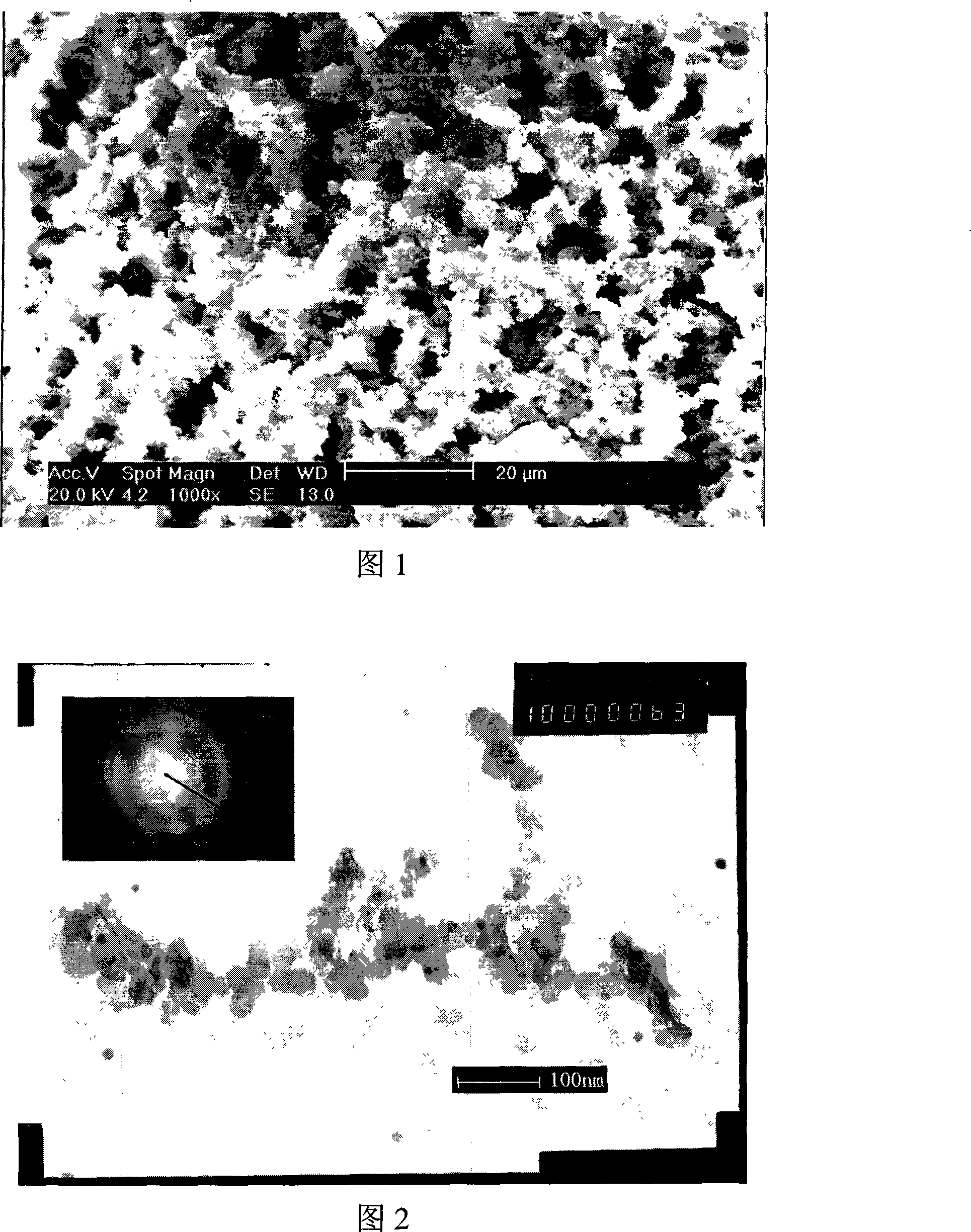
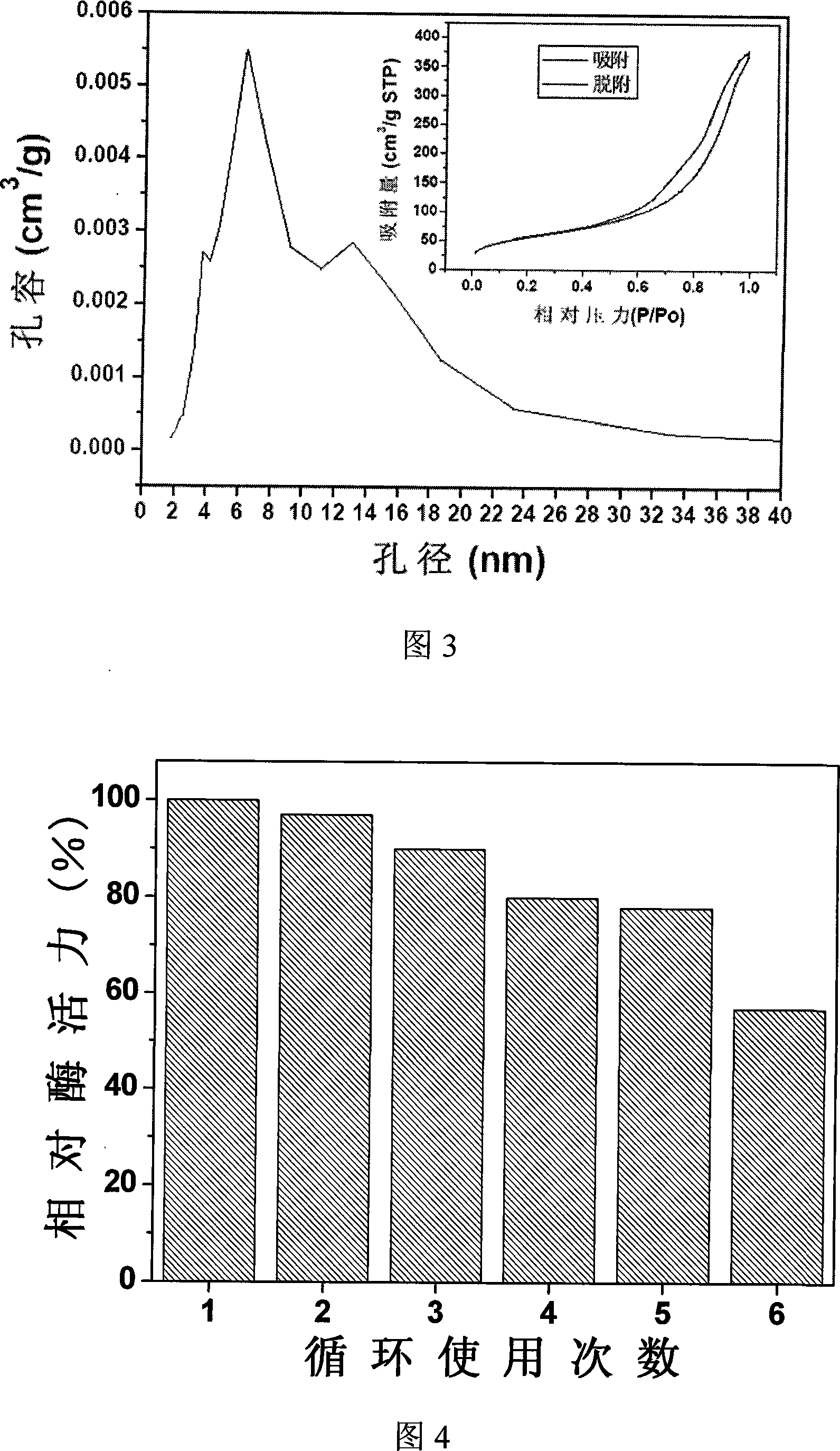
Nanometer grain preparation method of bionic SiO2 fixing Beta-glucuronide enzyme
A technology of aldolidase and silicon dioxide, which is applied in the direction of immobilization on or in the inorganic carrier, can solve the problems of activity decline and inactivation, and achieve good immobilization ability, mild preparation conditions and high activity maintenance rate Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Accurately weigh 1.0mg of β-glucuronidase, dissolve it in 0.05mol / L tris-hydrochloric acid (Tris-HCl) buffer solution, and dilute to 10mL to obtain 0.1mg / mL β-glucuronidase Acidase solution. Weigh 300 mg of protamine sulfate, dissolve it in 0.05 mol / L Tris-HCl buffer solution, and dilute to 20 mL to obtain a 15 mg / mL protamine sulfate solution. Sodium silicate was dissolved in deionized water, and the pH of the solution was adjusted to 7.0 with 2mol / L HCl to prepare a 10mmol / L sodium silicate solution.
[0017] Take 0.5ml each of β-glucuronidase solution and protamine sulfate solution, mix well and add to 7mL freshly prepared sodium silicate solution, let it stand for 5min, set the centrifuge speed to 4500r / min, and centrifuge for 10min . Pour out the supernatant, wash with deionized water, centrifuge for 10 min, and pour out the supernatant. Wash repeatedly 2-3 times. The content of β-glucuronidase in the supernatant was analyzed through the conversion reaction of ...
Embodiment 2
[0021] Accurately weigh 1.0mg of β-glucuronidase, dissolve it in 0.05mol / L tris-hydrochloric acid (Tris-HCl) buffer solution, and dilute to 10mL to obtain 0.1mg / mL β-glucuronidase Acidase solution. Weigh 40 mg of protamine sulfate, dissolve it in 0.05 mol / L Tris-HCl buffer solution, and dilute to 20 mL to obtain a 2 mg / mL protamine sulfate solution. Sodium silicate was dissolved in deionized water, and the pH of the solution was adjusted to 7.0 with 2mol / L HCl to prepare a 30mmol / L sodium silicate solution.
