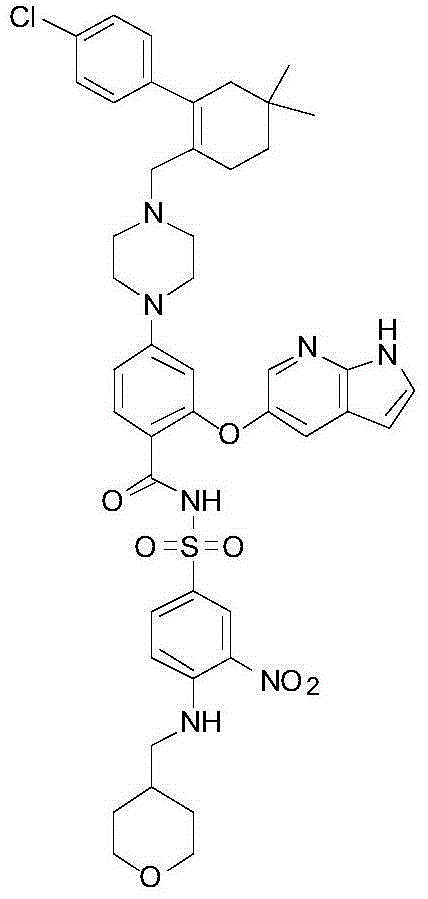
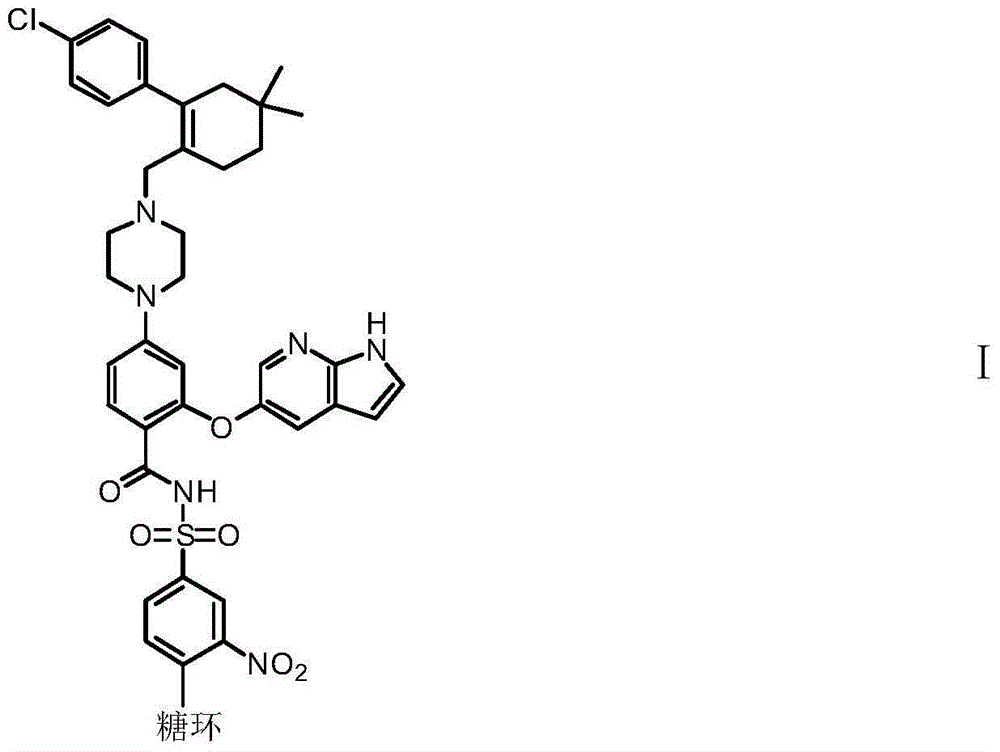
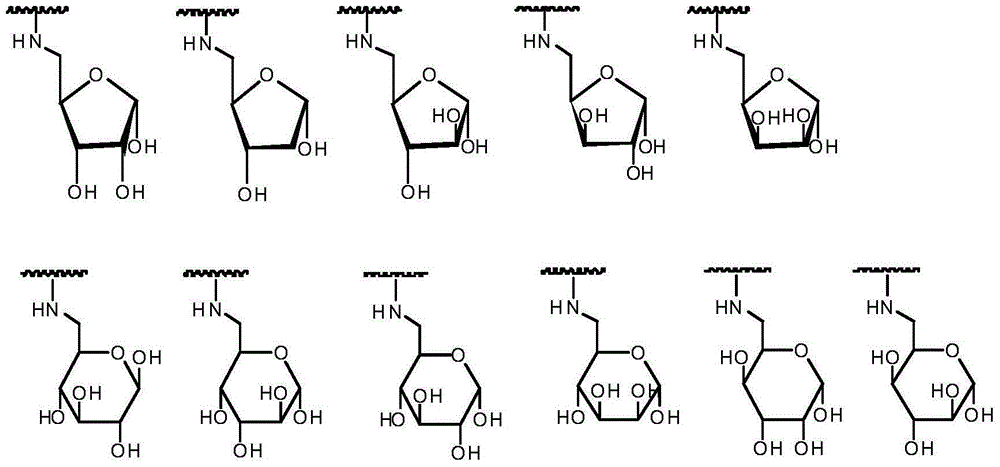
BCL-2 selective inhibitor with sugar ring structure and application thereof
A sugar ring, selected technology, applied in the field of new BCL-2 selective inhibitors, can solve the problems of poor water solubility of Venetoclax and increased occurrence of clinical side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0143] 3-nitro-4-((((2R,3R,4R,5R)-3,4,5-tris((trimethylsilyl)oxy)tetrahydrofuran-2-yl)methyl)amino)benzene Preparation of sulfonamide (intermediate 1)
[0144]
[0145] 4-Chloro-3-nitrobenzenesulfonamide (5.0g, 21.1mmoL), ((2R,3R,4R,5R)-3,4,5-tris((trimethylsilyl)oxy)tetrahydrofuran -2-yl)methylamine (11.2g, 30.5mmoL) and DIPEA (8.8g) were added to anhydrous acetonitrile, reacted overnight in an oil bath at 80°C, and the completion of the reaction was monitored by TLC. After cooling to room temperature, the solvent was distilled off under reduced pressure, and the residue was separated by flash column chromatography. The eluent was n-hexane / ethyl acetate=1 / 1 to obtain 8.0 g of white solid with a yield of 60%. 1 H NMR (DMSO-d6, 400MHz): 1 H NMR (DMSO-d6, 400MHz): δ8.62(t, 1H), 8.27(d, 1H), 7.68(dd, 1H), 7.29(s, 2H), 7.18(d, 1H), 5.56(d ,1H),4.06(m,1H),3.80(m,2H),3.74(m,1H),3.65(m,1H),0.12(m,27H).
Embodiment 2
