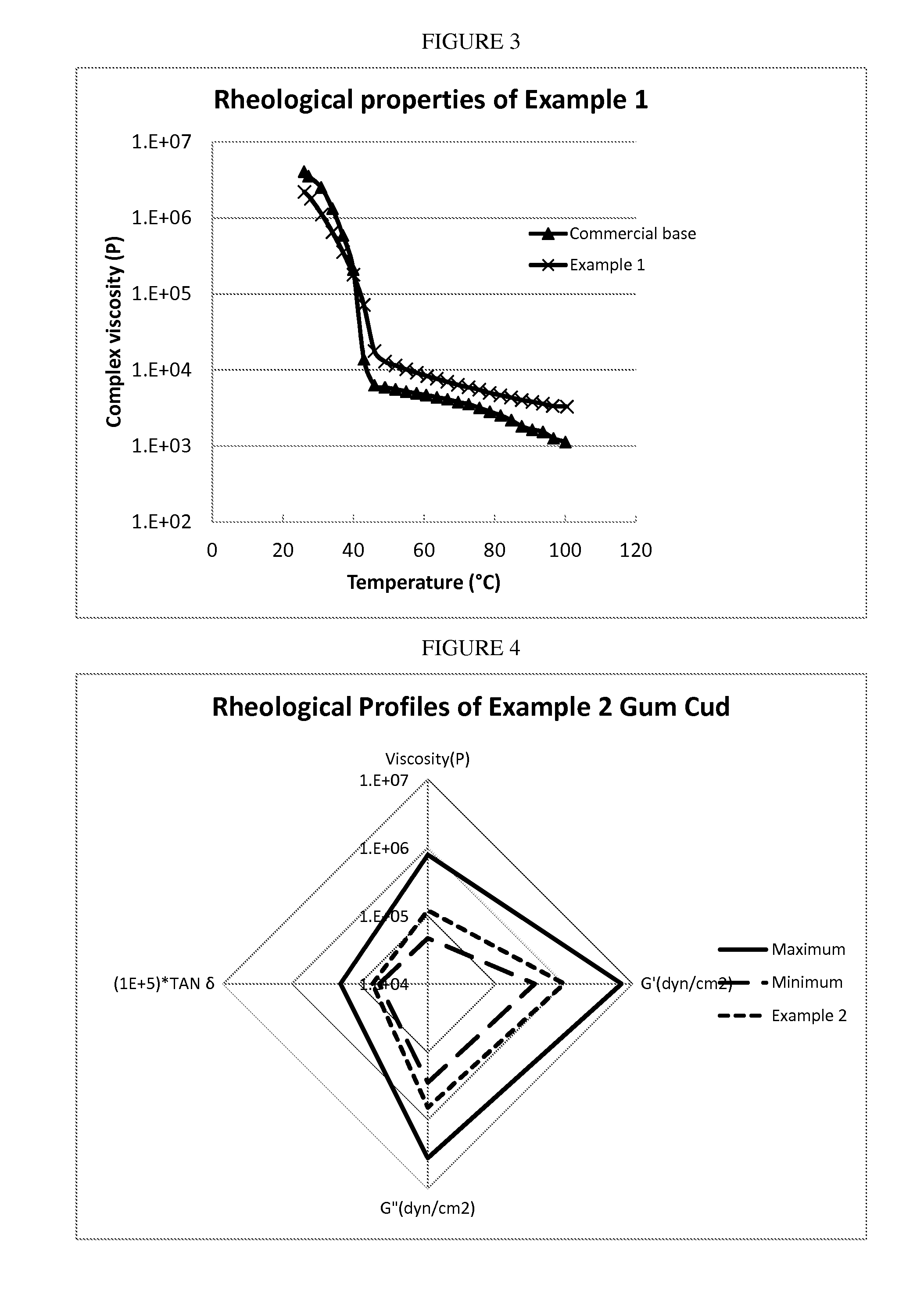
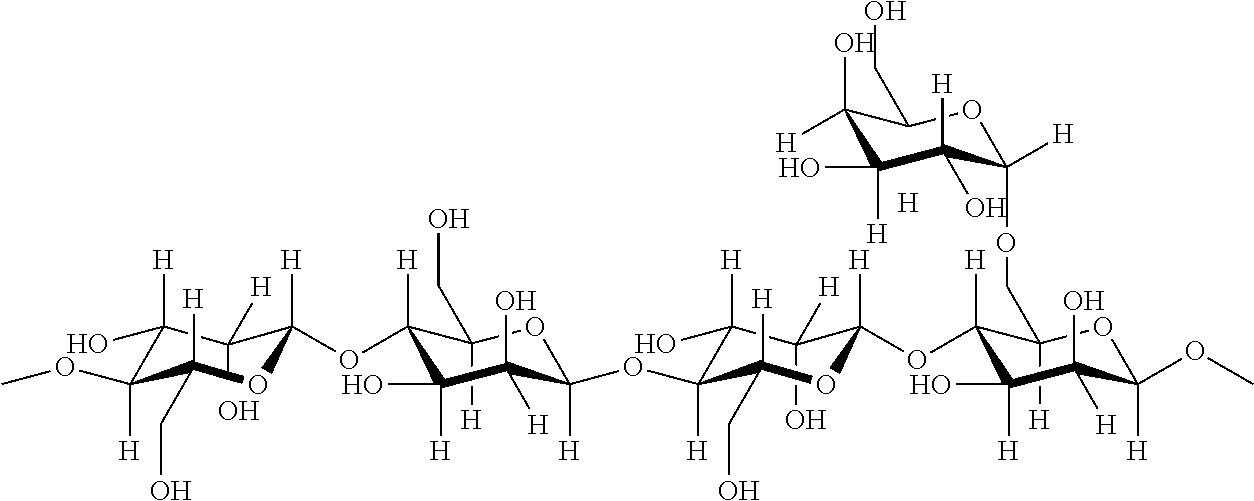
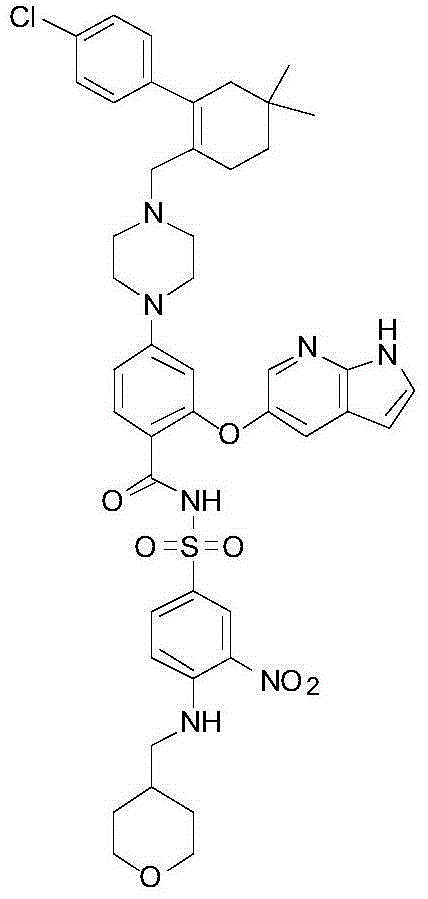
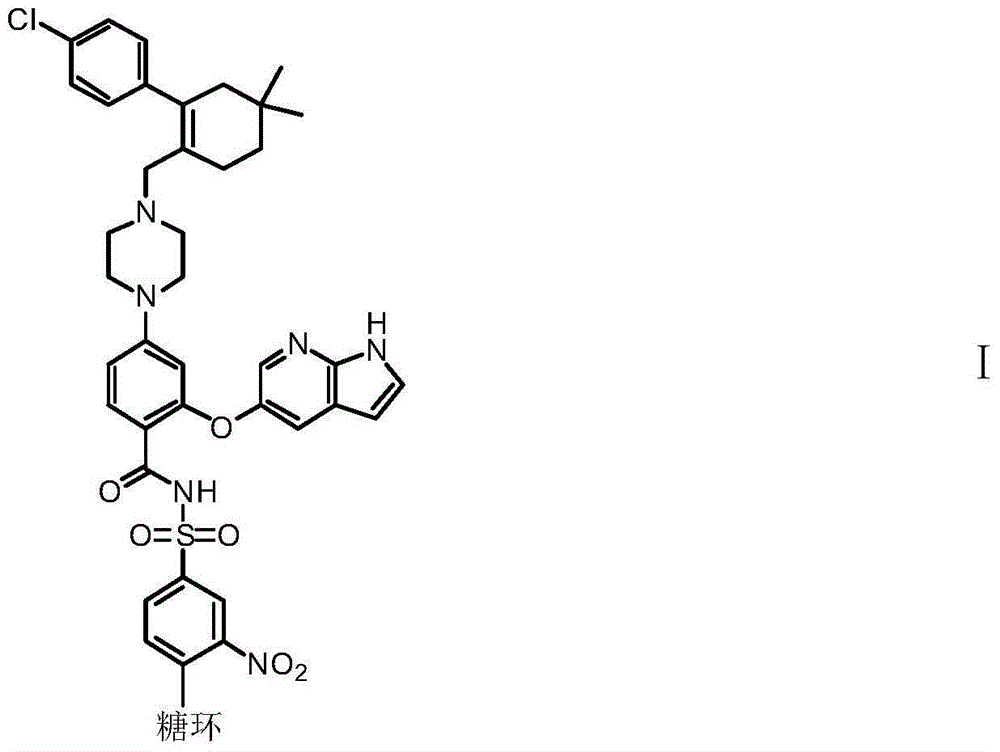
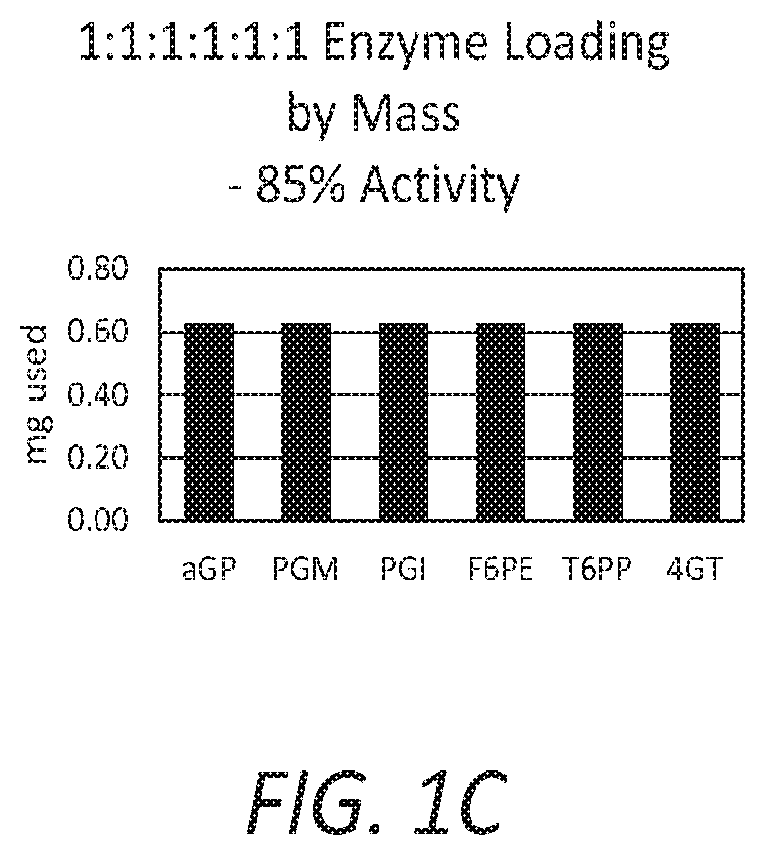
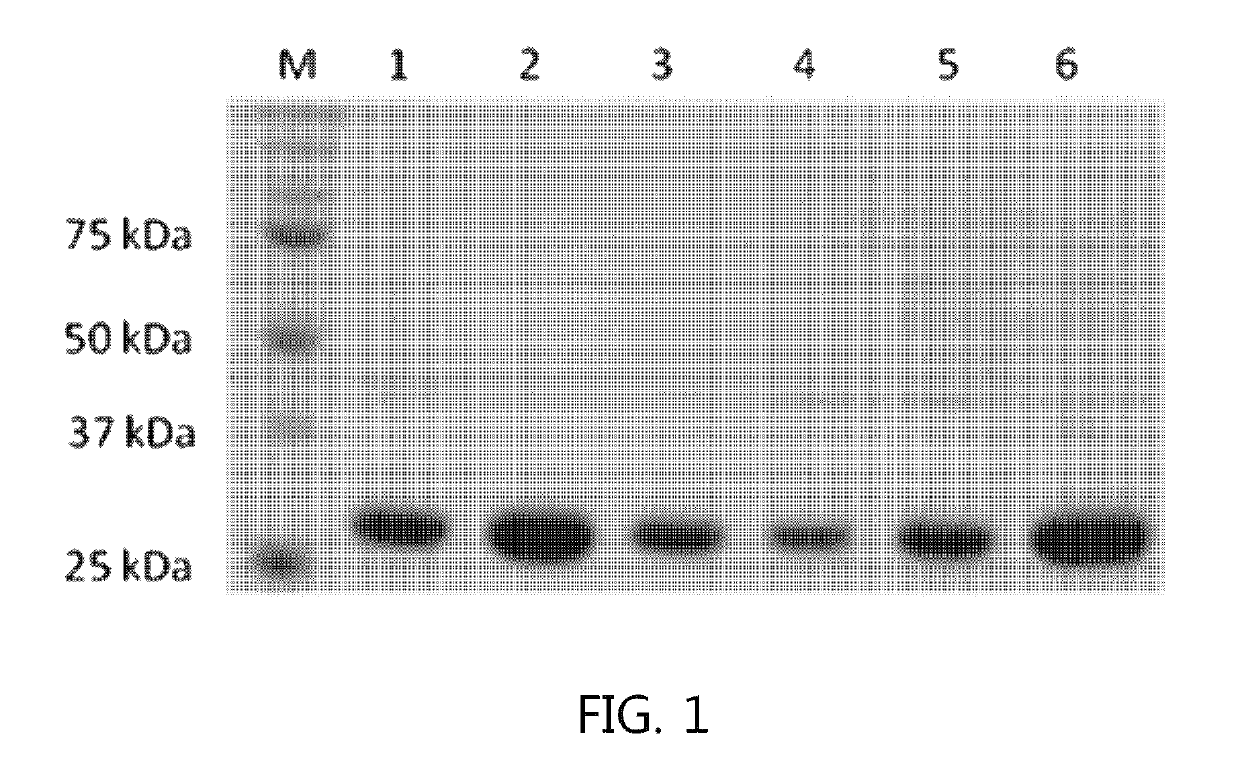
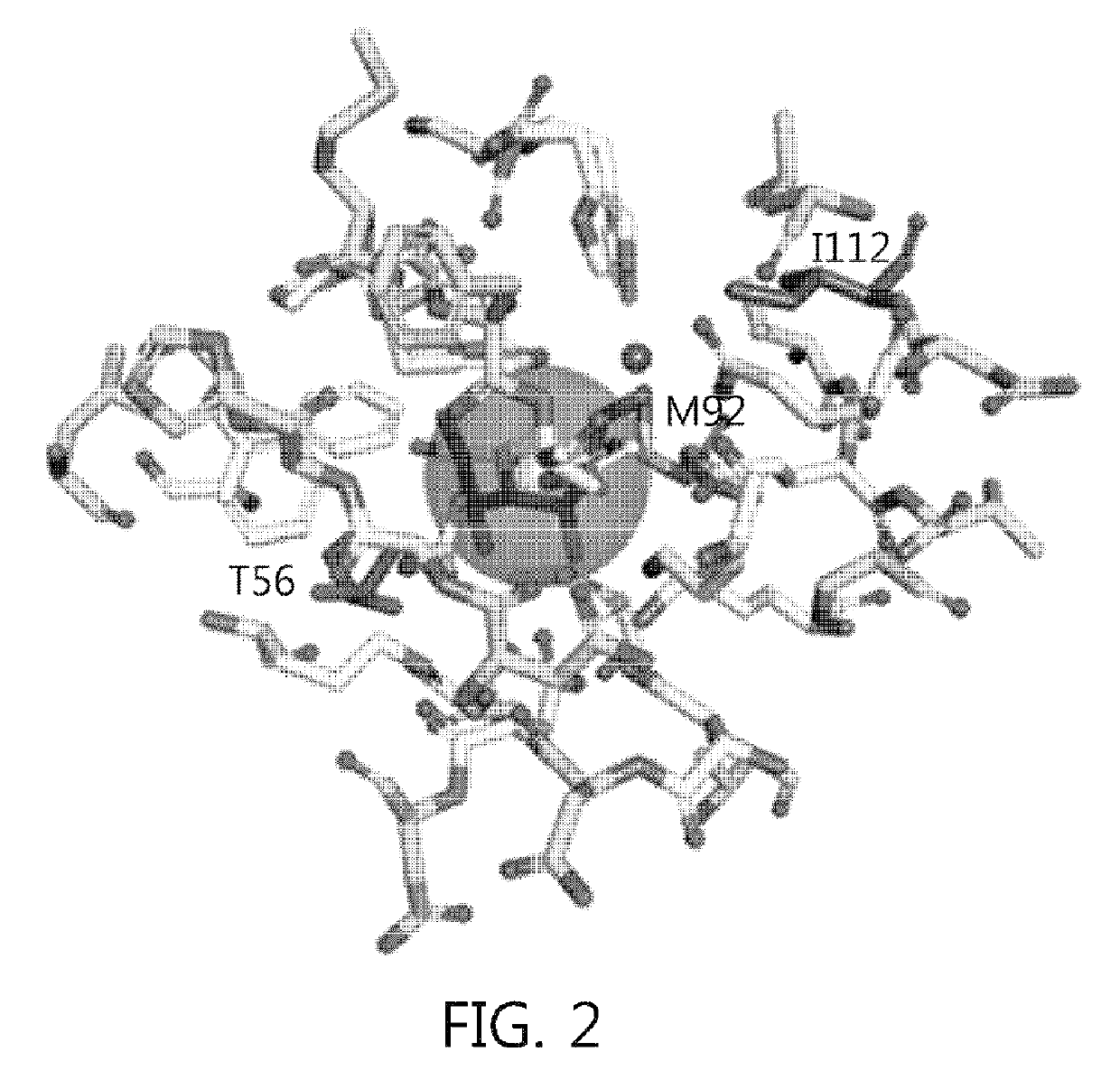
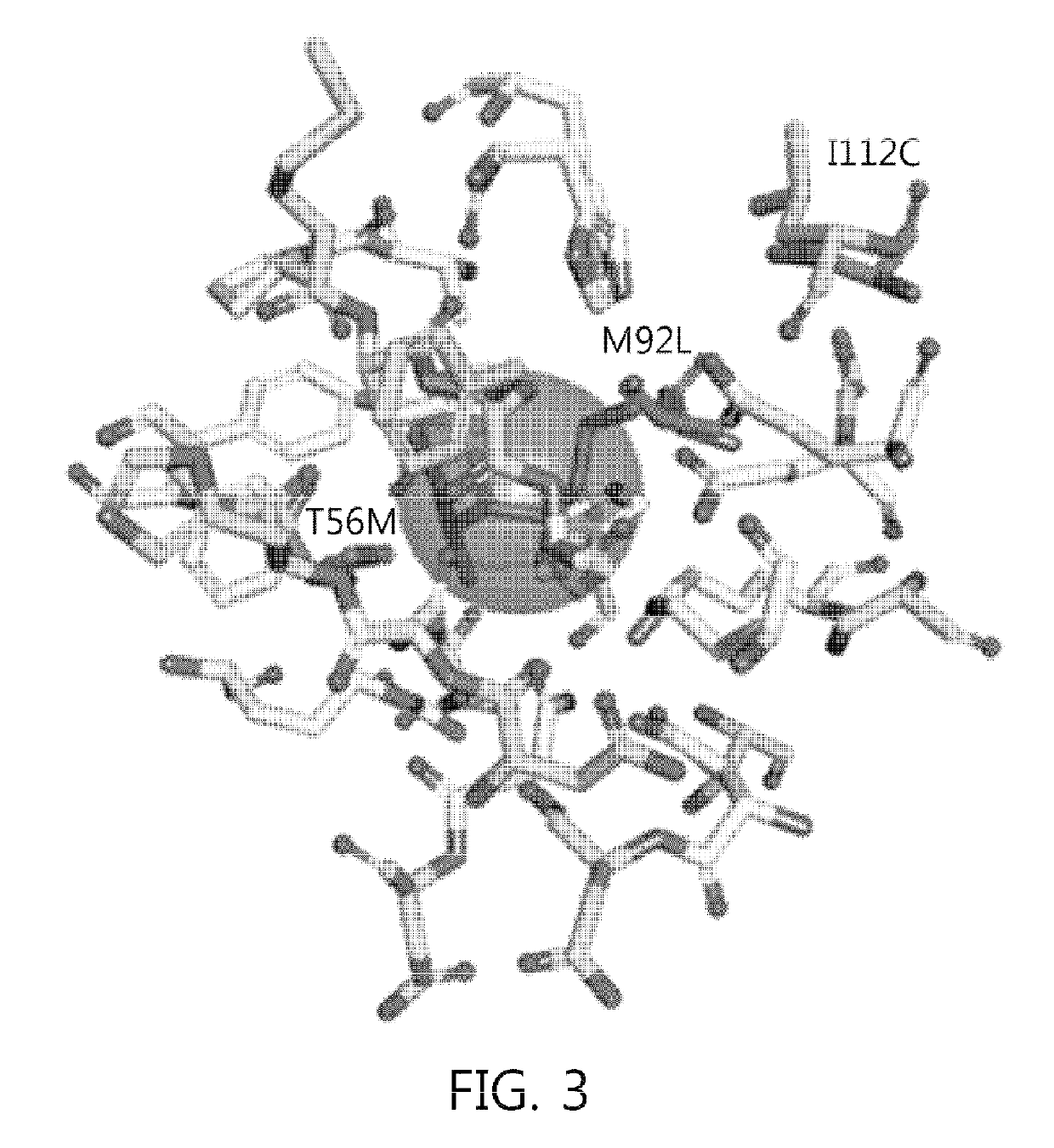
Patents
Literature
32 results about "Gulose" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Gulose is an aldohexose sugar. It is a monosaccharide that is very rare in nature, but has been found in archaea, bacteria and eukaryotes. It also exists as a syrup with a sweet taste. It is soluble in water and slightly soluble in methanol. Neither the d- and l-forms are not fermentable by yeast.
Ophthalmic and contact lens solutions containing simple saccharides as preservative enhancers
InactiveUS20070098818A1Effective preservationDegree of reductionBiocideHydroxy compound active ingredientsTagatoseSucrose
The present invention relates to an ophthalmic solution comprising 0.00001 to 10.0 weight percent of a simple saccharide, at least 0.00001 weight percent of a preservative, and not more than about 0.2 percent by weight chloride. The simple saccharide is chosen from the group consisting of: inositol; mannitol; sorbitol; sucrose; dextrose; glycerin; propylene glycol; ribose; triose; tetrose; erythrose; threose; pentose; arabinose; ribulose; xylose; xylulose; lyxose; hexose; allose; altrose; fructose; galactose; glucose; gulose; idose; mannose; sorbose; talose; tagatose; adlose; ketose; heptose; sedoheptulose; monosaccharides; disaccharides; sugar alcohols; xylitol; and polyol.
Owner:FXS VENTURES LLC
Dicycloglycosides compound, preparation method and application thereof
InactiveCN101104629AGood water solubilityIncrease resistanceOrganic active ingredientsSugar derivativesSolubilityAltrose
Disclosed is a preparation method of a bicyclol glycoside compound, which is characterized in that first bicyclol and glycine protected by Fmoc are condensed under the catalysis of 1-(3-dimethylamino propyl)-3-ethyl carbodiimide hydrochloride and 4-dimethylamino pyridine, and then protecting group of Fmoc is removed under the effect of acetonitrile diethylamine to obtain intermediate bicyclol-glycine ester which is the condensation of the bicyclol and the glycine. At