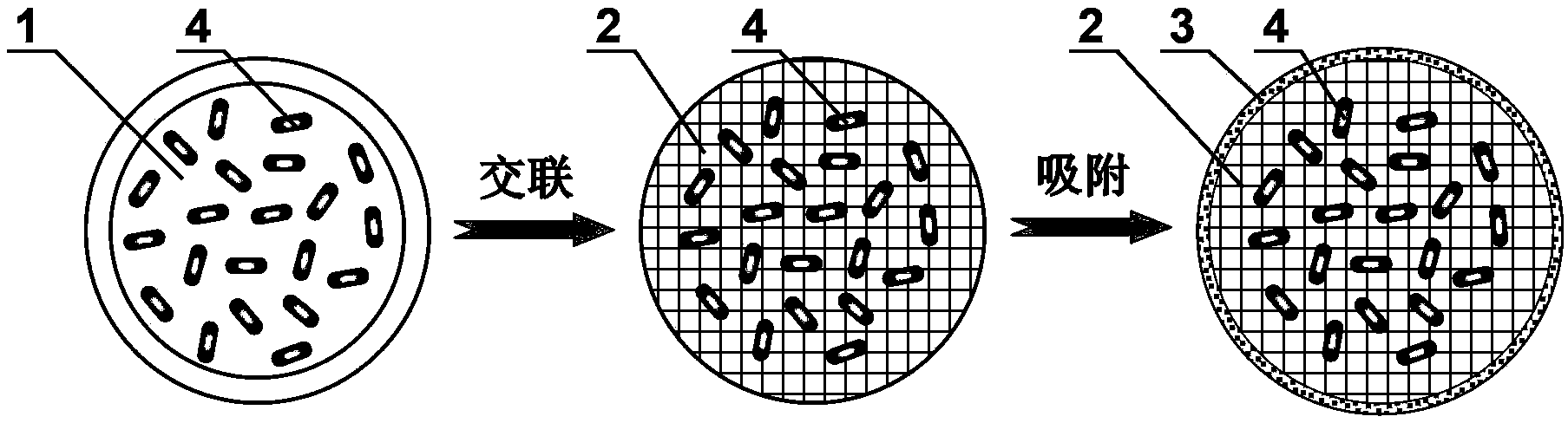
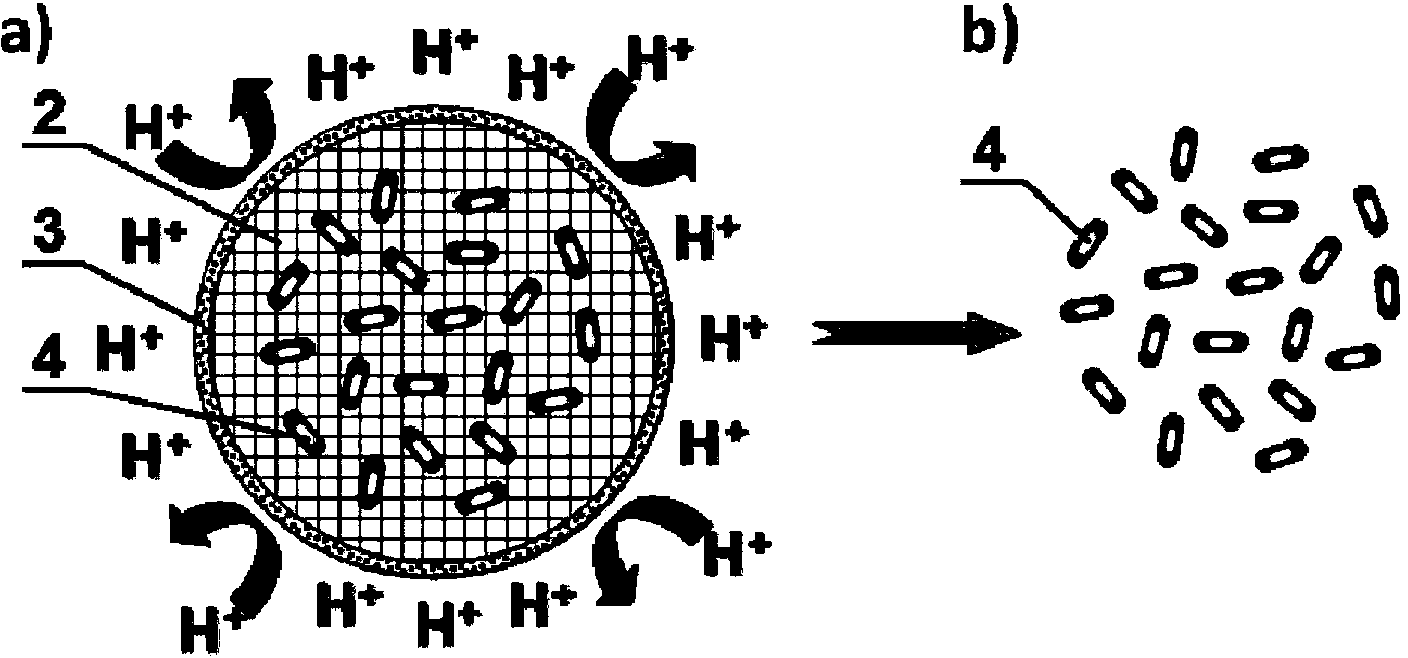
ActiveCN103100089BGood pH responsive intelligent protection functionFast dissolutionMetabolism disorderDigestive systemProtamine sulfateCalcium alginate
The invention belongs to the field of pH responsive targeting vectors, and provides an oral pH responsive intestinal targeting vector, wherein the vector consists of calcium alginate gel kernels and protamine sulfate layers wrapped by calcium alginate gel kernels, is spherical, and has the particle diameter of 4.1mm-4.6mm. The method for preparing the vector comprises the following steps of: (1) preparing an external phase fluid, an internal phase fluid, a calcium nitrate solution and a protamine sulfate solution; (2) preparing calcium alginate gel kernels; and (3) adsorbing protamine sulfate so as to form the protamine sulfate layers. The invention also provides applications of the oral pH responsive intestinal targeted vector in embedding probiotics, nutrients or medicaments.