[0147] 2-((1H-pyrrole[2,3-b]pyridin-5-yl)oxy)-4-(4-((4'-chloro-5,5-dimethyl-3,4,5, 6-tetrahydro-[1,1'-diphenyl]-2-yl)methyl)piperazin-1-yl)-N-((3-nitro-4-((((2R,3S,4R ,5S)-3,4,5-trihydro-2H-pyran-2-yl)methyl)amino)phenyl)sulfonyl)benzamide (compound 1)
[0148]
[0149] Intermediate 1 (2.42 g, 4.28 mmoL), DMAP (1.05 g, 8.59 mmoL), EDCI (1.07 g, 5.58 mmoL) and dichloromethane (30.0 mL) were added to Reaction Flask 1 . Then 4-[4-[[2-(4-chlorophenyl)-4,4-dimethyl-1-cyclohexen-1-yl]methyl]-1-piperazine]-2-( 1H-pyrrolo[2,3-b]pyridin-5-yloxy)benzoic acid (2.5g, 4.4mmoL), triethylamine (0.87g) and dichloromethane (12.0mL) were added to reaction flask 2 . Finally, slowly drop the solution in the reaction bottle 2 into the reaction bottle 1, stir overnight after the addition, and monitor the completion of the reaction by TLC. Add N,N'-dimethylethylenediamine (0.94g), heat the oil bath to 55°C, add 10% acetic acid (18.5mL), and stir overnight. Cool and separate the liquid. The ...
Embodiment 3
[0151] 4-((((2R,3S,5R)-3,5-bis((trimethylsilyl)oxy)tetrahydrofuran-2-yl)methyl)amino)-3-nitrobenzenesulfonamide ( Preparation of Intermediate 2)
[0152]
[0153] Except that instead of ((2R,3R,4R,5R)-3 , Except for 4,5-tris((trimethylsilyl)oxy)tetrahydrofuran-2-yl)methanamine, the preparation of intermediate 2 was the same as that of intermediate 1, and the yield was 78%. 1 H NMR (DMSO-d6, 400MHz): δ8.78(t, 1H), 8.32(d, 1H), 7.68(dd, 1H), 7.20(s, 2H), 7.32(d, 1H), 5.48(d ,1H),4.06(m,1H),3.80(m,2H),3.57(m,1H),2.27(m,2H),0.15(s,18H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com