Foot-and-mouth disease purification vaccine, preparation method and applications thereof
A technology for foot-and-mouth disease and foot-and-mouth disease, which is applied in the field of vaccine preparation, can solve the problems of unstable immune protection, undetectable neutralizing antibodies, and low effective antigen content, and achieve the effects of low cost, stable properties, and good immunity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
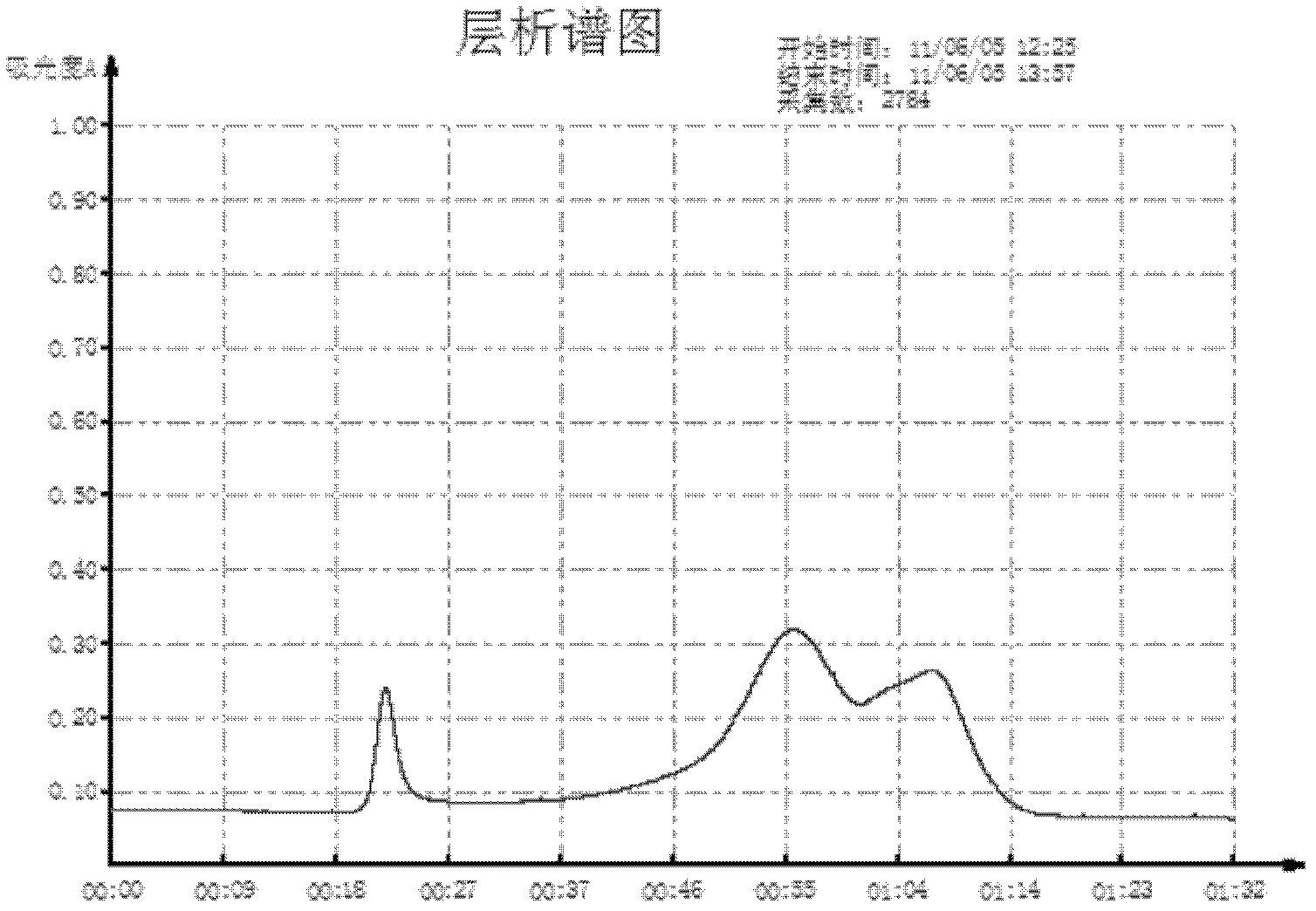
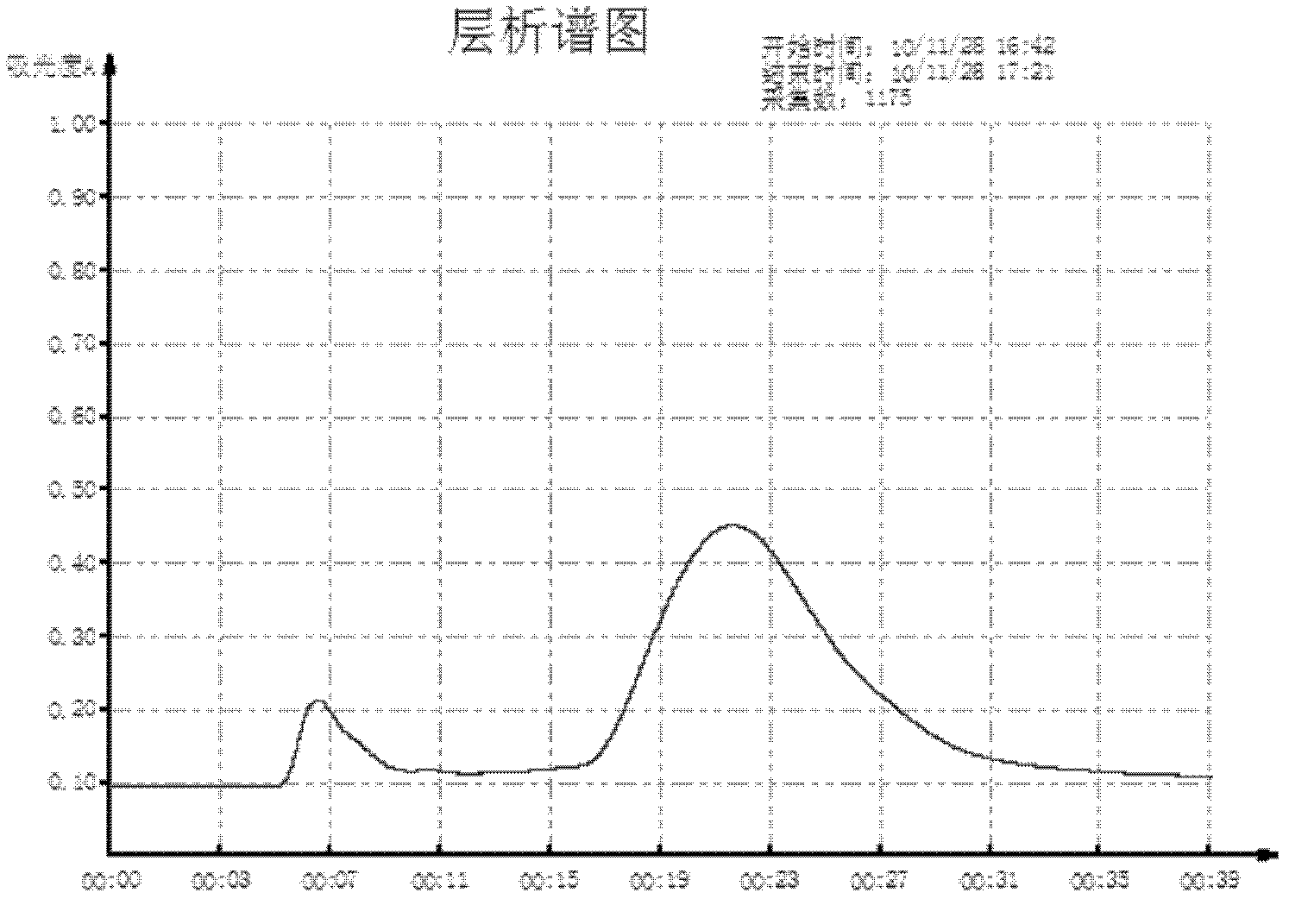
Image
Examples
Embodiment 1
[0032] The preparation of embodiment 1 bovine foot-and-mouth disease bivalent inactivated purified vaccine (ONXC strain+JSL strain)
[0033] 1 material
[0034]1.1 Vaccine strains: the virus strain used for the production of type O foot-and-mouth disease inactivated vaccine for this product is ONXC / 92 strain and the virus strain used for the production of Asian type I foot-and-mouth disease inactivated vaccine is Asia1 / JSL / GSZY / 06 strain, purchased from Lanzhou Veterinary Research Place.
[0035] 1.2 Chemical reagents: BHK21 cells were prepared and preserved according to existing conventional methods for this experiment; protamine sulfate (product number p4380), BEA, both products of sigma company; 206 adjuvant was purchased from Seppic company in France.