last, Alpha or Beta glycosyl carboxyl methyl glycoside of glucose, galactose, mannose, allose, altrose, gulose, idose, talose, acetyl-glucosamine or acetyl galactosamine has condensation reaction with the intermediate bicyclol-glycine ester in solvent N-methyl pyrrolidone and under the effect of condensing agent and alkali. The water solubility of the bicyclol can be increased with the invention, and the bioavailability of the bicyclol is improved, which can provide enough raw material and effective compound route for the study of the anti-hepatitis drug. The invention has the advantages that the preparation method is simple and convenient; the condition is easy to control.
Owner:OCEAN UNIV OF CHINA
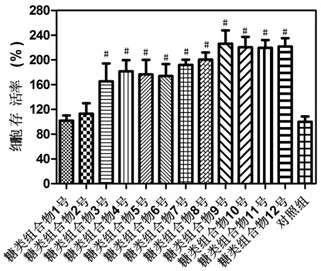
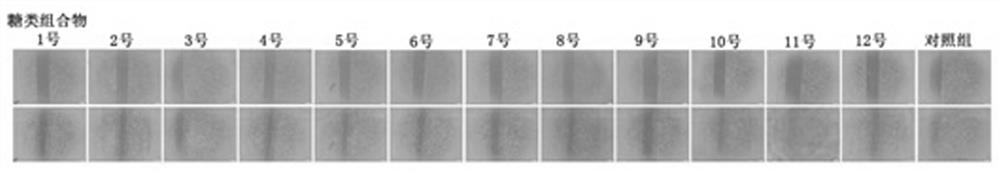
Carbohydrate composition with wound healing promoting effect and application of carbohydrate composition
ActiveCN108553481ARaw materials are easy to getEasy to manufactureOrganic active ingredientsDermatological disorderCarbohydrate compositionOligosaccharide
The invention provides a carbohydrate composition with a wound healing promoting effect and application of the carbohydrate composition. The carbohydrate composition contains at least five types of carbohydrate including carboxymethyl chitosan, rhizoma bletillae polysaccharides, fucoidan from fucus vesiculosus, heparan sulfate, chondroitin sulfate, oligosaccharides of hyaluronan and gulose aldehyde acid oligosaccharides. The carbohydrate composition and the application have the advantages that VEGFA (vascular endothelial growth factor A), FGF2 (fibroblast growth factor 2) and VE-cadherin protein mRNA (messenger ribonucleic acid) expression can be obviously improved by the carbohydrate composition, VEGF (vascular endothelial growth factor) secretion and skin fibroblast (HSF) and human immortalized keratinocyte (HaCaT) proliferation can be obviously promoted by the carbohydrate composition, and the obvious wound healing promoting effect can be realized by the carbohydrate composition; raw materials required by the carbohydrate composition are easily available, composition methods for the carbohydrate composition are simple, the carbohydrate composition is low in cost, easy to industrialize and wide in application range, and the like.
