Preparation of drug particles using evaporation precipitation into aqueous solutions
a technology of evaporative precipitation and drug particles, which is applied in the field of drug particles, can solve the problems of poor bioavailability, difficult to produce highly uniform submicron particles, and wet milling techniques exhibit problems, and achieve high drug activity, high dissolution rate, and high potency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
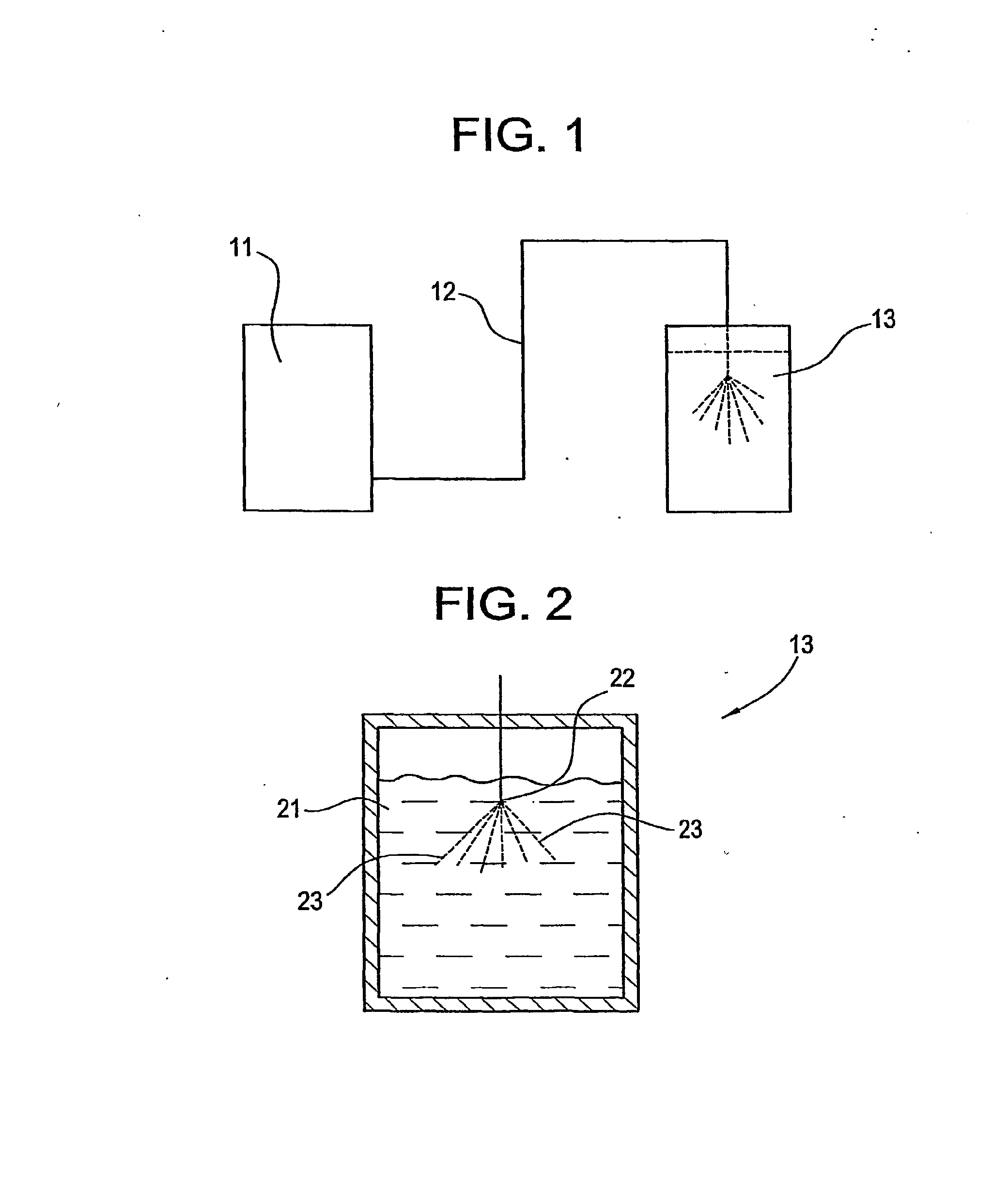
[0067]For the following examples, the apparatus shown in FIG. 1 is used. The drug / organic mixture was fed via a Constametric 3200 HPLC pump through a preheating coil into a 30 mL receiving tank containing the required amount of aqueous solution. The nozzle used for the spraying was made by cutting 1 / 16″ stainless steel tubing to form an elliptical conical geometry at the end. The end of the tube was filed to obtain the desired flow rate. Nitrogen was continuously flowed downward to break up foam in cases where it formed. For all of the examples, particle size was measured by dynamic light scattering techniques within 4 hours of the spray.