[0022] Take 0.5mL of β-glucuronidase solution and protamine sulfate solution, mix well and add to 7mL freshly prepared sodium silicate solution, let it stand for 10min, set the centrifuge speed to 4500r / min, and centrifuge for 10min . Pour out the supernatant, wash with deionized water, centrifuge for 10 min, and pour out the supernatant. Wash repeatedly 2-3 times. The content of β-glucuronidase in the supernatant was analyzed through the conversion reaction of baical...
Embodiment 3
[0026] Accurately weigh 1.0 mg of β-glucuronidase, dissolve it in deionized water, and dilute to 10 ml to obtain 0.1 mg / ml β-glucuronidase solution. Weigh 300 mg of protamine sulfate, dissolve it in deionized water, and dilute to 20 ml to obtain a 15 mg / ml protamine sulfate solution. Sodium silicate was dissolved in deionized water, and the pH of the solution was adjusted to 7.0 with 2mol / L HCl to prepare a 0.03mmol / L sodium silicate solution.
[0027] Take 0.5mL of β-glucuronidase solution and protamine sulfate solution, mix well and add to 10mL freshly prepared sodium silicate solution, let it stand for 5min, set the centrifuge speed to 4500r / min, and centrifuge for 10min . Pour out the supernatant, wash with deionized water, centrifuge for 10 min, and pour out the supernatant. Wash repeatedly 2-3 times. The content of β-glucuronidase in the supernatant was analyzed through the conversion reaction of baicalin, and it was found that there was no β-glucuronidase in the supe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com