Owner:OCEAN UNIV OF CHINA
Method of Controlling the Proliferation of Vascular Endothelial Cells and Inhibiting Lumen Formation
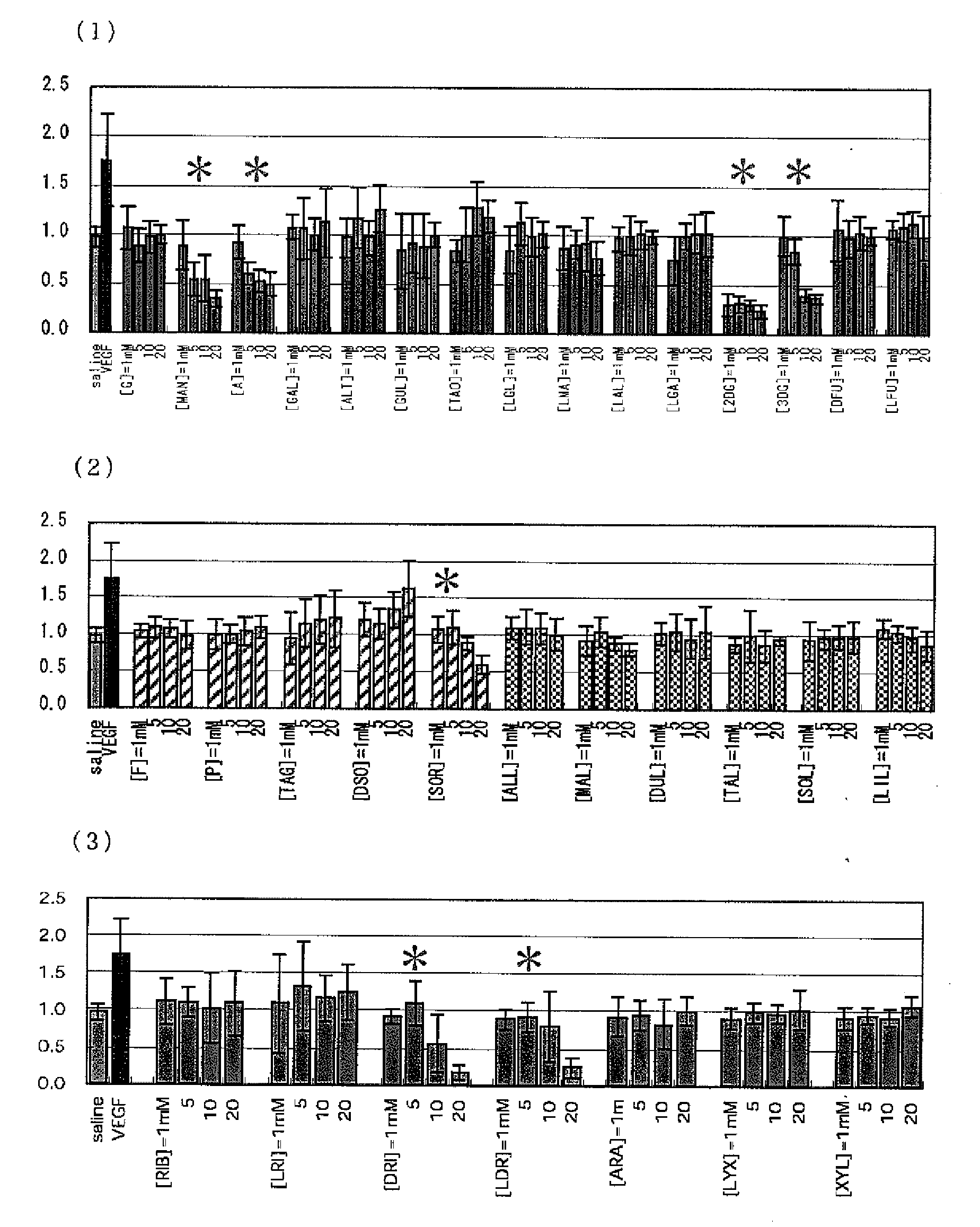
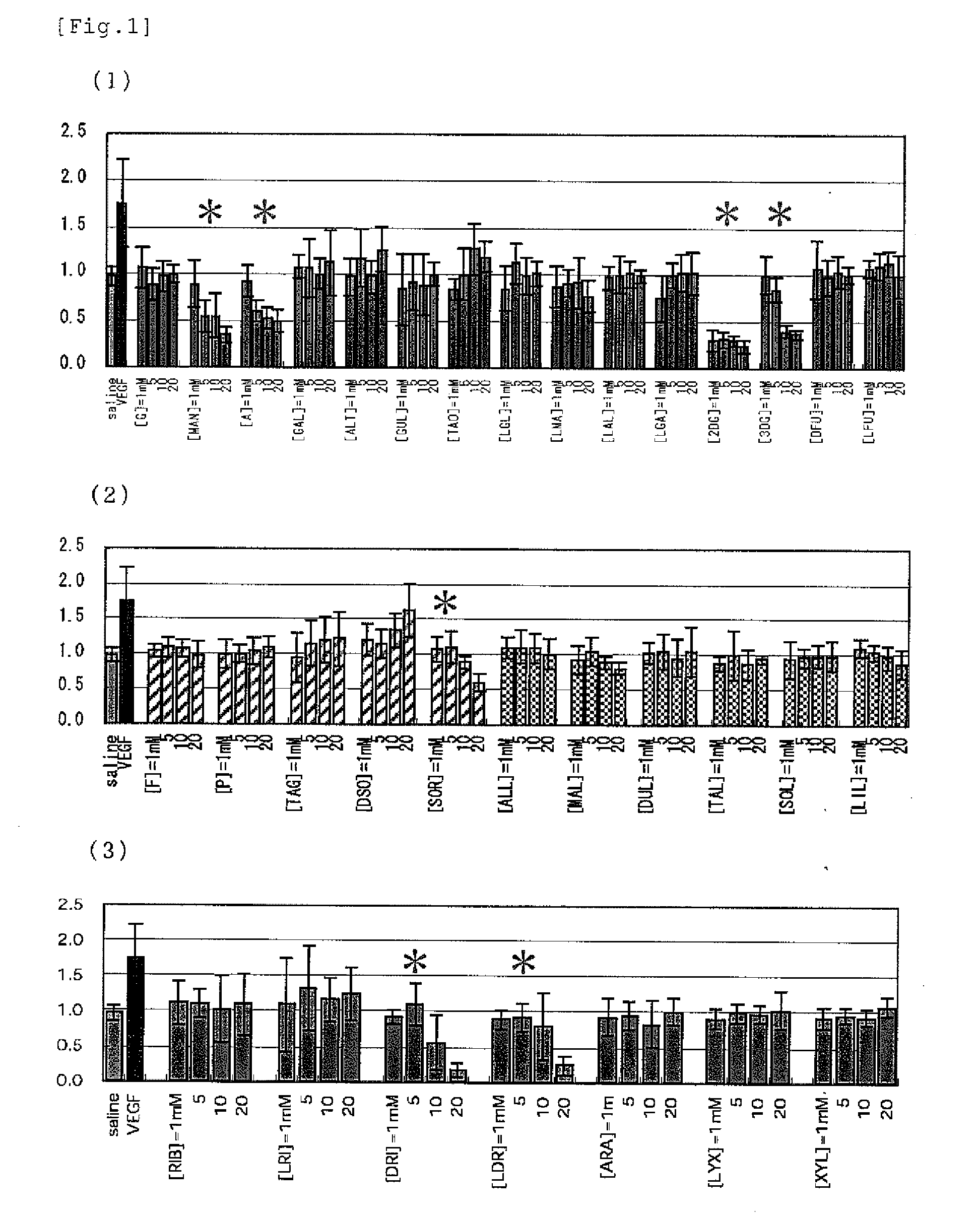
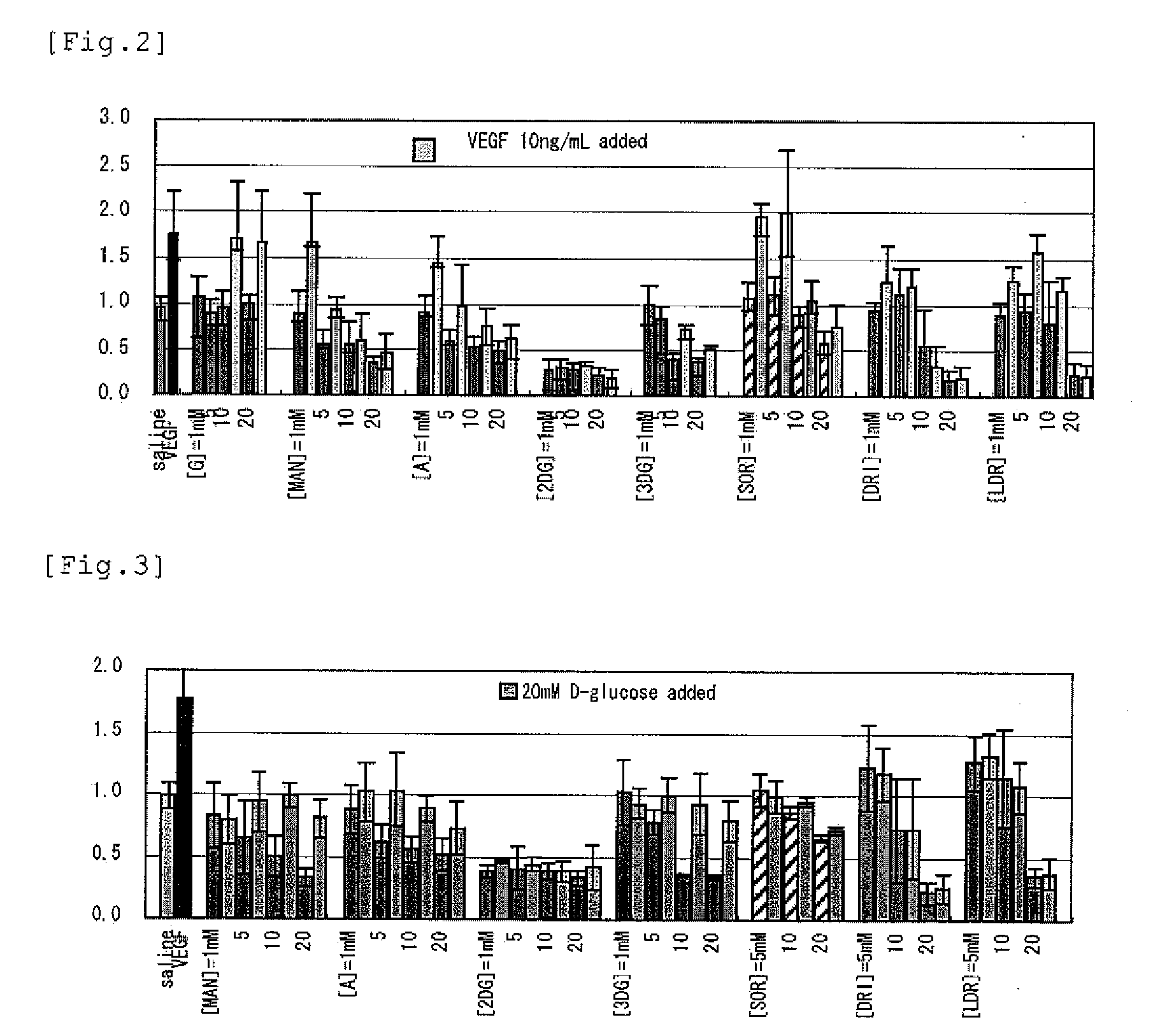
[PROBLEMS] To find out a specific rare sugar having effects of inhibiting the proliferation of vascular endothelial cells and lumen formation and utilize these effects. To provide this rare sugar as a preventive / remedy for diseases with angiogenesis, a cosmetic or a functional food.[MEANS FOR SOLVING PROBLEMS] A method of controlling the proliferation of vascular endothelial cells characterized by utilizing the vascular endothelial cell proliferation-controlling effect of D-mannose, D-allose, 2-deoxy-D-glucose, 3-deoxy-D-glucose, L-sorbose, 2-deoxy-D-ribose and / or 2-deoxy-L-ribose. A method of inhibiting lumen formation of vascular endothelial cells characterized by utilizing the vascular endothelial cell lumen formation-inhibiting effect of D-allose, D-altrose, D-gulose, D-talose, L-allose, 2-deoxy-D-glucose, 3-deoxy-D-glucose, D-ribose, L-ribose, 2-deoxy-D-ribose and / or 2-deoxy-L-ribose.
Owner:RARE SUGAR PRODN TECHN RES LAB
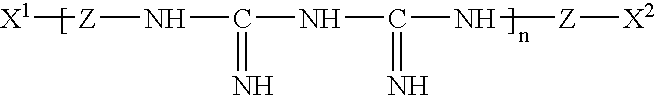
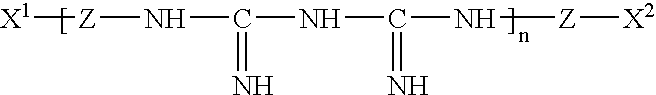
Antineoplastic new usage of cardiac glycoside compound in antiar
InactiveCN101156865AEnhanced inhibitory effectOrganic active ingredientsAntineoplastic agentsAlloseChemical compound
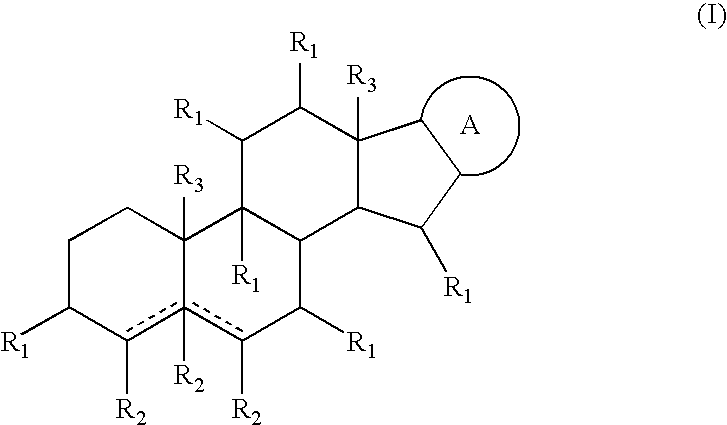
The invention relates to the medicine technology field, in particular relates to a new application of cardiac glycoside chemical compound having general formula (I) in preparing oncotherapy drug, wherein, R equals to 2-O-methyl-Beta-D-mycose, 6-deoxidation-Beta-D-gulose or 6-deoxidation-2-O-methyl-Beta-D-allose. The cardiac glycoside chemical compound can be obtained through a separation from upas in various conventional separation methods or be obtained through a synthesis or semisynthesis method.
Owner:INST OF TROPICAL BIOSCI & BIOTECH CHINESE ACADEMY OF TROPICAL AGRI SCI
Chewing gum base containing substituted polysaccharides and chewing gum products made there from
A chewing gum base comprises food acceptable substituted polysaccharides wherein substituents on the saccharide units in the polysaccharides produce a degree of substitution of at least I.0. The polysaccharides may have branches with an average length of 1 to 15 saccharide units per branch. The polysaccharides may be linked saccharide units such as allose, altrose, mannose, gulose, idose, galactose, 3,6 anhydro galactose, glucuronic acid, mannuronic acid, galacturonic acid, aldobiouronic acid, fucose, rhamnose, arabinose, xylose, talose, acyl substituted glucose, fructose, lactose and combinations thereof.
Owner:WM WRIGLEY JR CO
BCL-2 selective inhibitor with sugar ring structure and application thereof
The invention discloses a BCL-2 selective inhibitor with a sugar ring structure and application of the BCL-2 selective inhibitor in medicine for treating diseases like tumors, immune and autoimmune diseases, and the like, caused by BCL-2 overexpression. The BCL-2 selective inhibitor is characterized in that a sugar ring is monosaccharide and alkyl, thioether or alkenyl derivatives thereof, or oligosaccharide and alkyl, thioether or alkenyl derivatives thereof; monosaccharide is selected from glucose, mannose, galactose, fructose, xylose, arabinose, ribose, desoxyribose, allose, altrose, gulose, idose, talose or threose; and oligosaccharide is selected from saccharose, maltose, cellobiose, lactose or raffinose.