Owner:SICHUAN UNIV
Two-phase isophane zinc recombinant human insulin injection and preparation method thereof
InactiveCN101732245AControl blood sugar levelsGood treatment optionsPeptide/protein ingredientsMetabolism disorderMedicineInsulin injection
The invention relates to a two-phase isophane zinc recombinant human insulin injection and a preparation method thereof. A specification I comprises the following components in part by weight: 300,000IU of recombinant human insulin, 42 to 54g of isosmotic agent, 7.5 to 1.5g of preservative, 14.4 to 15.0g of pH regulator and 0.45 to 0.9g of protamine sulfate. A specification II comprises the following components in part by weight: 400,000IU of recombinant human insulin, 140 to 180g of isosmotic agent, 25 to 35g of preservative, 48 to 50g of pH regulator and 1.5 to 3.0g of protamine sulfate. Both preparation methods of both specifications of the two-phase isophane zinc recombinant human insulin comprise a step of mixing solution A and solution B, wherein the solution A is prepared by adding water for injection into the recombinant human insulin, the isosmotic agent, the preservative and the pH regulator for dissolving; then slowly adding an appropriate amount of water for injection with the pH of 2.0 into the solution until the pH is between 6.9 and 7.8; and filtering the solution with a 0.22 mu m filter membrane and sterilizing; and the mixing ratio of the solution A to the solution B is 10 / 90-75 / 25. The recombinant human insulin is more favorable for absorbing.
Owner:JIANGSU WANBANG BIOPHARMLS +1
A mild lead-acid storage battery additive and its preparation method and application
ActiveCN108461830BAccelerated vulcanizationExtended service lifeLead-acid accumulatorsProtamine sulfateStearic acid
The invention provides a mild condition lead-acid battery additive and a preparation method and application thereof, and relates to the technical field of lead-acid batteries. The additive for lead-acid batteries with mild conditions comprises, by weight, 8-12 parts of chitooligosaccharide stearic acid, 12-36 parts of protamine sulfate, 1-3 parts of nitric acid, 1-3 parts of sodium silicate, 2-6 parts of acetylene black, 0.2 to 0.6 part of polyvinylpyrrolidone, 0.3-0.6 part of decylethylamine hydrochloride, 0.5-1.5 parts of zwitterionic surfactant and 100-120 parts of water. The additive has reasonable formula, small amount of use and wide application range, can effectively alleviate the polarization phenomenon of an electrode, enhance the charge acceptance ability of the battery, and improve the cycling times of the battery. The service life of the lead-acid battery can be prolonged, the preparation and use of additives are simple, and the conditions of use are mild.
Owner:TIANNENG GRP HENAN ENERGY TECH
Protamine sulfate titer measuring method
ActiveCN103604779AReduce experiment costEasy to operateTransmissivity measurementsMedicineTransmittance
"Chinese pharmacopoeia" (2010 edition) regulates that protamine sulfate titer measurement should adopt bioassay methods. A titer of a sample is measured by comparing the clotting times of fresh rabbit bloods, pig plasmas, or rabbit plasmas which are individually added with a heparin standard substance (S) and the sample (T). The market price of rabbit plasma is high, one dose of rabbit plasma costs 10 yuan, and contains 0.5ml of rabbit plasma; and at least 60 doses are needed to measure a protamine sulfate sample. An ultraviolet spectrophotometer is adopted, the solution light-transmittance is measured under a light with a wavelength of 550 nm, then the protamine sulfate titer is measured, in the operation rabbit plasma is not needed, thus the cost is reduced, and moreover the operation is more convenient.
Owner:QINGDAO JIULONG BIO PHARMA
Double timephase neo-insulin zinc injection (30%) and prepn process thereof
ActiveCN1931360BReduce the number of injectionsRelieve painPeptide/protein ingredientsMetabolism disorderDouble-timeInsulin injection
The present invention is double time phase protamine zinc insulin injection (30 %) and its preparation process, and belongs to the field of medicine preparation technology. The double time phase protamine zinc insulin injection (30 %) consists of insulin 100000 IU, isosmotic agent 35-45 g, preservative 5.5-7 g, pH regulator 12-12.5 g and fish protamine sulfate 0.21-0.42 g, and has pH value of 6.9-7.8. Its preparation process includes the steps of preparing neutral insulin injection, preparing low protamine zinc insulin injection, and mixing these two kinds of injection. The double time phase protamine zinc insulin injection (30 %) has two forms of insulin, including one in solution for quick acting and the other in precipitate with combined fish protamine sulfate as the middle acting phase. It has less required injection times.
Owner:JIANGSU WANBANG BIOPHARMLS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com