Preparation method of paricalcitol
A technology of paricalcitol and its compounds, which is applied in the field of preparation of paricalcitol, can solve the problems of poor selectivity, low yield of Julia coupling olefination, and non-environmental protection, and achieve convenient operation and reagent Simple, short route effect
Inactive Publication Date: 2010-11-10
CHONGQING TAIHAO PHARM CO LTD
View PDF3 Cites 8 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
The second route is longer (WO2008053961, JP053392300), with more than seventeen steps
Among them, the Julia coupling olefination yield used when connecting the side chain is low, and the use of Na-Hg is not environmentally friendly; the several-step reaction used when converting the extracyclic double bond is not very good, such as dihydroxy The oxidation reaction may oxidize the double bond of the side chain, etc.
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment Construction
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
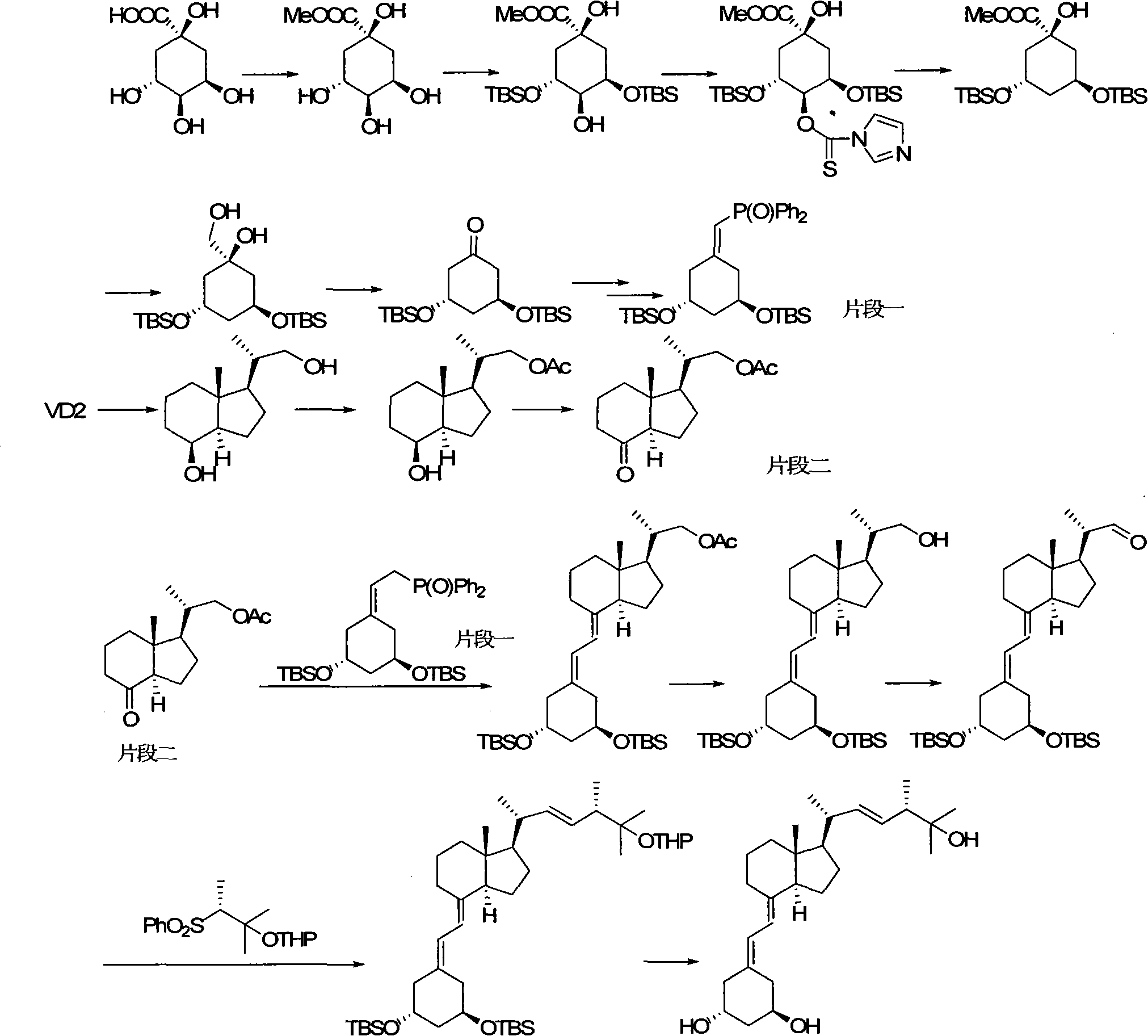
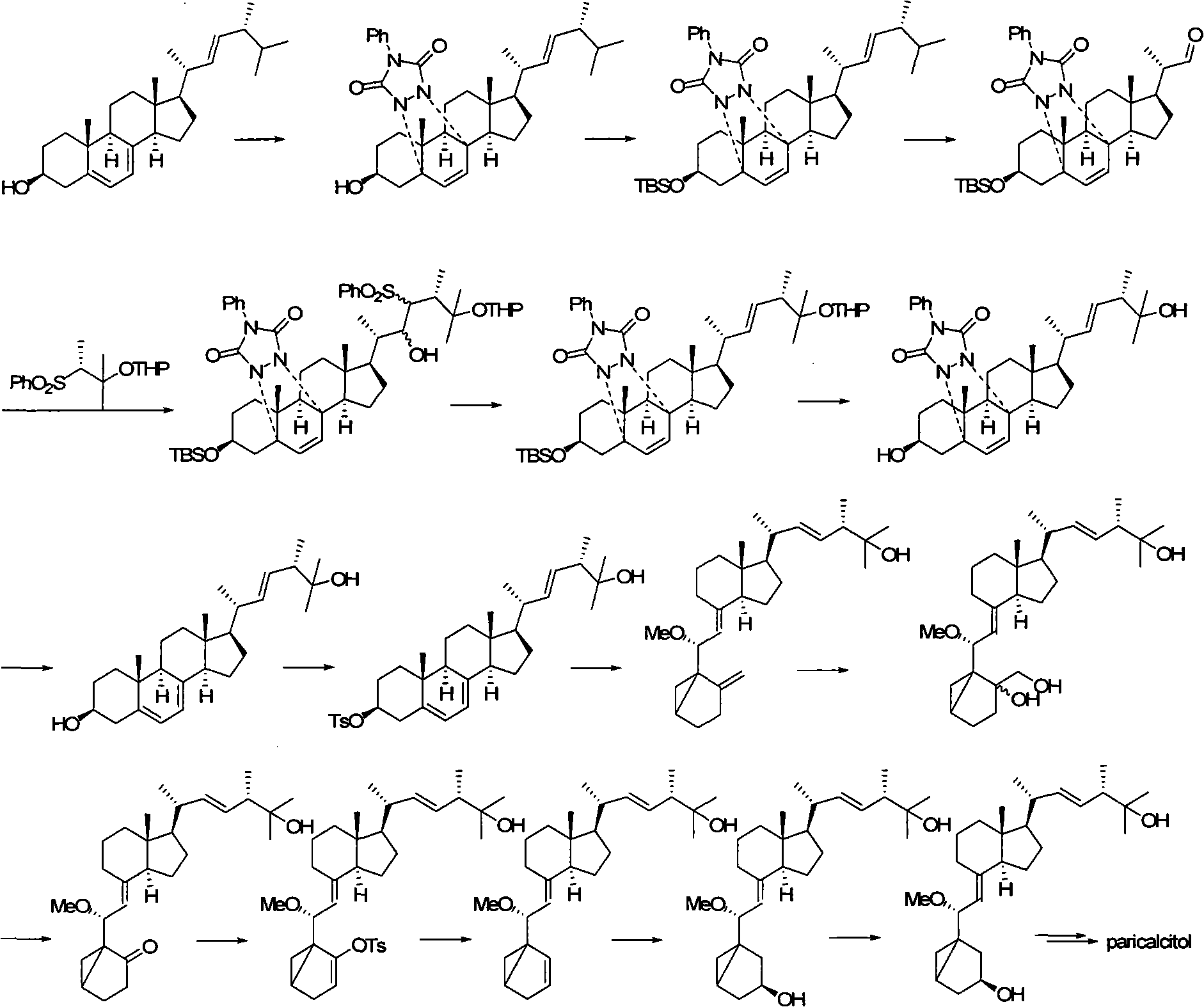