Owner:SUZHOU GUOKUANG PHARMTECH CO LTD
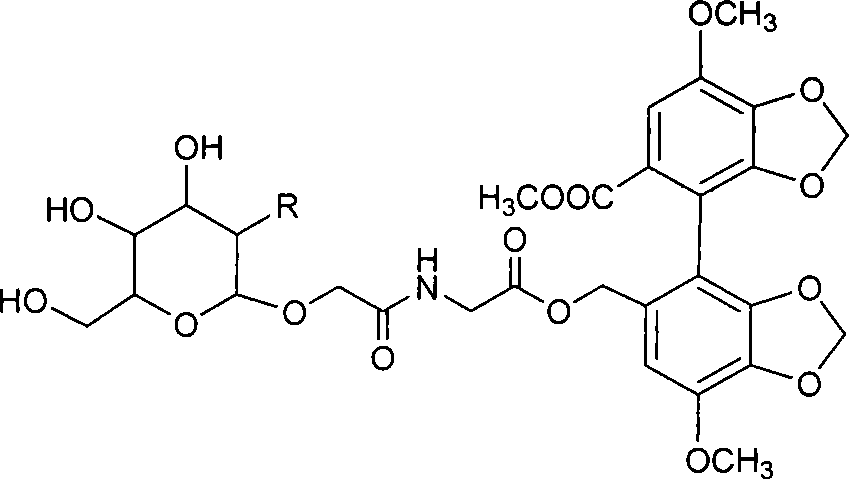
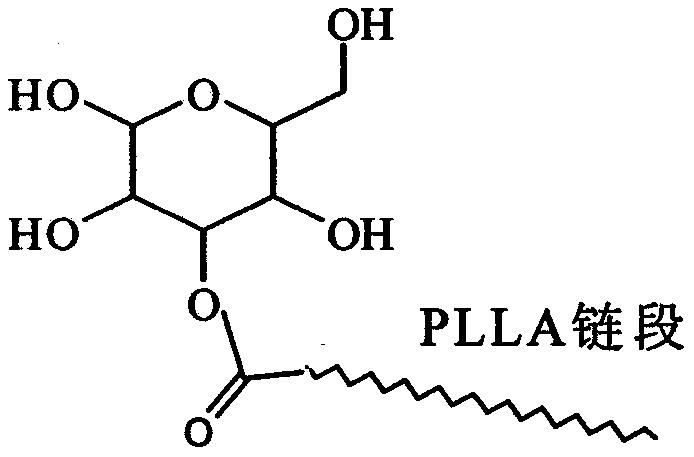
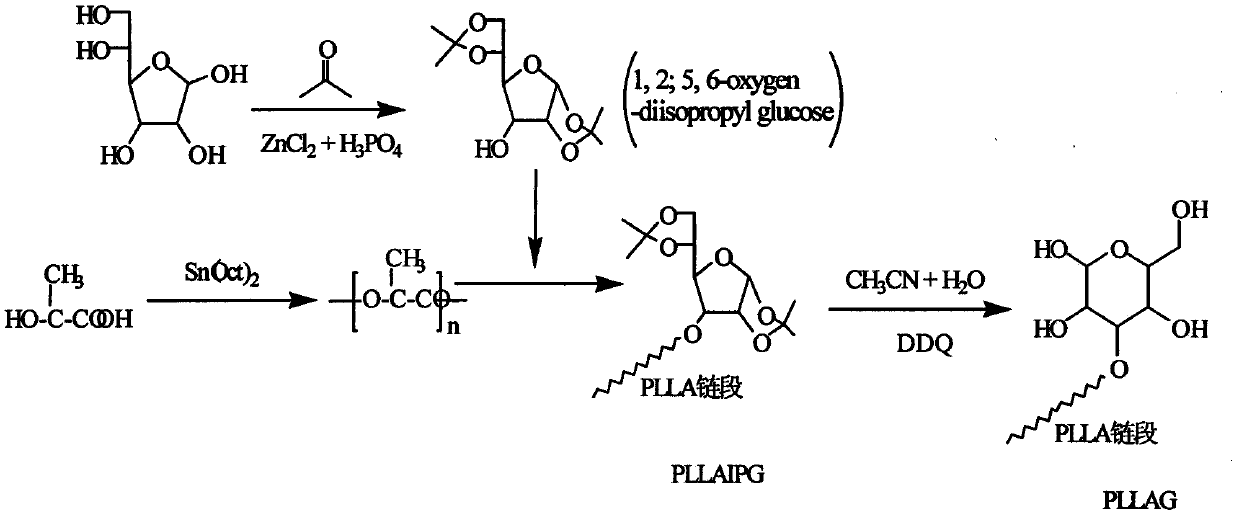
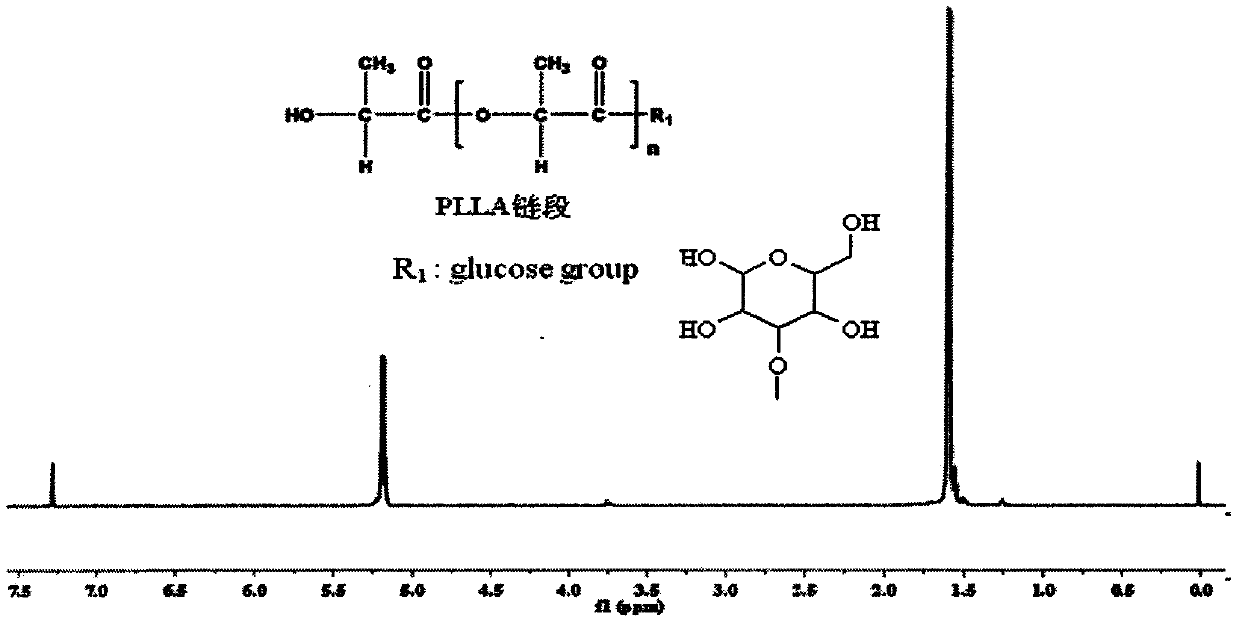
Glucose group-terminated poly(L-lactic acid) (PLLA) diblock copolymer material and preparation method thereof
InactiveCN111040145AControl lengthControl chain structurePharmaceutical non-active ingredientsPolymer scienceOrganic chemistry
The invention discloses a glucose group-terminated PLLA diblock copolymer material and a preparation method thereof, belonging to the technical field of high polymer materials. The preparation methodcomprises the following steps: firstly, subjecting PLLA and 1,2:5,6-di-O-isopropylidene-D-gulose (IPG) serving as raw materials to a reaction under the conditions that a molar ratio of IPG to PLLA isgreater than 4: 1, a polymerization pressure is 10-300 Pa, and a polymerization temperature is 160-200 DEG C; and carrying out a melt polymerization reaction for 4-12 hours to prepare a poly(L-lacticacid)-isopropylidene glucose diblock copolymer (PLLAIPG), and removing a hydroxyl protection group of the PLLAIPG to obtain the glucose group-terminated PLLA diblock copolymer (PLLAG). The PLLAG material has a melting temperature of about 150 DEG C and a crystallinity of about 50%, which are similar to those of PLLA, but the hydrophilicity of the PLLAG material is superior to the hydrophilicity ofPLLA; and the contact angle of the PLLAG material is about 65 degrees while the contact angle of PLLAG is 88 degrees.
Owner:NANJING FORESTRY UNIV
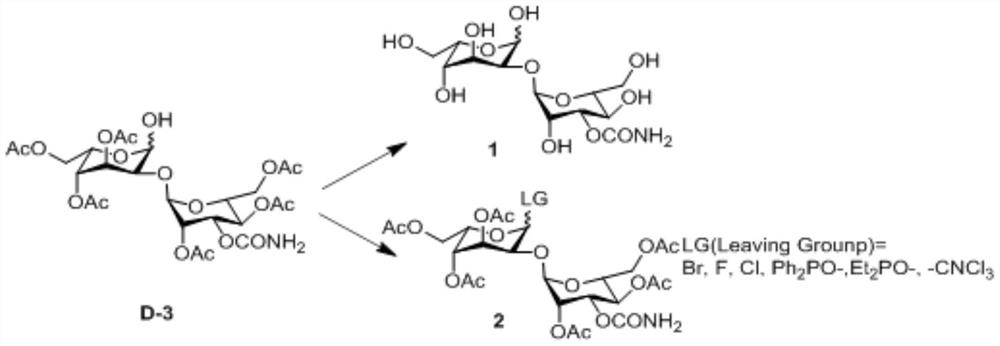
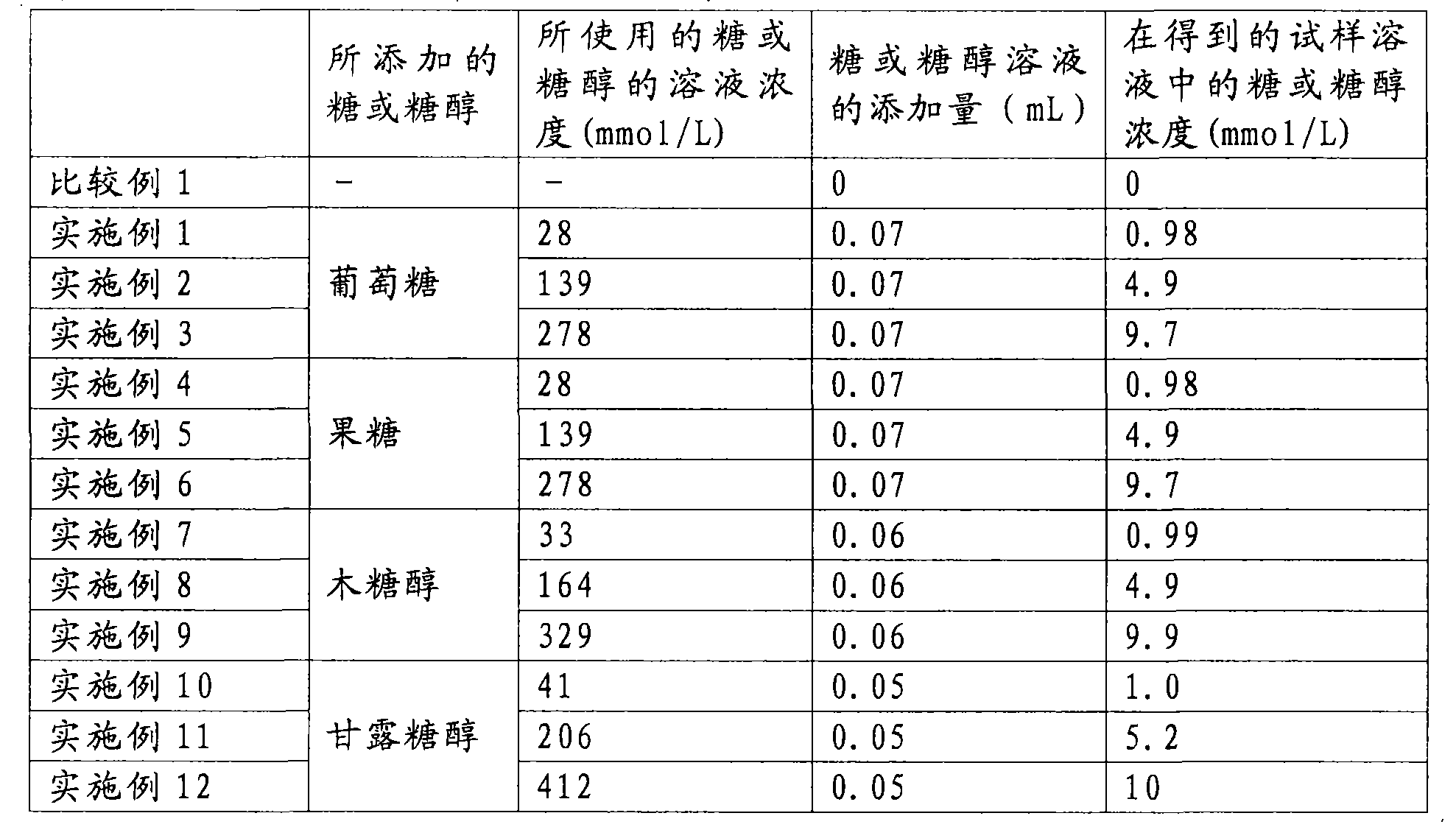
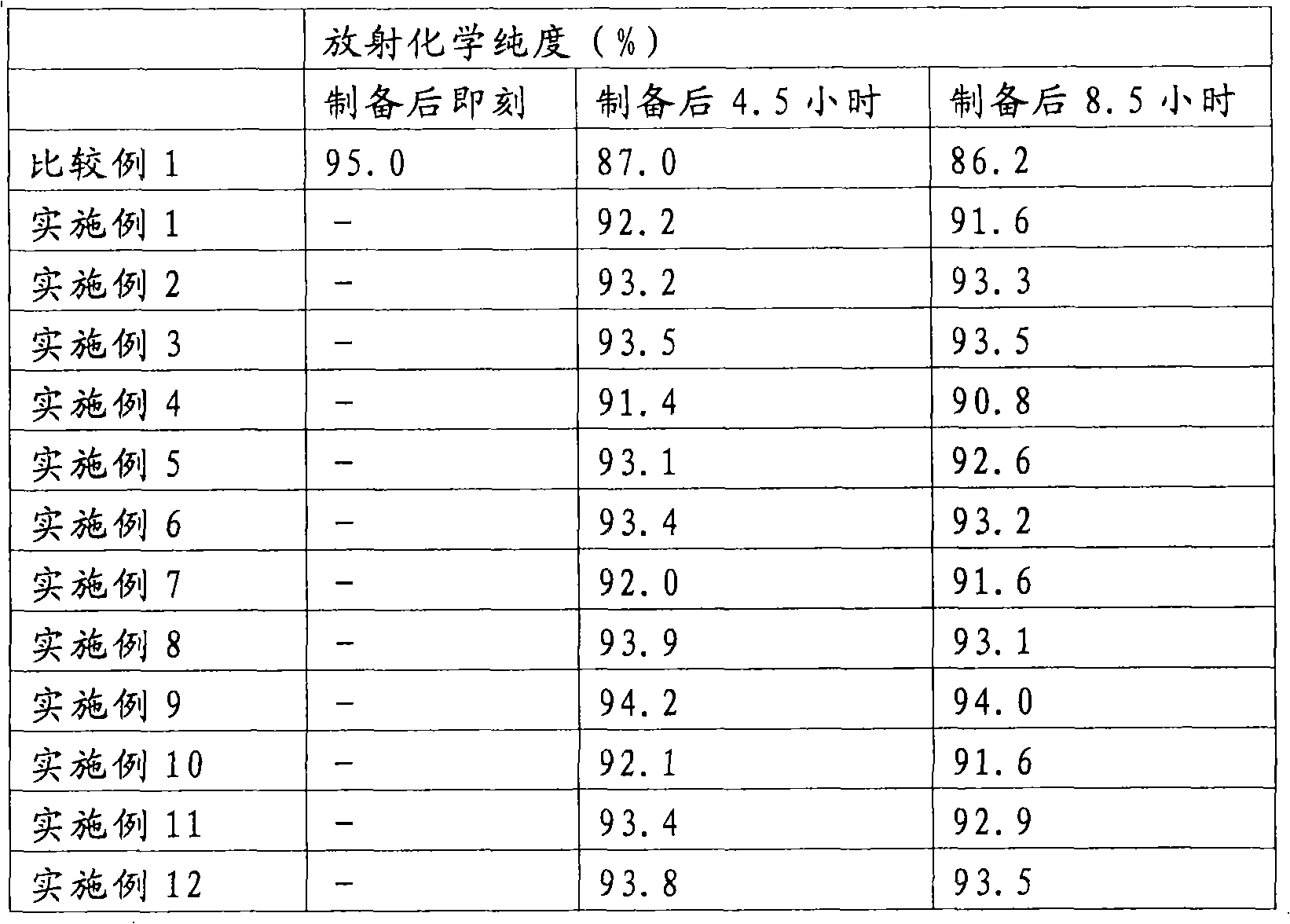
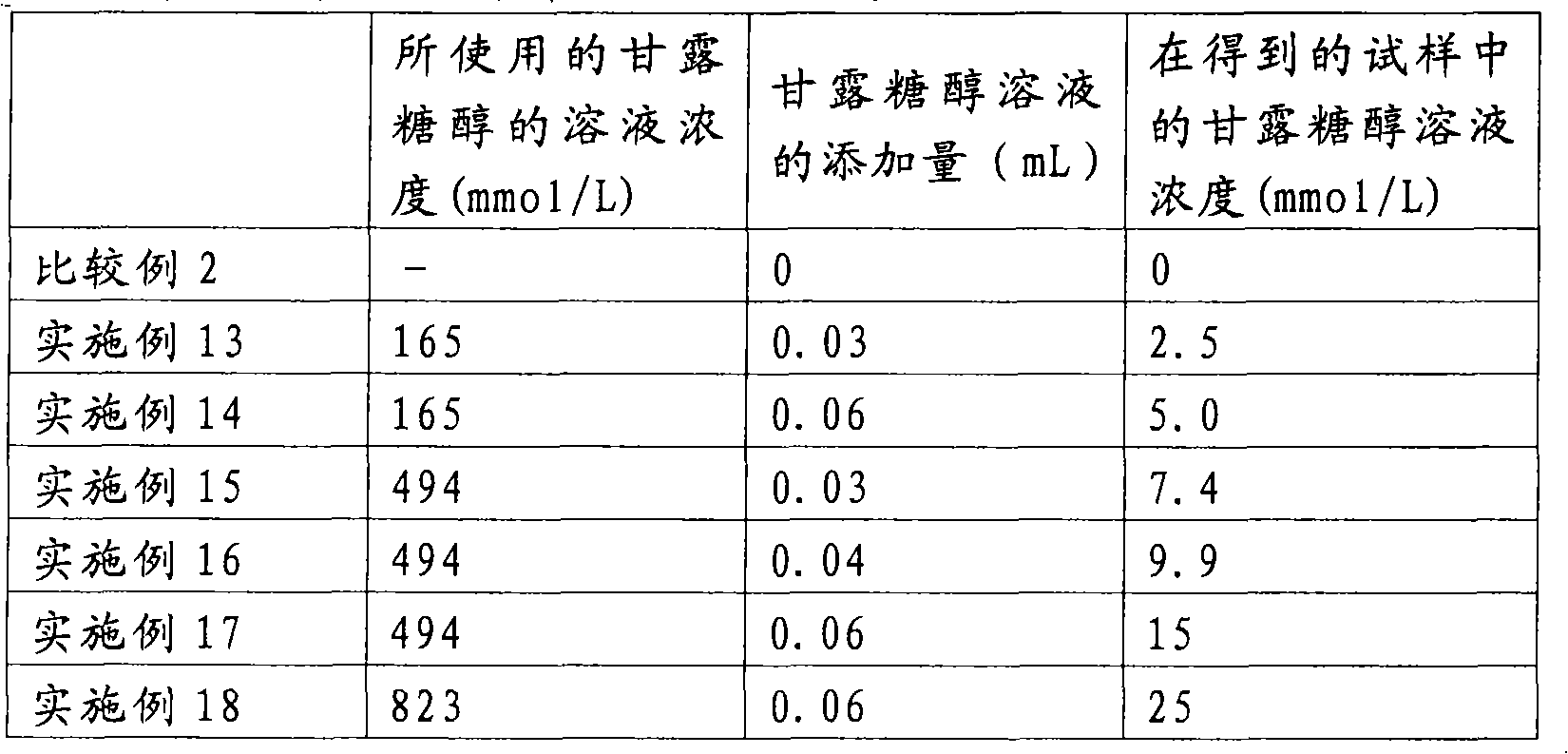
Radioactive Diagnostic Imaging Agent
InactiveUS20080193379A1Low puritySugar derivativesRadioactive preparation carriersTagatoseImaging agent
It is intended to provide a radioactive diagnostic imaging agent comprising a radioactive halogen-labeled compound as an active ingredient, in which the active ingredient is prevented from radiolysis and its stability is improved. This is achieved by adding a biologically-acceptable sugar or sugar alcohol to the radioactive diagnostic imaging agent in an amount effective to prevent radiolysis. The amount of the sugar or sugar alcohol to be added is preferably 10 (mmol / L) / GBq / mL or more, and more preferably 50 (mmol / L) / GBq / mL or more. The sugar is preferably selected from the group consisting of erythrose, threose, ribose, arabinose, xylose, lyxose, allose, altrose, glucose, mannose, gulose, idose, galactose, talose, erythrulose, ribulose, xylulose, psicose, fructose, sorbose, and tagatose. The sugar alcohol is preferably selected from the group consisting of erythritol, xylitol, sorbitol, and mannitol.
