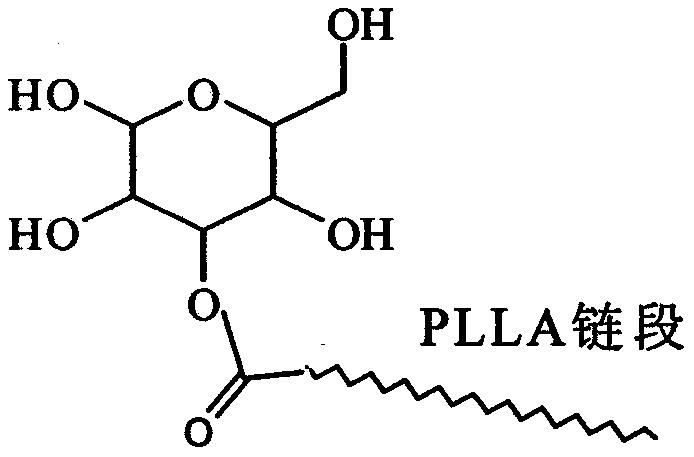
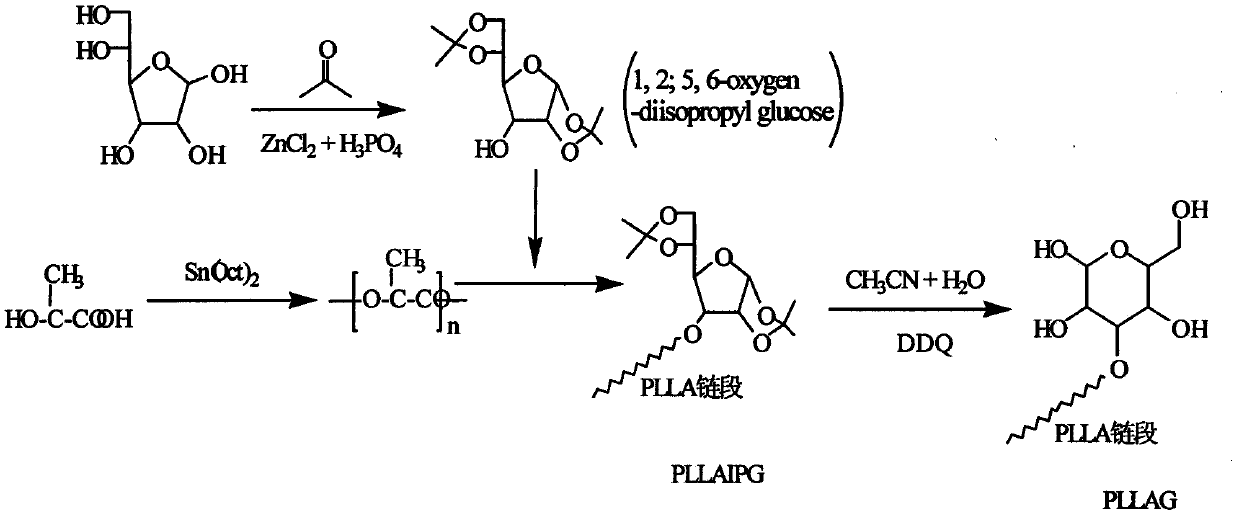
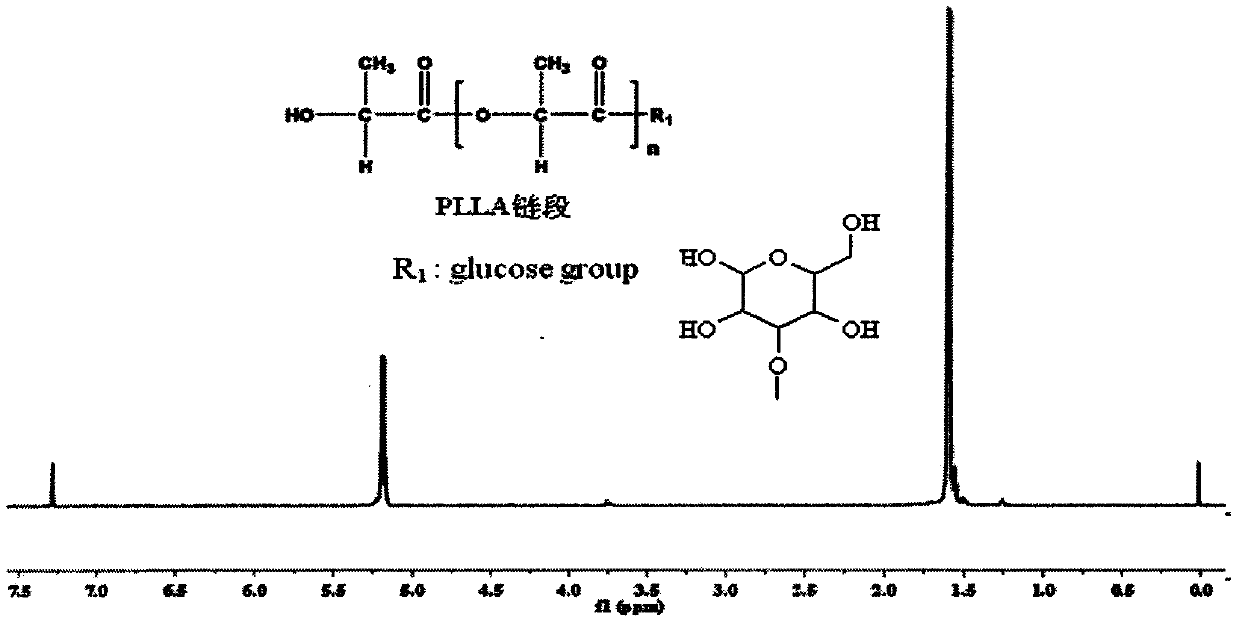
Glucose group-terminated poly(L-lactic acid) (PLLA) diblock copolymer material and preparation method thereof
A diblock copolymer and glucose-based technology, which is applied in the field of poly-L-lactic acid diblock copolymer materials and their preparation, can solve the problems of difficult control of copolymer properties, unstable material properties, and difficult chain structure control.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] use as figure 2 The synthetic route shown prepares a poly-L-lactic acid-isopropylidene glucose diblock copolymer. (1) Preparation of 1,2;5,6-oxo-diisopropylidene glucose (IPG) by hydroxyl protection method. Add 10 g of glucose and 200 ml of acetone into the reactor, stir and add an appropriate amount of zinc chloride and phosphoric acid (the molar ratio of which is 1:0.1) in sequence, and react for 30 h at room temperature and normal pressure. Add 50% NaOH aqueous solution dropwise under rapid stirring to adjust the pH of the reaction system to 8.0, filter with suction, wash with acetone for 2 to 3 times until the filtrate is colorless and transparent, rotary evaporate the filtrate, dissolve the obtained solid in chloroform, wash and extract with water 2 to 3 times, the obtained chloroform solution was rotary evaporated, and the obtained sample was recrystallized 2 to 3 times with ethyl acetate and petroleum ether to obtain white needle-shaped IPG. (2) Preparation of...
Embodiment 2
[0023] The method in Example 1 was used to prepare IPG. The L-lactic acid that takes by weighing 100g is added in the reactor, is heated up to 150 ℃, normal pressure constant temperature reaction 1h, then depressurizes to 200Pa reaction 4h, then adds catalyst stannous octanoate (mass is 0.5wt% of PLLA), heats