The invention relates to a preparation method of paricalcitol, which is characterized in that after hydroxyl in the vitamin D2 is protected by p-toluenesulfonates, in the presence of alkali, intramolecular cyclization reaction happens in methanol to generate a compound 5; the compound 5 undergoes allylic oxidation and hydroxyl is protected to obtain a key intermediate 7; in the presence of ozone,the side chains and exocyclic terminal double bonds of the key intermediate 7 are cut off to obtain a compound 8; the primary hydroxyl in the compound 8 is selectively protected, a three-membered ring is opened in the presence of acid and then hydroxyl is protected to obtain a key intermediate 11; after the secondary hydroxyl in the key intermediate 11 is protected by sulphonate, a compound 12 isobtained through reduction by LiAlH4; a compound 13 is obtained after the compound 12 is subjected to Swern oxidation and carries out Wittig reaction with the compound 12 to obtain a compound 14; andthe target compound can be obtained by removing the protective group in the compound 14. The reagents used in the method are simple and are convenient to operate, the reactions concerning regioselectivity and stereoselectivity are few, the route is shorter and 12 steps of reactions are carried out.
Description
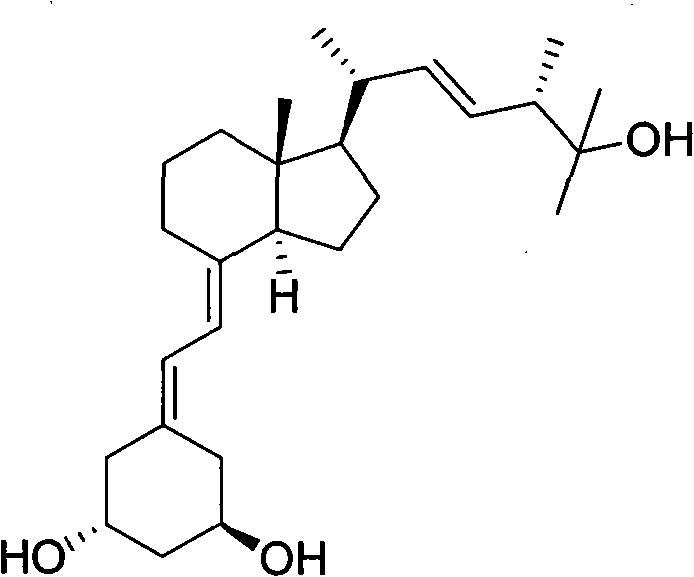
The preparation method of paricalcitol technical field The present invention relates to the synthesis of a compound, in particular to a preparation method of paricalcitol. Background technique Paricalcitol (active vitamin D treatment drug, Paricalcitol) is a drug for the prevention and treatment of secondary hyperparathyroidism (SHPT), which is effective for stage III and IV chronic kidney disease before dialysis and transplantation. SHPT in patients with CKD has shown preventive and therapeutic effects, and has become the most widely used drug for the prevention and treatment of SHPT in patients with dialysis. Serum phosphorus levels had minimal impact, and PTH reduction was a key indicator of efficacy of SHPT treatment. The binding of paricalcitol See formula 1 for construction: 1 Paricalcitol formula 1 Synthetic Paricalcitol mainly contains the following two methods in the prior art: The first route (US5281731, US5086191) adopts the strategy of converging to ca...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): C07C401/00
CPCY02P20/55
Inventor 李瀛薛吉军张宪恕徐少军
Owner CHONGQING TAIHAO PHARM CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com