Owner:NIHON MEDI PHYSICS CO LTD
Novel oils having antibacterial activity
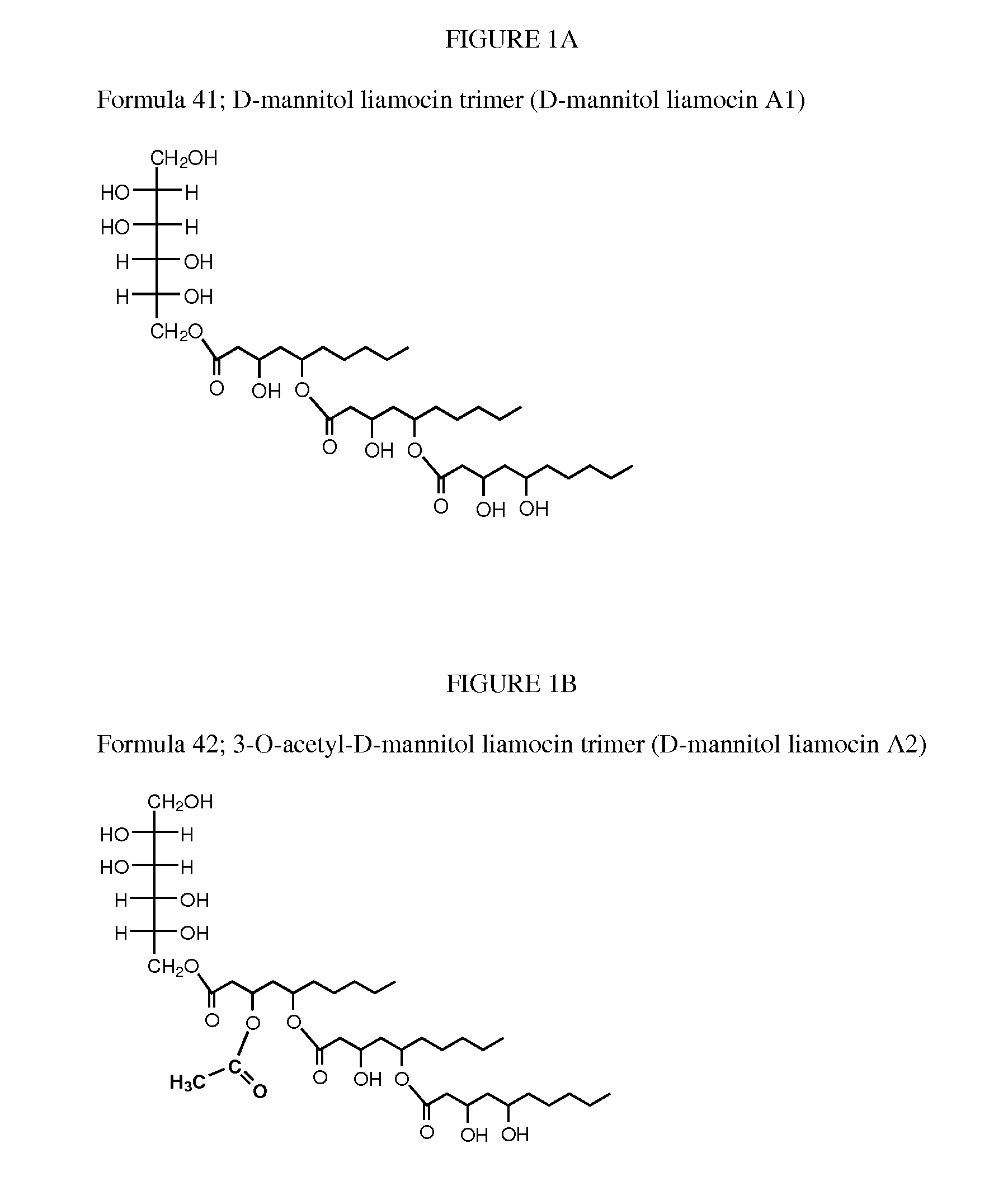
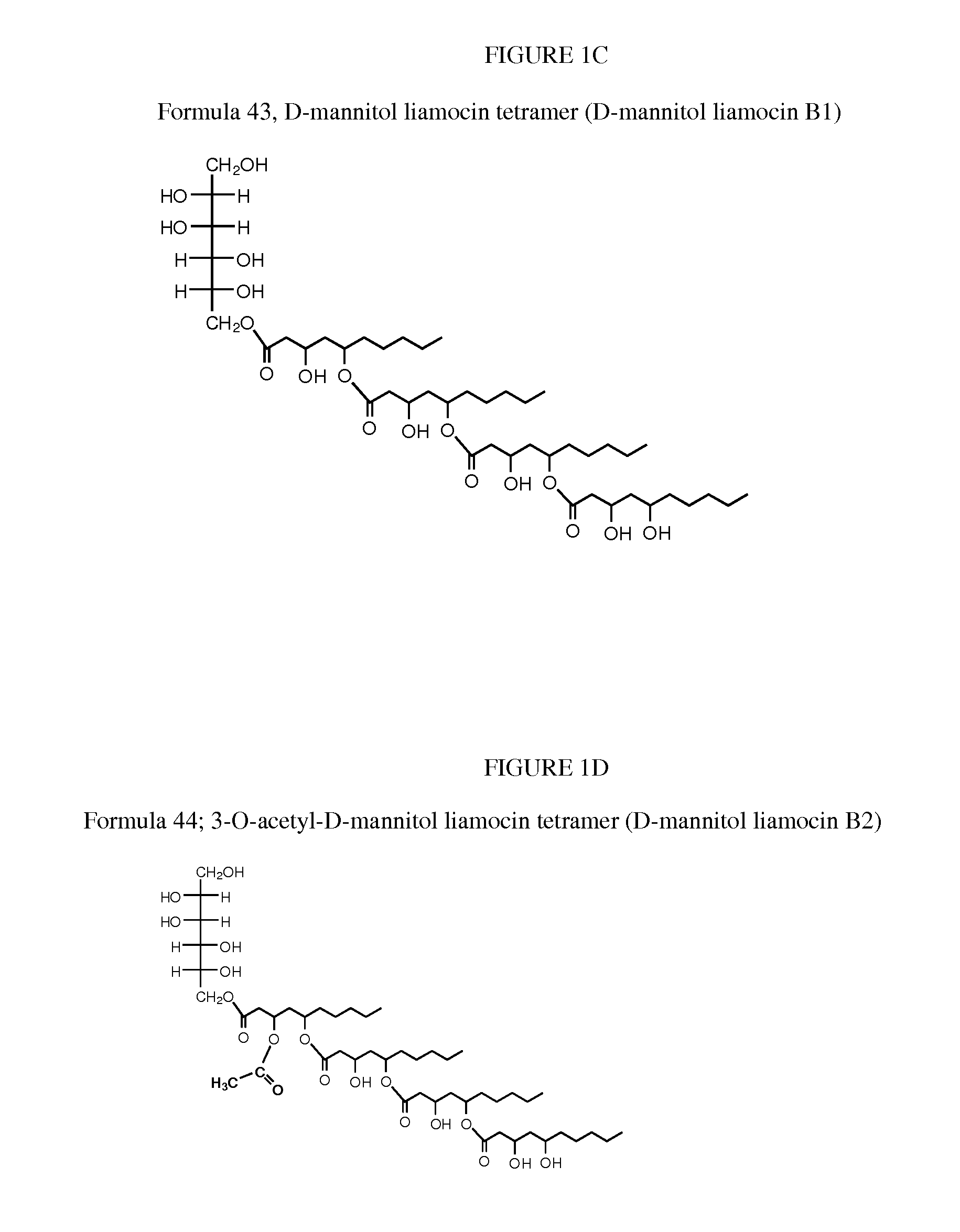
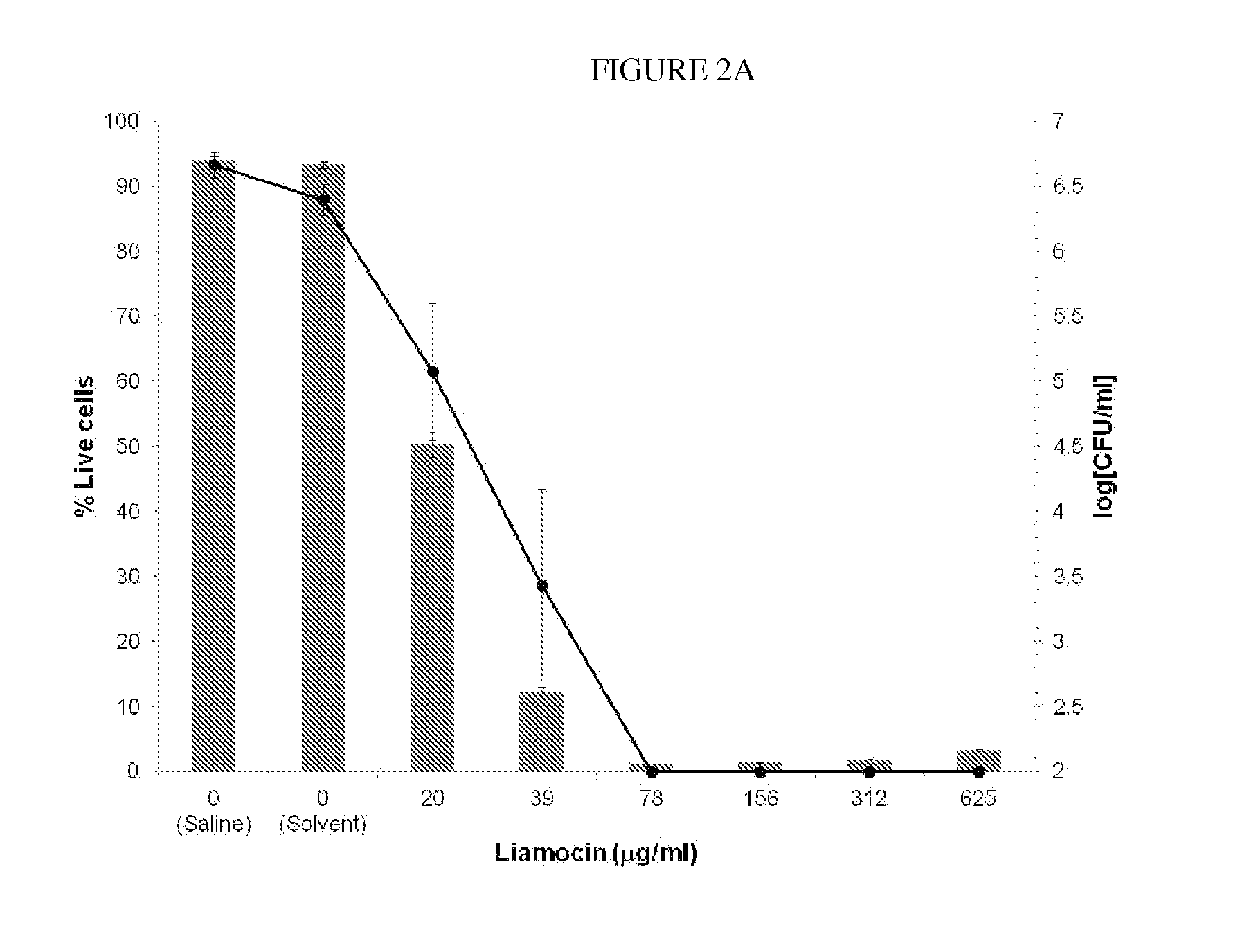
Novel compounds, called liamocins from Aureobasidium pullulans, having the general structure in Formula 1 are disclosed.where R1 is either COCH3 or H; and R2 is between two to ten O-linked 3,5-dihydroxydecanoate; and R3 can be a polyol (e.g., L- or D-glycerol, L- or D-threitol, L- or D-erythritol, L- or D-arabitol, L- or D-xylitol, L- or D-lyxitol, L- or D-ribitol, L- or D-allitol, L- or D-altritol, L- or D-mannitol, L- or D-iditol, L- or D-gulitol, L- or D-glucitol (also called sorbitol), L- or D-galactitol (also called dulcitol), and L- or D-talitol), 2-amino-D-mannitol, 2N-acetylamino-D-mannitol, L-rhamnitol, or D-fucitol; except when R3 is D-mannitol, R2 is not 2 nor 3 O-linked 3,5-dihydroxydecanoate chains. These liamocins described above in addition to D-mannitol liamocin A1, D-mannitol liamocin A2, D-mannitol liamocin B1, and D-mannitol liamocin B2, alone or in combination with each other, can be used to kill certain bacteria and to treat certain bacterial infections.