up to React at 180°C for 10h under reduced pressure to below 10Pa to obtain poly-L-lactic acid, then add IPG to the reaction system, the molar ratio of IPG to PLLA is 8:1, and react for 6h at 10Pa and 200°C. The obtained product was dissolved in chloroform, excess methanol was precipitated, and the precipitate was vacuum-dried to obtain PLLAIPG. The method in Example 1 was used to remove the hydroxyl protecting group of PLLAIPG to obtain PLLAG. use 1 H-NMR spectrum analysis showed that the molecular chain of PLLAG had a double-block structure of PLLA and IPG. The weight-average relative molecular mass of the diblock copolymer is 31600, the melting point is 152°C, the c...
Embodiment 3
[0025] The method in Example 1 was used to prepare IPG. The L-lactic acid that takes by weighing 100g is added in the reactor, is heated up to 150 ℃, normal pressure constant temperature reaction 1h, then depressurizes to 200Pa reaction 4h, then adds catalyst tin protochloride (mass is 0.3wt% of PLLA), heats up to React at 180°C for 6 hours under reduced pressure to below 10Pa to obtain poly-L-lactic acid, then add IPG to the reaction system, the molar ratio of IPG to PLLA is 10:1, and react for 8 hours at 10Pa and 200°C. The obtained product was dissolved in chloroform, excess methanol was precipitated, and the precipitate was vacuum-dried to obtain PLLAIPG. The method in Example 1 was used to remove the hydroxyl protecting group of PLLAIPG to obtain PLLAG. use 1 The molecular chain of diblock copolymer obtained by H-NMR spectrum analysis has PLLA and IPG diblock structure. The weight-average relative molecular weight of the diblock copolymer is 21200, the melting point is...
PUM
| Property | Measurement | Unit |
|---|---|---|
| crystallinity | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| crystallinity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com