Macrophage targeting carrier system and preparation method thereof
A carrier system and macrophage technology, applied in the field of macrophage targeting carrier system and its preparation, can solve the problems of lack of cell specificity of protamine carrier, increase macrophage uptake, etc., achieve good nuclear localization performance, good Targeted, mild and controllable reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
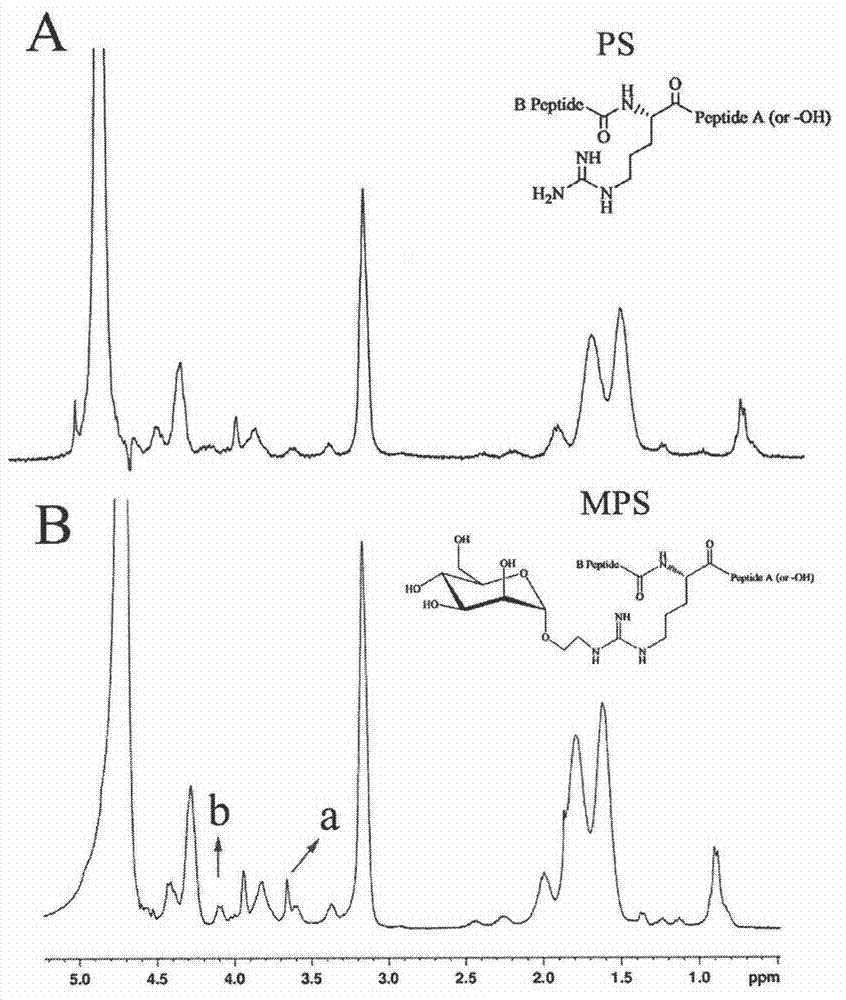
[0038] Example 1: Synthesis and Characterization of Mannosylated Protamine
[0039] Add 1-α-formylmethyl-mannopyranoside (50 mg, 0.23 mM) and protamine sulfate (PS, 113.6 mg, 0.02 mM) into 15 mL of distilled water, and stir at room temperature until completely dissolved. Sodium triacetoxyborohydride (474.75 mg, 2.3 mM) was added to the reaction solution, and stirred at room temperature for 48 h (TLC monitored the reaction progress, methanol: triethylamine: water = 3:2:1, v / v / v) . After the reaction was complete, the solution was dialyzed in distilled water at 4° C. for 48 h, and then freeze-dried to remove the solvent to obtain 69 mg of white final product mannosaccharated protamine (MPS), with a yield of 65%.
[0040] The synthesized MPS is carried out to proton nuclear magnetic resonance spectrum analysis, the result shows that the hydroxyl proton peak signal on the mannose ligand appears at 3.6 and 4.1ppm, and the degree of substitution of the calculated mannose is 6.4% (a...
Embodiment 2
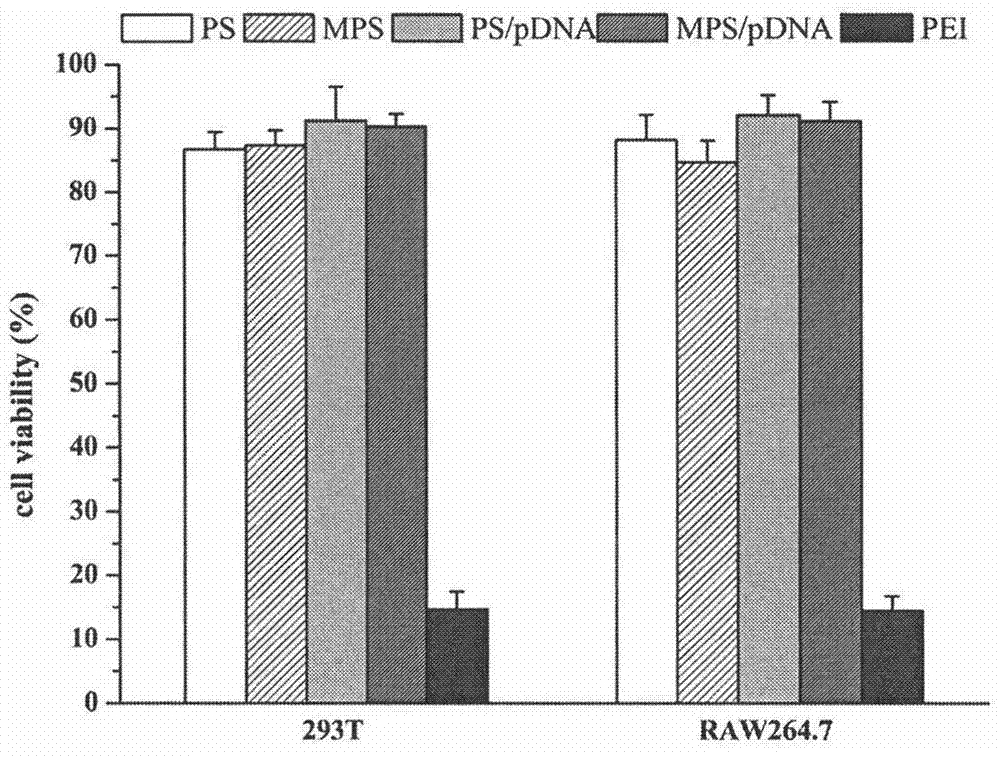
[0042] Embodiment 2: Preparation of MPS / pDNA nanoparticles
[0043] The pDNA aqueous solution (100 μg / mL) was added dropwise to an equal volume of the mannosaccharified protamine aqueous solution (56 μg / mL) of Example 1 under stirring, and allowed to stand at room temperature for 30 minutes to form a nanoparticle prepared with an N / P ratio of 0.8. particle.
Embodiment 3
[0044] Embodiment 3: Preparation of MPS / pDNA nanoparticles
[0045] The pDNA aqueous solution (100 μg / mL) was added dropwise to an equal volume of the mannosaccharified protamine aqueous solution (70 μg / mL) of Example 1 under stirring, and allowed to stand at room temperature for 30 minutes to form a nanoparticle prepared with an N / P ratio of 1. particle.
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of substitution | aaaaa | aaaaa |
| encapsulation rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com