Owner:US SEC AGRI
Glycoalkaloid compositions and various uses thereof
InactiveUS20050227928A1Remove the subjects ability to fall pregnantBiocideOrganic active ingredientsTagatoseGlycerol
A composition comprising at least two glycoalkaloids of formula I: wherein: either one or both of the dotted lines represents a double bond, and the other a single bond, or both represent single bonds; A: represents a radical selected from the following radicals of general formulae (II) to (V): each of R1 is a radical separately selected from the group consisting of hydrogen, amino, oxo and OR4; each of R2 is a radical separately selected from the group consisting of hydrogen, amino and OR4; each of R3 is a radical separately selected from the group consisting of hydrogen, carbohydrate and a carbohydrate derivative; “X” is a radical selected from the group comprising —CH2—, —O— and —NH2—; and wherein the compound includes at least one R4 group that is a carbohydrate or a derivative thereof selected from the group comprising glyceric aldehyde, glycerose, erythrose, threose, ribose, arabinose, xylose, lyxose, altrose, allose, gulose, mannose, glucose, idose, galactose, talose, rhamnose, dihydroxyactone, erythrulose, ribulose, xylulose, psicose, fructose, sorbose, tagatose, and other hexoses, heptoses, octoses, nanoses, decoses, deoxysugars with branched chains, (e.g. apiose, hamamelose, streptose, cordycepose, mycarose and cladinose), compounds wherein the aldehyde, ketone or hydroxyl groups have been substituted (e.g. N-acetyl, acetyl, methyl, replacement of CH2OH), sugar alcohols, sugar acids, benzimidazoles, the enol salts of the carbohydrates, saccharinic acids, sugar phosphates; wherein the ratio of said glycoalkaloids is between 6:1 and 1:6 and on the proviso that when the glycoalkaloids are solamargine and solasonine and they are present in a 1:1 ratio the solamargine and solasonine are isolated.
Owner:SOLBEC PHARMA LTD
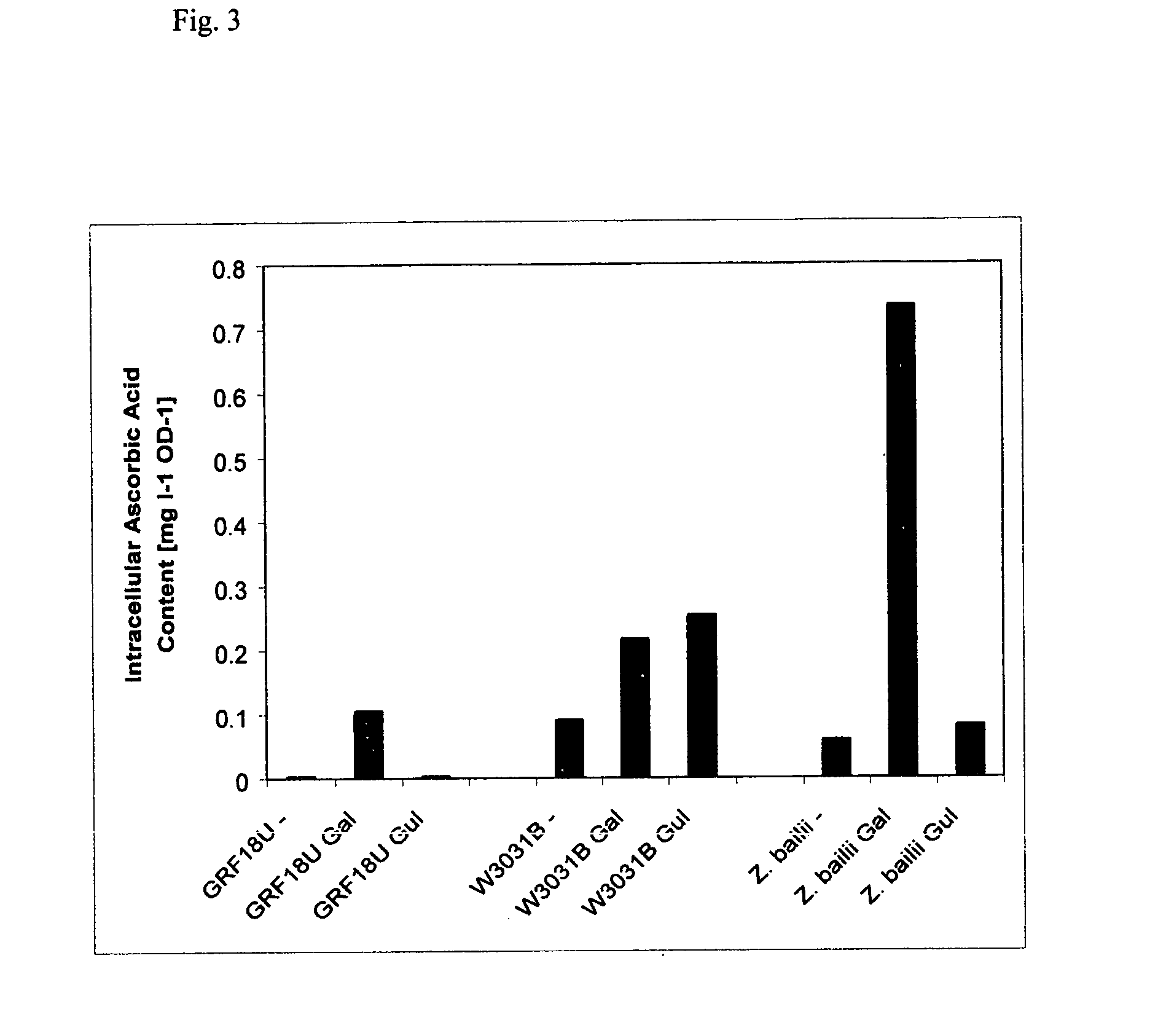
Ascorbic acid production from yeast
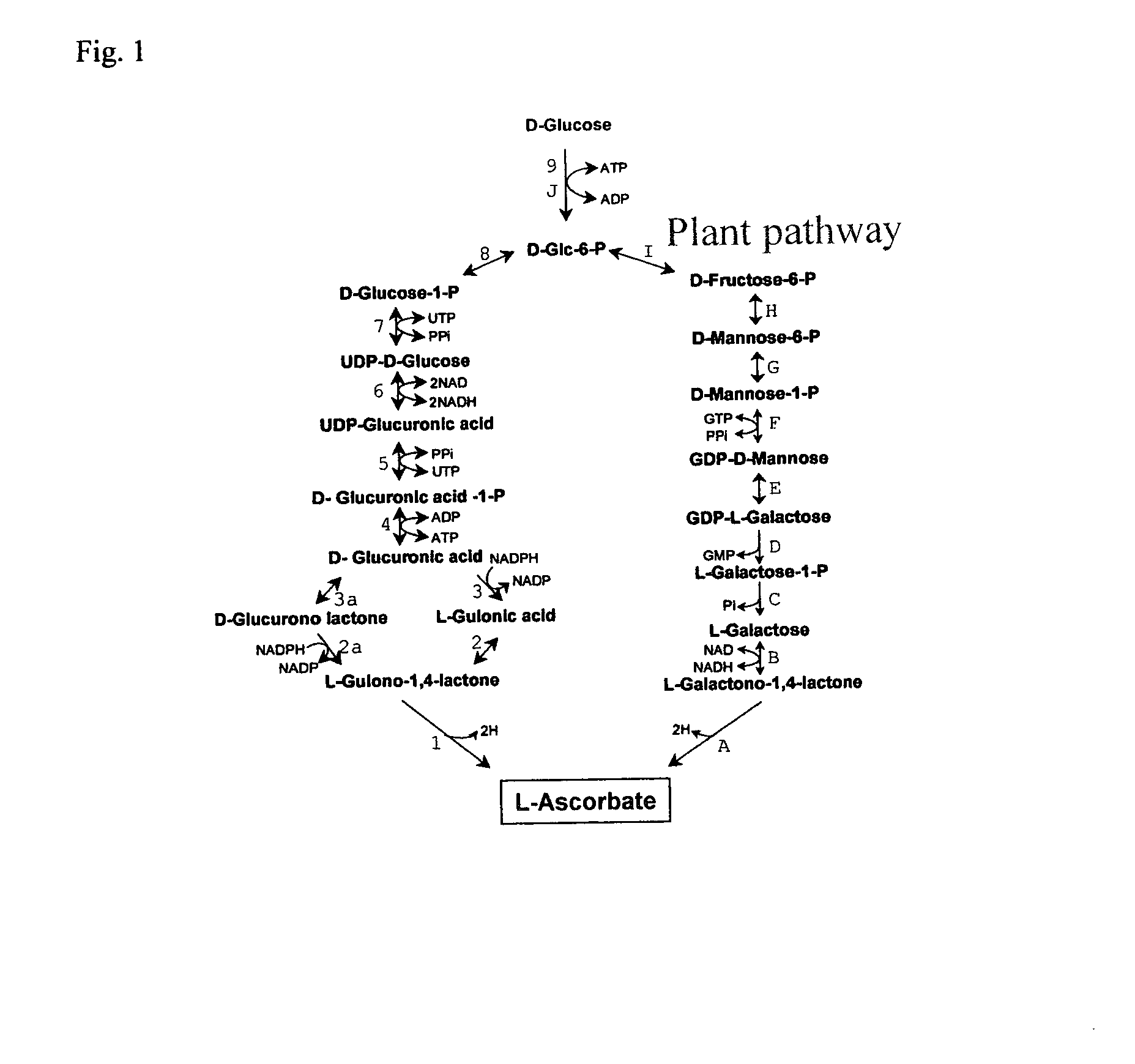
Herein is disclosed a method of generating ascorbic acid from yeast. In one embodiment, the yeast is a Zygosaccharomyces spp. or a Kluyveromyces spp. growing in a medium comprising an ascorbic acid precursor. In a second embodiment the yeast is a recombinant yeast growing in a medium comprising an ascorbic acid precursor. Preferably the recombinant yeast is transformed with a coding region encoding an enzyme selected from L-galactose dehydrogenase (LGDH), L-galactono-1,4-lactone dehydrogenase (AGD), D-arabinose dehydrogenase (ARA), D-arabinono-1,4-lactone oxidase (ALO) or L-gulono-1,4-lactone oxidase (RGLO). The ascorbic acid precursor is preferably D-glucose, L-galactose, L-galactono-1,4-lactone, or L-gulono-1,4-lactone. In another preferred embodiment the ascorbic acid is accumulated in the medium at levels greater than background. Preferably, the yield of the conversion of the precursor to ascorbic acid is preferably at least about 35%.
Owner:UNIV DEGLI STUDI DI MILANO BICOCCA
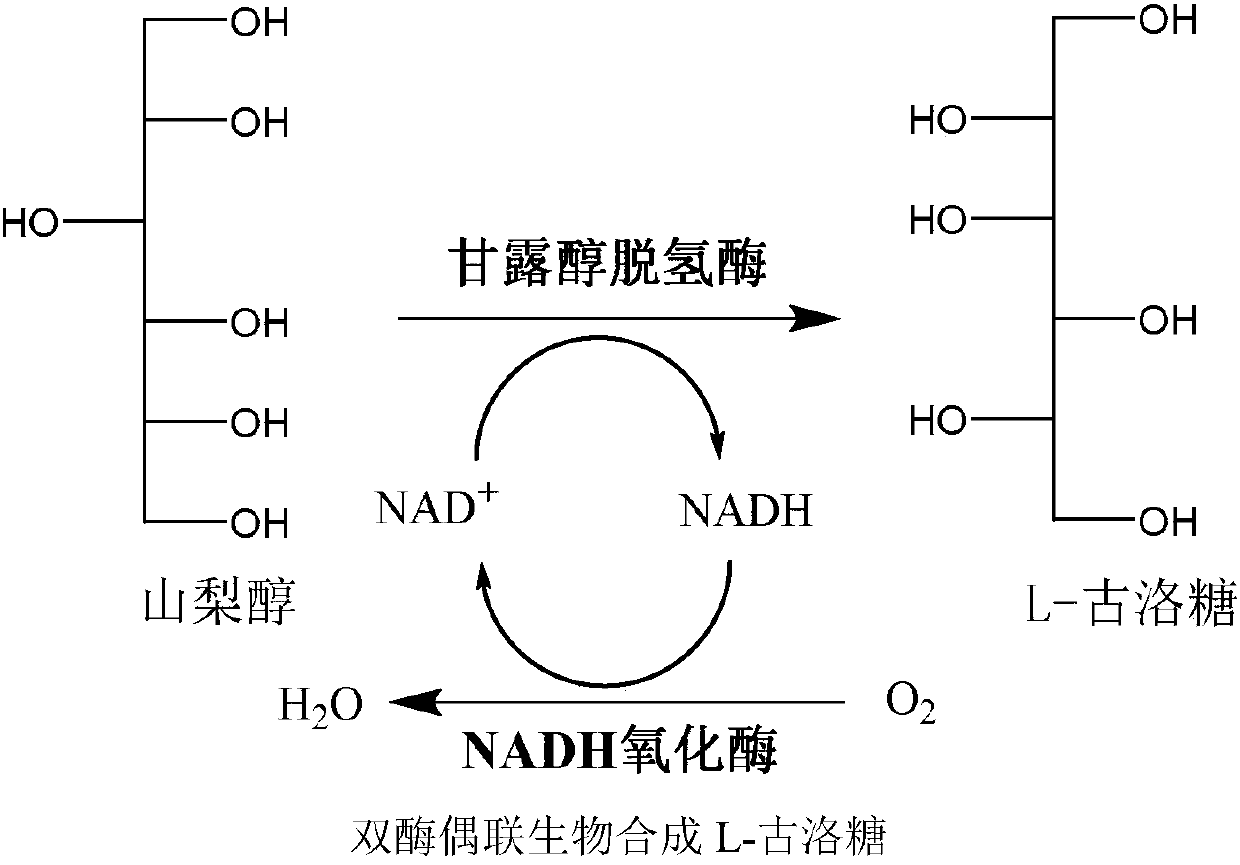
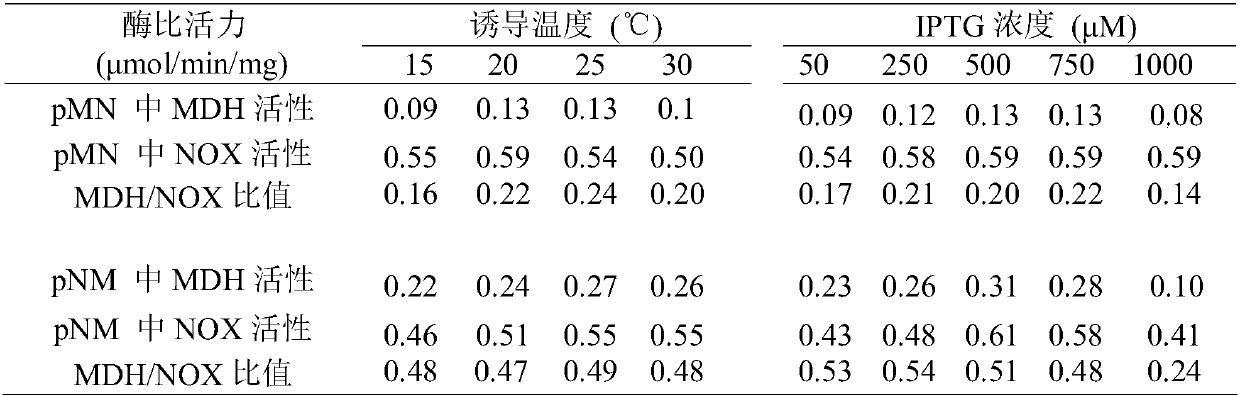
Preparation method for L-gulose
InactiveCN107937455AEfficient Green BiosynthesisAchieve inhibitionFermentationEscherichia coliVitamin C
L-gulose is a rare aldohexose, which can be used as a synthetic precursor of vitamin C and a drug intermediate for the synthesis of nucleoside antiviral drugs, with high potential economic value. In view of the shortcomings of the existing gulose synthesis process, the present invention co-expressed NAD+-dependent mannitol dehydrogenase and NADH oxidase in Escherichia coli to construct a whole-cell biocatalyst. A green biosynthetic process for the preparation of L-gulose.