[0068]Dissolution testing for the following examples was carried out using a Vankel dissolution apparatus following the USP Apparatus II paddle method. During all dissolution tests, to ensure sink conditions, only 10-30 percent of the saturation solubility of the drug was added to the dissolution apparatus. The appropriate amount of final drug prepa...
examples 1-8
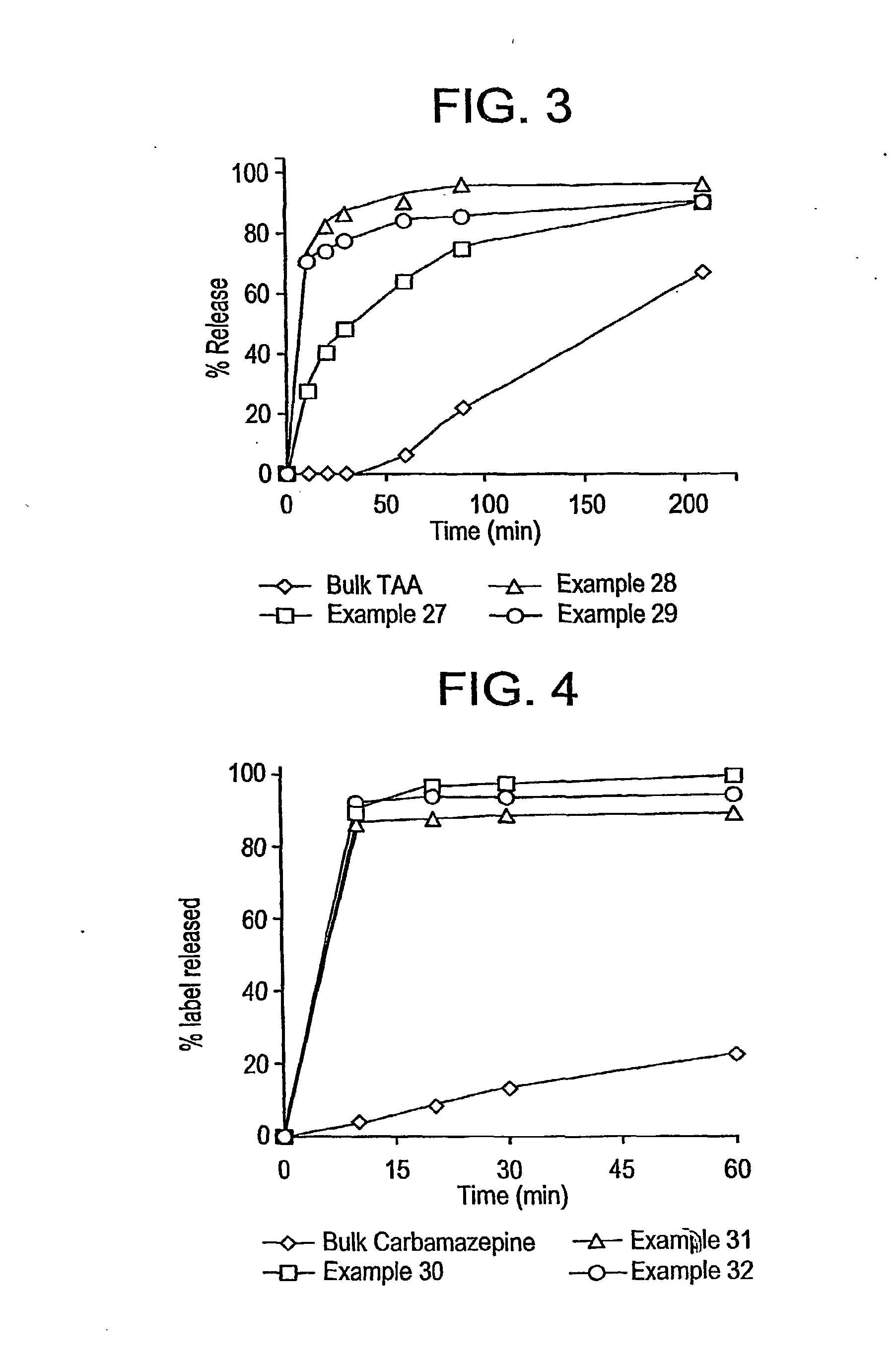
[0069]The drug was cyclosporine, the organic was diethylether, and the concentration of the drug / organic mixture was 5.0 weight percent cyclosporine in diethylether. For the aqueous solution, Tween-80, a polyoxyethylene sorbitan monolaurate (ACROS) was a surfactant, or other excipient, which was used as the particle stabilizer. The drug / organic mixture was sprayed into 10 mL of aqueous solution at a rate of 1 ml / min. Table A lists processing parameters and the resulting particle sizes.
TABLE ADrug / organicAqueousSprayTween-80Medianpercenttemp.temptimeconc (wtparticleparticles Ex(° C.)(° C.)(min)percent)size (nm)median size165551014701126555101608643656510111146146565101759415706510170633670651017964377065101932508706520532214
examples 9-13
[0070]The drug was cyclosporine, the organic was diethylether, and the concentration of the drug / organic mixture was as shown below in Table B. For the aqueous solution, phosphatidyl choline (10 wt percent), a Sigma egg lecithin, 60 percent pure, was a surfactant, or other excipient, used as a particle stabilizer. The drug / organic mixture was sprayed into 10 mL aqueous solution at a rate of 1 ml / min. The temperature of the aqueous solution is 75° C., while the drug / organic mixture was sprayed into the aqueous solution at a temperature of 75° C. Table B lists some processing parameters and the resulting particle sizes.
TABLE BDrug concDrug conc in Spray in aqueousParticle sizeorganic solntime(mg / mlDrug / surfactantrangeEx(wt percent)(min)water)ratio(nm)912014.50.13135-3901021930.80.28120-5751151037.10.33 90-3001251034.90.32215-5901310629.70.30170
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com