Antineoplastic new usage of cardiac glycoside compound in antiar
A technology of cardiac glycosides and compounds, applied in the field of medicine, can solve the problem of no inhibition of tumor cell activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
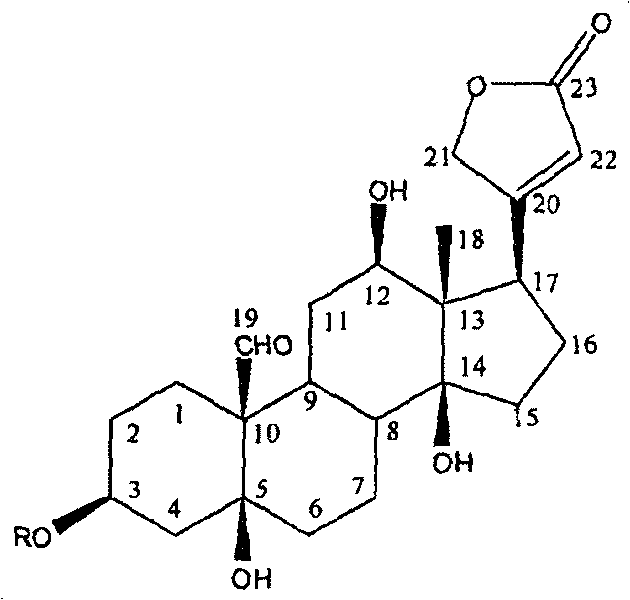
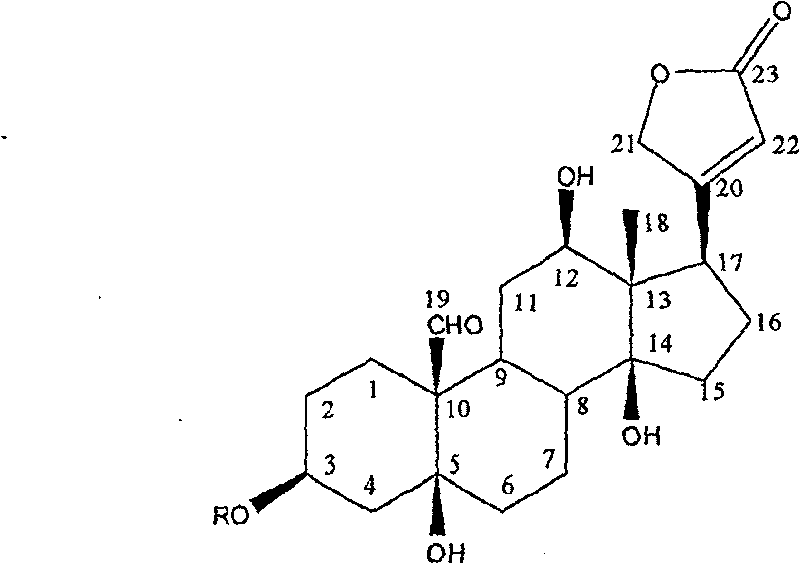
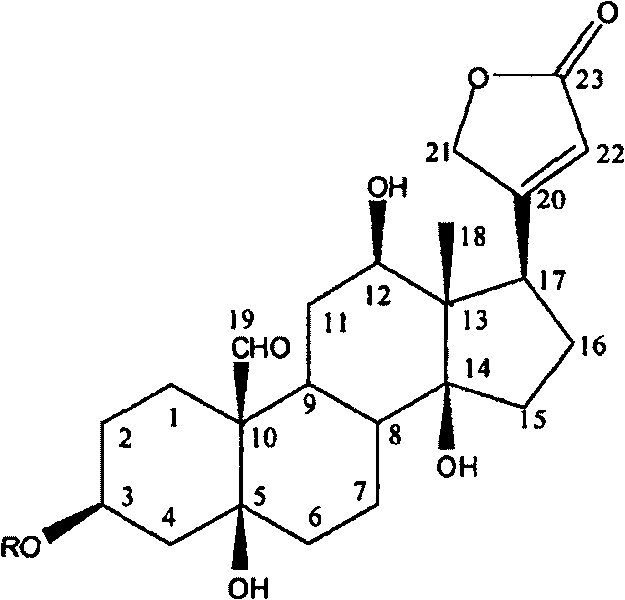
[0013] Example 1: Cardiac glycoside compounds toxicarioside A (toxicarioside A), α-antiaroside (α-antiarin), see the extraction and separation of antiarojavoside:
[0014] See blood seal throat milk (4L) with 95% ethanol at room temperature leaching 3 times, and recover ethanol under reduced pressure until it has no alcohol smell. The ethanol extract was dispersed in water to form a suspension, extracted with petroleum ether to obtain the petroleum ether part (6.87g), and then extracted with ethyl acetate to obtain the ethyl acetate part (8.69g), and the remaining aqueous solution was filtered and placed on the Macroporous adsorption resin (D-101) column chromatography, eluting with water and methanol successively, collecting the methanol eluate, and concentrating to obtain the methanol fraction (228.4g). The obtained ethyl acetate part and the methanol part were divided into several fractions through a decompression column respectively, and then repeated column chromatography...
Embodiment 2
[0015] Example 2: Structural identification of cardiac glycosides toxicarioside A (toxicarioside A), α-antiarojarin, and antiarojavoside:
[0016] Utilizing spectroscopic techniques, including ultraviolet, infrared, nuclear magnetic resonance and high-resolution mass spectrometry, the cardiac glycosides in Example 1 were identified as toxoside, α-toxoside, and toxoside. Structure. For the first time, 2D-NMR technology was used to analyze certain compounds, such as the sugars on sugars such as blood seal throat java glycosides. 13 C-NMR, 1 H-NMR data were assigned. (see Attachment 1, 2)
Embodiment 3
[0017] Example 3: Cardiac glycoside compounds toxicarioside A (toxicarioside A), α-antiarojavoside (α-antiarin), antiarojavoside in vitro tumor inhibition experiment:
[0018] MTT method was used to test the effects of cardiac glycosides Dujianxuefengroside A, α-seexuefengyroside, and Xuefengyaoside on human liver cancer cells SMMC-7721, gastric cancer cells SGC-7901, and breast cancer cells. In vitro tumor inhibitory activity of MCF-7 and chronic myelogenous leukemia cells K562 (see attached table 3 for results). Activity testing method is as follows: experiment is established negative control group (water), DMSO solvent control group, positive control group (mitomycin C) and 8 different concentrations (0.8,1.6,3.2,6.3,12.5,25,50,100 ) of the samples to be tested, two parallels were set for each concentration. Cells in the logarithmic growth phase were collected, counted on a hemocytometer, and inoculated in a 96-well flat-bottomed cell culture plate according to the amount ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com