Owner:CHINA PHARM UNIV
Beta-elemene substituted ethyl peracetylated sugar complex, beta-elemene substituted ethyl sugar complex, and preparation methods and use
InactiveCN103772453AImprove solubilityLess irritatingSugar derivativesSugar derivatives preparationSolubilityLactose
The invention provides a beta-elemene substituted ethyl peracetylated sugar complex, a beta-elemene substituted ethyl sugar complex, and preparation methods and use. The beta-elemene substituted ethyl peracetylated sugar complex has a structure in a general formula I, wherein the complex is peracetylated galactose, peracetylated fucose, peracetylated rhamnose, peracetylated gulose, peracetylated lactose or peracetylated maltose when X is S or Se; and the complex is peracetylated glucose, peracetylated mannose, peracetylated galactose, peracetylated fucose, peracetylated rhamnose, peracetylated gulose, peracetylated lactose or peracetylated maltose when X is O or NH. The water-solubility is enhanced by leading in a polar ground to a beta-elemene structure; the beta-elemene substituted ethyl peracetylated sugar complex has important significance on research and application of the beta-elemene in the field of a medicine.
Owner:DALIAN UNIVERSITY
Gulose tablet excipient, medicine tablet and preparation method of medicine tablet
InactiveCN102671204ANo toxicityPromote degradationPill deliveryPharmaceutical non-active ingredientsSolubilityAdditive ingredient
The invention discloses gulose tablet excipient, which consists of the following ingredients in percentage by weight: 88 percent to 96 percent of gulose, 1 percent to 5 percent of starch octenyl succinate anhydride, 1 percent to 5 percent of silicon dioxide and 1 percent to 5 percent of porcellanite, and has the advantages that the mobility is good, the forming degree is good, the demolding performance is good, and the gulose tablet excipient can be directly tableted with medicine and water for preparing medicine tablets. The invention also discloses a medicine tablet, which is prepared from the following ingredients in percentage by weight: 75 percent to 85 percent of medicine active ingredients, 5 percent to 15 percent of gulose tablet excipient and 5 percent to 15 percent of water raw materials, wherein the gulose tablet excipient and the medicine active ingredients do not have incompatibility and reaction, in addition, the solvability is good, the flowability is good, and the medicine tablet is suitable for the direct tableting of various kinds of medicine. The invention also discloses a preparation method of the medicine tablet, which has the advantages that the preparation is simple, the implementation is easy, and the operation and the control are easy.
Owner:安吉东来药用辅料有限责任公司
Thermostable L-ribose isomerase and method for producing same and use of same
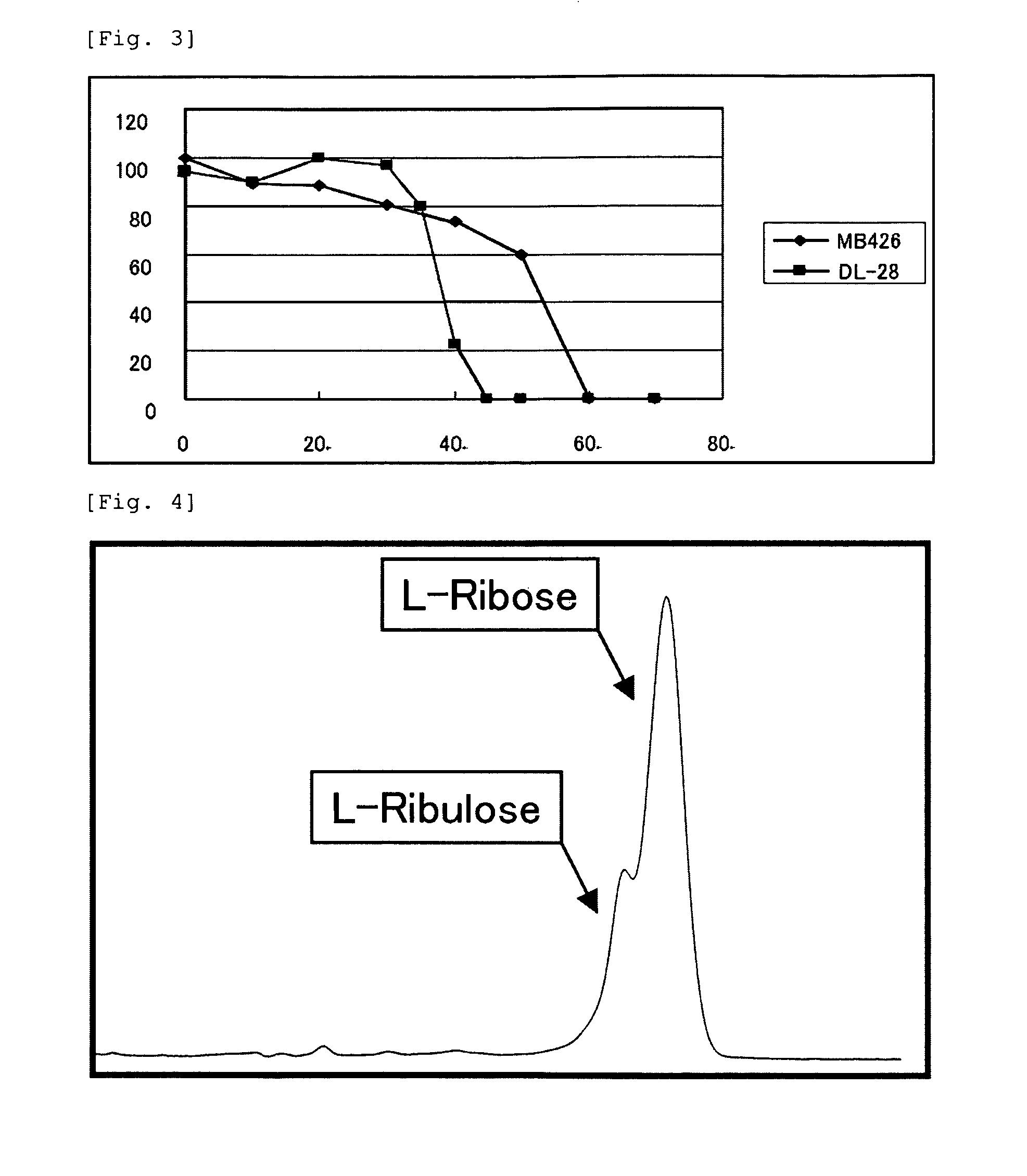
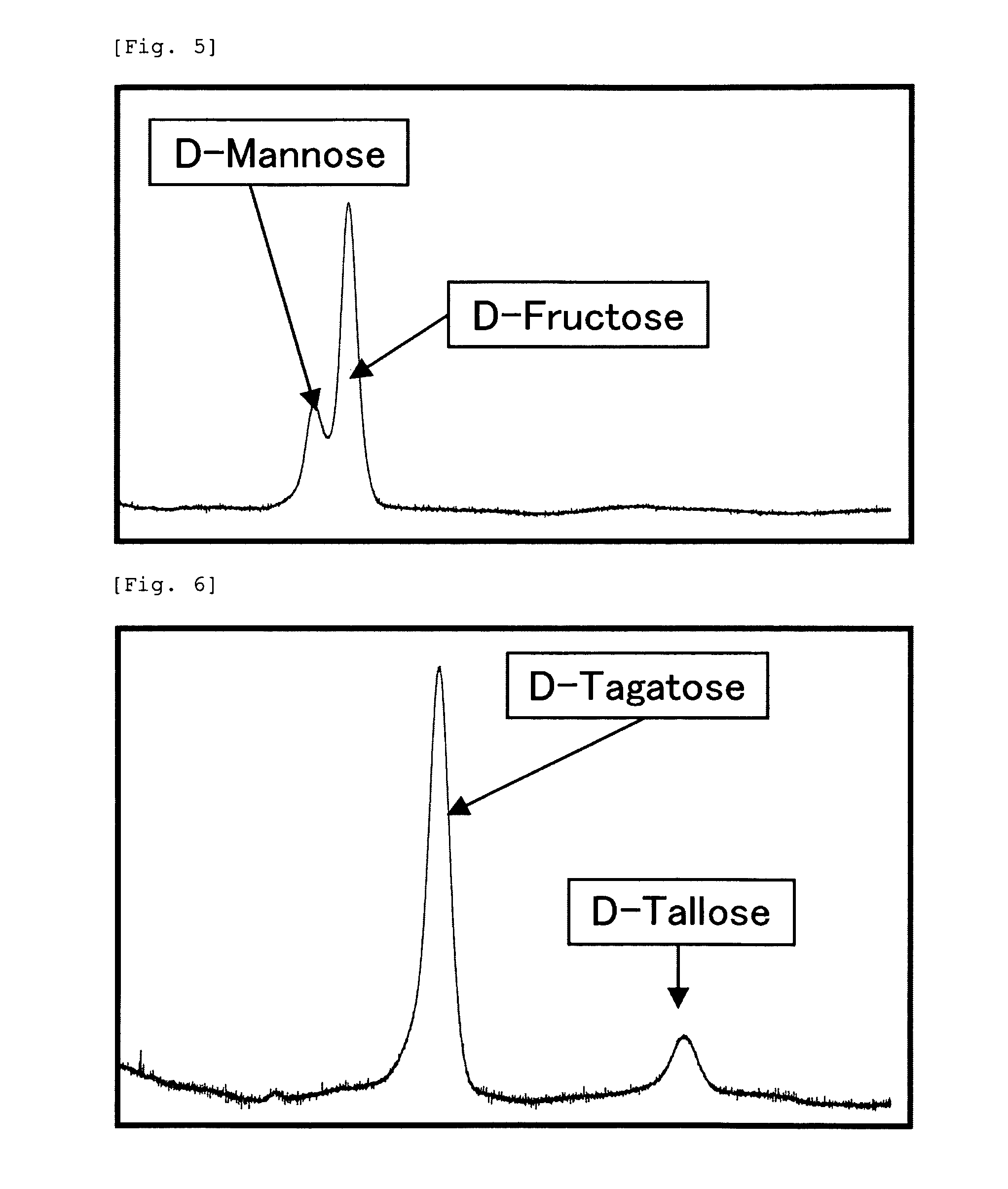
Object: To provide a thermostable L-ribose isomerase.Means for Resolution: The thermostable L-ribose isomerase with MW. 32,000 (by SDS-PAGE), optimal temperature of 45° C., optimal pH of pH 9.0 (glycine-NaOH buffer), and stable physicochemical properties such as temperature stability up to 45° C. during thermal treatment at pH 9.0 for 10 minutes, and with an action to isomerize L-ribose to generate L-ribulose or of inversely to isomerize L-ribulose to generate L-ribose. A conversion method between an aldose and a ketose comprising allowing the thermostable L-ribose isomerase as an enzyme derived from (1) Raoultella ornithinolytica strain MB426 (NITE BP-277) to interact with an aldose selected from L-ribose, D-lyxose, D-tallose, D-mannose, L-allose and L-gulose to isomerize the aldose to generate a ketose selected from the individually corresponding L-ribulose, D-xylulose, D-tagatose, D-fructose, L-psicose and L-sorbose or to interact with a ketose selected from L-ribulose, D-xylulose, D-tagatose, D-fructose, L-psicose and L-sorbose to isomerize the ketose to generate an aldose selected from the individually corresponding L-ribose, D-lyxose, D-tallose, D-mannose, L-allose and L-gulose.
Owner:KAGAWA UNIVERSITY +1
Process for the manufacture of 2-keto-l-gulonic acid
InactiveUS20050019881A1Inhibition formationOrganic chemistryMicroorganismsGluconic acid2-keto-L-gulonic acid
The present invention discloses a process for producing sodium 2-keto-L-gulonate from sorbitol and recovering 2-keto-L-gulonic acid in high recovery yields, by controlled cation exchange treatment of the micro-organism free fermentation broth and / or adjusting Ph of said purified fermentation broth, followed by direct crystallization of 2-keto-L-gulonic acid monohydrate.
Owner:CERESTAR HLDG
Immobilized enzyme composition for hexose production
The invention relates to an immobilized enzyme composition for preparing hexose. The hexoses include, for example, tagatose, psicose, fructose, allose, mannose, galactose, antrose, talose, sorbose, gulose, idulose, and inositol. The invention also relates to an enzymatic process for the preparation of hexose from a saccharide by contacting a starch derivative with an immobilized enzyme composition of the invention.
Owner:BONUMOSE INC
Radioactive diagnostic imaging agent
InactiveCN101198359APrevent radioactive decompositionLow purityIn-vivo radioactive preparationsSugar derivativesImaging agentAlcohol sugars
In a radioactive diagnostic imaging agent comprising a radioactive halogen-labeled compound as an active ingredient, to prevent the radiation degradation of the active ingredient to increase the stability of the radioactive diagnostic imaging agent. A biologically acceptable saccharide or sugar alcohol is added to the radioactive diagnostic imaging agent in an amount effective for the prevention of the radiation degradation. The amount of the saccharide or sugar alcohol to be added is preferably 10 (mmol / L) / GBq / mL or more, more preferably 50 (mmol / L) / GBq / mL or more. The saccharide is preferably selected from the group consisting of erythrose, threose, ribose, arabinose, xylose, lyxose, allose, altrose, glucose, mannose, gulose, idose, galactose, talose, erythrulose, ribulose, xylulose, psicose, fructose, sorbose and tagatose. The sugar alcohol is preferably selected from the group consisting of erythritol, xylitol, sorbitol and mannitol.
Owner:NIHON MEDI PHYSICS CO LTD
Antineoplastic new usage of cardiac glycoside compound in antiar
InactiveCN101156865BEnhanced inhibitory effectOrganic active ingredientsAntineoplastic agentsAlloseChemical compound
The invention relates to the medicine technology field, in particular relates to a new application of cardiac glycoside chemical compound having general formula (I) in preparing oncotherapy drug, wherein, R equals to 2-O-methyl-Beta-D-mycose, 6-deoxidation-Beta-D-gulose or 6-deoxidation-2-O-methyl-Beta-D-allose. The cardiac glycoside chemical compound can be obtained through a separation from upasin various conventional separation methods or be obtained through a synthesis or semisynthesis method.