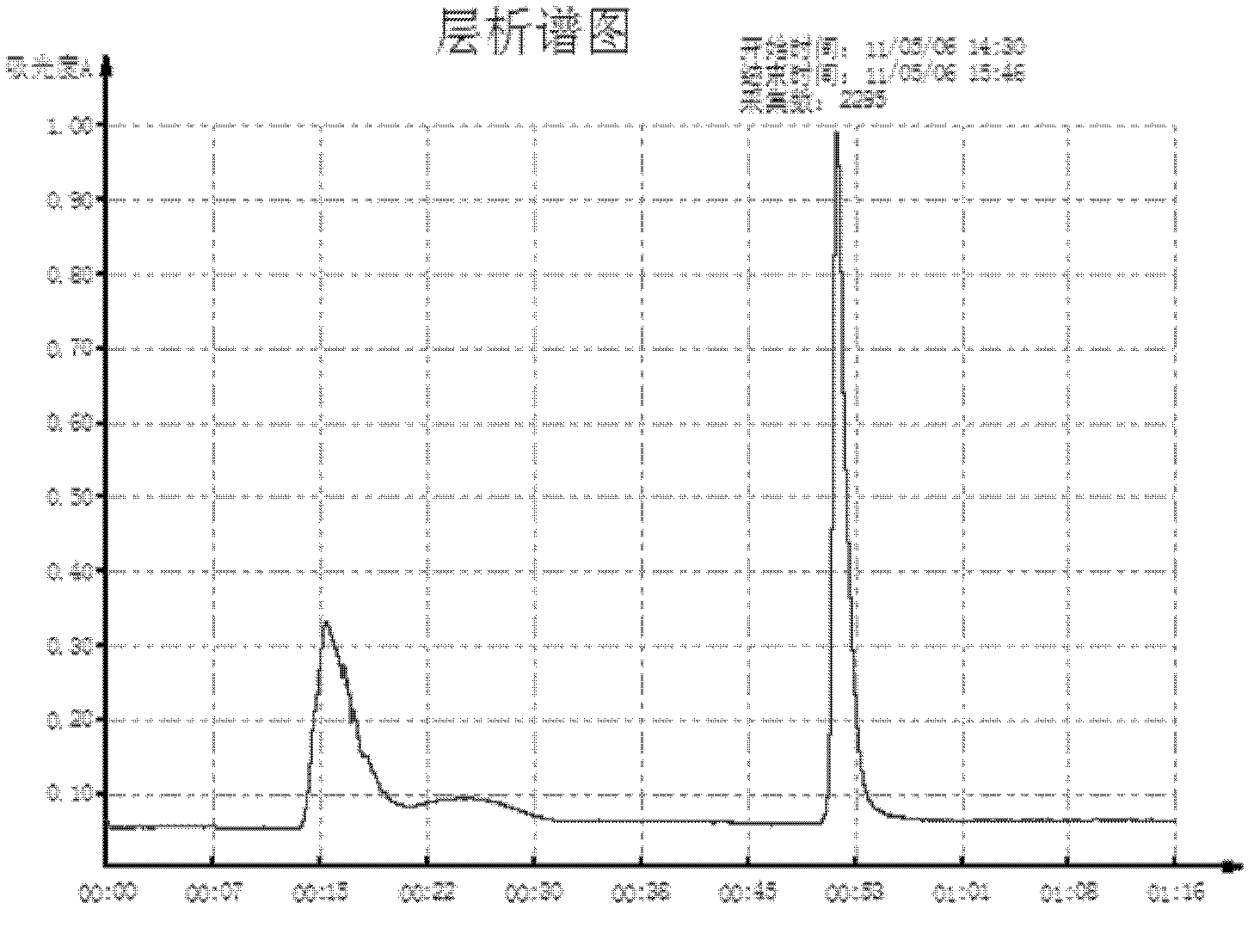
[0036] 1.3 Chromatographic column and gel medium: the gel medium packing sepphacryl S-300 and the empty column XK26 are all products of GE;
[0037] 1.4 Microporous filtration system: filter elements with a pore size of 5...
Embodiment 2
[0046] The preparation of embodiment 2 porcine foot-and-mouth disease O type inactivated purified vaccine (OR / 80 strain+O / ZK / 93-08 strain)
[0047] 1 material
[0048] 1.1 Vaccine strains: The foot-and-mouth disease virus used to manufacture this product is O-type foot-and-mouth disease inactivated vaccine production strain OR / 80 and O-type foot-and-mouth disease inactivated vaccine production strain O / ZK / 93-08 purchased from Lanzhou Veterinary Medicine graduate School.
[0049] 1.2 Chemical reagents: BHK21 cells were prepared and preserved according to existing conventional methods for this experiment; protamine sulfate (product number p4380), BEA, both products of sigma company; 206 adjuvant was purchased from Seppic company in France.
[0050] 1.3 Chromatographic column and gel medium: empty column XK26; gel medium packing: sepphacryl S-300 are all products of GE;
[0051] 1.4 Microporous filtration system: Hollow fiber columns with a pore size of 5 microns, 1 micron and ...
Embodiment 3
[0060] The preparation of embodiment 3 porcine foot-and-mouth disease O type inactivated purified vaccine (OR / 80 strain+O / ZK / 93-08 strain)
[0061] 1 material
[0062] 1.1 Vaccine strains: The foot-and-mouth disease virus used to manufacture this product is O-type foot-and-mouth disease inactivated vaccine production strain OR / 80 and O-type foot-and-mouth disease inactivated vaccine production strain O / ZK / 93-08 purchased from Lanzhou Veterinary Medicine graduate School.
[0063] 1.2 Chemical reagents: BHK21 cells were prepared and preserved according to existing conventional methods for this experiment; protamine sulfate (product number p4380), BEA, both products of sigma company; 206 adjuvant was purchased from Seppic Company of France; PEG6000 was purchased from AMRESCO Company.
[0064] 1.3 Chromatographic column and gel medium: empty column XK26; gel medium packing: sepphacryl S-300 are all products of GE;
[0065] 1.4 Microporous filtration system: Hollow fiber columns ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com