Owner:INST OF TROPICAL BIOSCI & BIOTECH CHINESE ACADEMY OF TROPICAL AGRI SCI
Immobilized enzyme compositions for the production of hexoses
The invention relates to immobilized enzyme compositions for the preparation of a hexose. Hexoses include, for example, tagatose, psicose, fructose, allose, mannose, galactose, altrose, talose, sorbose, gulose, idose, and inositol. The invention also relates to an enzymatic process for preparing a hexose from a saccharide by contacting a starch derivative with an immobilized enzyme composition of the invention.
Owner:BONUMOSE INC
Mutant sugar isomerase with improved activity, derived from E. coli, and production of L-gulose using the same
ActiveUS10253341B2Reduced activityImprove enzymatic activityIsomerasesFermentationEscherichia coliIsomerase
Owner:KONKUK UNIV IND COOP CORP
Gulose tablet excipient, medicine tablet and preparation method of medicine tablet
InactiveCN102671204BNo toxicityPromote degradationPill deliveryPharmaceutical non-active ingredientsOctenyl succinateSuccinic acid
The invention discloses gulose tablet excipient, which consists of the following ingredients in percentage by weight: 88 percent to 96 percent of gulose, 1 percent to 5 percent of starch octenyl succinate anhydride, 1 percent to 5 percent of silicon dioxide and 1 percent to 5 percent of porcellanite, and has the advantages that the mobility is good, the forming degree is good, the demolding performance is good, and the gulose tablet excipient can be directly tableted with medicine and water for preparing medicine tablets. The invention also discloses a medicine tablet, which is prepared from the following ingredients in percentage by weight: 75 percent to 85 percent of medicine active ingredients, 5 percent to 15 percent of gulose tablet excipient and 5 percent to 15 percent of water raw materials, wherein the gulose tablet excipient and the medicine active ingredients do not have incompatibility and reaction, in addition, the solvability is good, the flowability is good, and the medicine tablet is suitable for the direct tableting of various kinds of medicine. The invention also discloses a preparation method of the medicine tablet, which has the advantages that the preparation is simple, the implementation is easy, and the operation and the control are easy.
Owner:安吉东来药用辅料有限责任公司
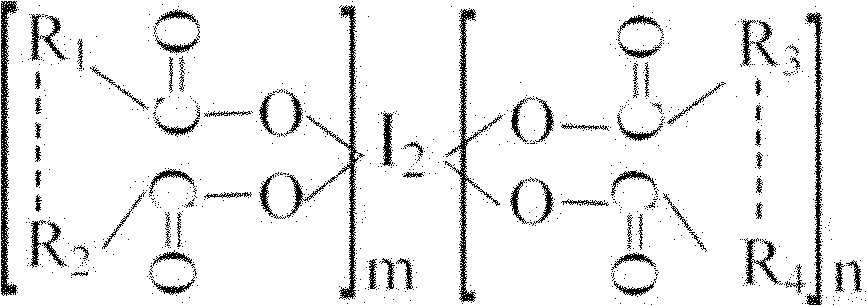
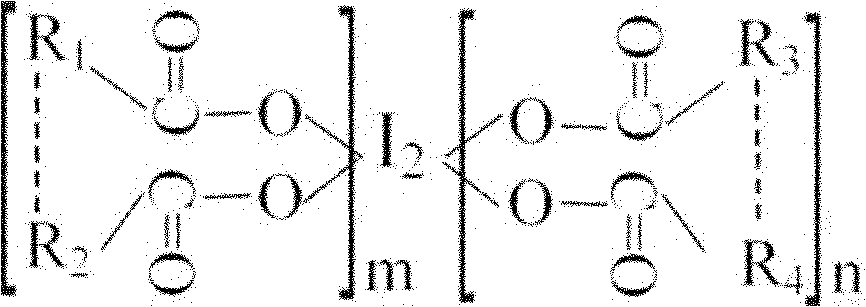
Oligomeric acid iodine as well as preparation method and application thereof
ActiveCN101967203BGuaranteed stabilityRapid and long-lasting prevention and treatmentBiocideFungicidesSolventStructural formula
The invention discloses oligomeric acid iodine as well as a preparation method and application thereof. The molecular structural formula of the provided oligomeric acid iodine is shown in the formula I, wherein m>=2, n>=2, and R1, R2, R3 and R4 are oligomeric glucose (acid) groups, oligomeric N-acetyl glucose (acid) groups, oligomeric D-amino glucose (acid) groups, oligomeric galactose (acid) groups, oligomeric gulose (acid) groups, oligomeric mannose (acid) groups or oligomeric xylose (acid) groups. The preparation method of the oligomeric acid iodine comprises the following steps of: mixingoligomeric acid or oligomeric acid salt with iodine in a mixed solvent of water and alcohol, stirring, controlling the temperature at 50-70 DEG C, and reacting to obtain the oligomeric acid iodine. The oligomeric acid iodine ensures the stability of iodine in the structural matter and has extremely strong effect of contact skilling plant pathogenic bacteria, keeps and strengthens the plant disease resistance induced by oligose, and has rapid and persistent effect for controlling plant diseases. The formula I is disclosed in the specification.
Owner:SHANGHAI GUANFA MARINE BIOTECH CO LTD
A kind of sugar composition with promoting effect of wound healing and application thereof
ActiveCN108553481BRaw materials are easy to getEasy to manufactureOrganic active ingredientsDermatological disorderCarbohydrate compositionOligosaccharide
The invention provides a carbohydrate composition with a wound healing promoting effect and application of the carbohydrate composition. The carbohydrate composition contains at least five types of carbohydrate including carboxymethyl chitosan, rhizoma bletillae polysaccharides, fucoidan from fucus vesiculosus, heparan sulfate, chondroitin sulfate, oligosaccharides of hyaluronan and gulose aldehyde acid oligosaccharides. The carbohydrate composition and the application have the advantages that VEGFA (vascular endothelial growth factor A), FGF2 (fibroblast growth factor 2) and VE-cadherin protein mRNA (messenger ribonucleic acid) expression can be obviously improved by the carbohydrate composition, VEGF (vascular endothelial growth factor) secretion and skin fibroblast (HSF) and human immortalized keratinocyte (HaCaT) proliferation can be obviously promoted by the carbohydrate composition, and the obvious wound healing promoting effect can be realized by the carbohydrate composition; raw materials required by the carbohydrate composition are easily available, composition methods for the carbohydrate composition are simple, the carbohydrate composition is low in cost, easy to industrialize and wide in application range, and the like.
Owner:OCEAN UNIV OF CHINA
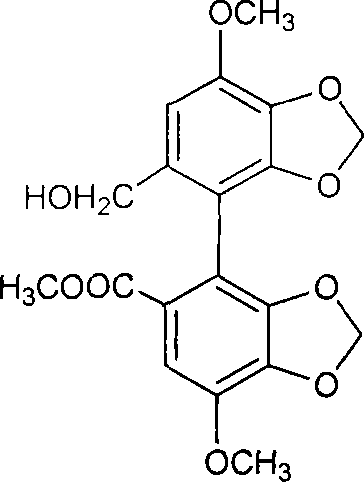
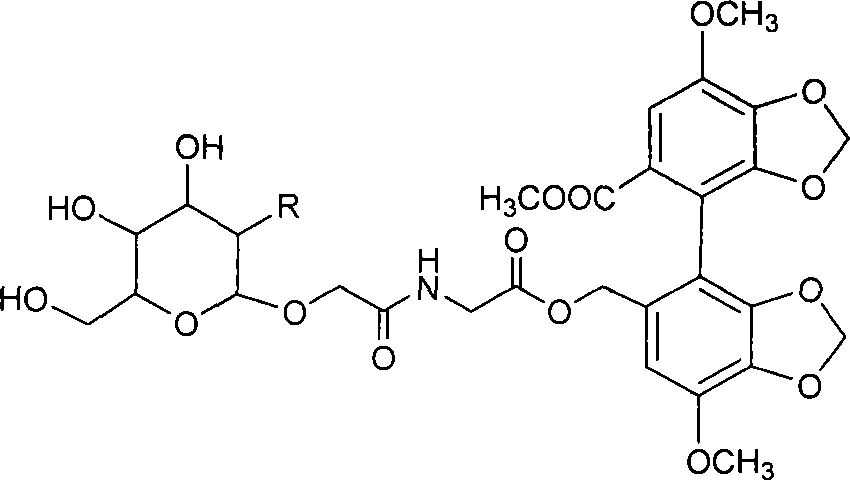
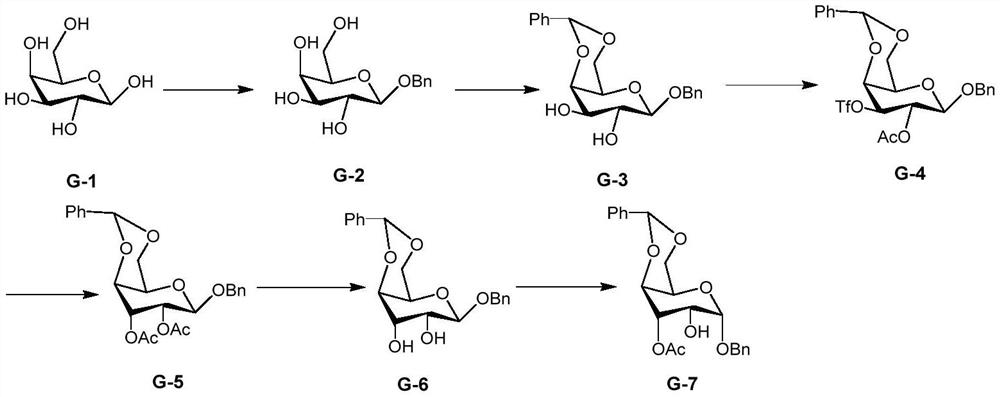
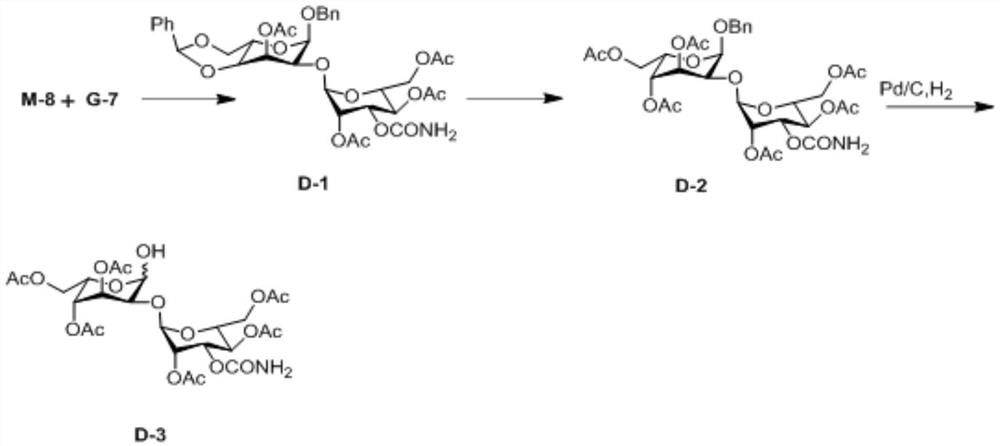
A kind of preparation method of 2-hydroxygulose receptor derivative, bleomycin disaccharide and its precursor
ActiveCN108948106BRaw materials are cheap and easy to getIncrease production capacityEsterified saccharide compoundsSugar derivativesReceptorIndustrial scale
The invention discloses a preparation method of 2-hydroxygulose acceptor derivatives, which comprises protecting benzyl-β-galactoside benzyl fork group, inversion of 3-position configuration, deacetylation and selective acetylation series of reactions. At the same time, the invention also discloses a method for preparing bleomycin disaccharide and its precursor by using the 2-hydroxygulose receptor derivative prepared by the method as the receptor. The preparation method of the 2-hydroxygulose acceptor derivative of the present invention solves the defects of rare natural L-gulose source, high cost, unsuitable for industrialization, etc., and simultaneously solves the problem of bleomycin disaccharide and its precursor yield Low, poor operability and repeatability of the reaction, unsuitable for industrialization and other problems. It has the advantages of cheap and easy-to-obtain raw materials, high yield, strong operability, easy control of conditions, industrial scale-up, high efficiency and low cost.
Owner:GUANGZHOU UNIVERSITY OF CHINESE MEDICINE
Radioactive diagnostic imaging agent
InactiveCN101198359BPrevent radioactive decompositionLow purityIn-vivo radioactive preparationsSugar derivativesImaging agentAlcohol sugars
In a radioactive diagnostic imaging agent comprising a radioactive halogen-labeled compound as an active ingredient, to prevent the radiation degradation of the active ingredient to increase the stability of the radioactive diagnostic imaging agent. A biologically acceptable saccharide or sugar alcohol is added to the radioactive diagnostic imaging agent in an amount effective for the prevention of the radiation degradation. The amount of the saccharide or sugar alcohol to be added is preferably 10 (mmol / L) / GBq / mL or more, more preferably 50 (mmol / L) / GBq / mL or more. The saccharide is preferably selected from the group consisting of erythrose, threose, ribose, arabinose, xylose, lyxose, allose, altrose, glucose, mannose, gulose, idose, galactose, talose, erythrulose, ribulose, xylulose, psicose, fructose, sorbose and tagatose. The sugar alcohol is preferably selected from the group consisting of erythritol, xylitol, sorbitol and mannitol.
Owner:NIHON MEDI PHYSICS CO LTD
Method for synthesizing 1,3,4,6-tetraacetyl-l-gulose
InactiveCN103848874BHigh yieldDoes not involve the use ofEsterified saccharide compoundsSugar derivativesHemiacetalBiochemical engineering
The invention discloses a method for synthesizing 1,3,4,6-tetraacetyl-L-gulose. According to the method, a compound, namely, 1,3,4,6-tetraacetatyl-L-gulose is prepared from glucuronolactone serving as a raw material by performing the steps of acetonide protection, benzyl protection, lactone reduction, hemiacetal hydroxyl protection, acetonide de-protection, lactone reduction, hemiacetal hydroxyl de-protection, acetyl protection and benzyl deprotection. In the method, experience reagents are not used, so that the cost is reduced; involved operation is simple and convenient, conditions are easy to control, and industrial production is easy; the total yield is high, and can be magnified to gram grade and over.
Owner:EAST CHINA NORMAL UNIV
Process for the manufacture of 2-keto-L-gulonic acid
The present invention discloses a process for producing sodium 2-keto-L-gulonate from sorbitol and recovering 2-keto-L-gulonic acid in high recovery yields, by controlled cation exchange treatment of the micro-organism free fermentation broth and / or adjusting PII of said purified fermentation broth, followed by direct crystallisation of 2-keto-L-gulonic acid monohydrate.
Owner:CERESTAR HLDG
Microbial production of vitamin C
The present invention provides a process for the production of vitamin C from different substrates like D-sorbitol, L-sorbose, L-sorbosone or L-gulose using a microorganism selected from the group consisting of Gluconobacter oxydans DSM 4025 (FERM BP-3812), a microorganism belonging to the genus Gluconobacter and having identifying characteristics of G. oxydans DSM 4025 (FERM BP-3812) and mutants thereof.
Owner:DSM IP ASSETS BV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com