Controlled release preparation containing cilostazoland process for the preparation thereof
A controlled-release preparation, the technology of cilostazol, which is applied in the field of controlled-release preparations of cilostazol, can solve the problems of large daily dose, inconvenience for patients to take comfortably, and complicated preparation methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0017] Preparation of drug granules
[0018] In step (1) according to the present invention, cilostazol or a pharmaceutically acceptable salt thereof is mixed with a solubilizer, and the resulting mixture is subjected to a solid dispersion method to obtain particles in which the drug is uniformly dispersed.
[0019] The solid dispersion method can be any conventional melting or solvent method. Where the melting method is used, solubilized drug particles can be prepared by mixing cilostazol with a solubilizing agent, heating the mixture until either the cilostazol or the solubilizing agent does not melt to result in cilostazol The temperature of the molecular level mixing of azole and solubilizing agent, slow cooling of the mixture to form solid clusters, and processing of the solid clusters to obtain particles of the desired size. When the solvent method is used, the surface of the cilostazol-containing particles is modified by a solubilizing agent, which can be prepared by ...
Embodiment 1 to 9
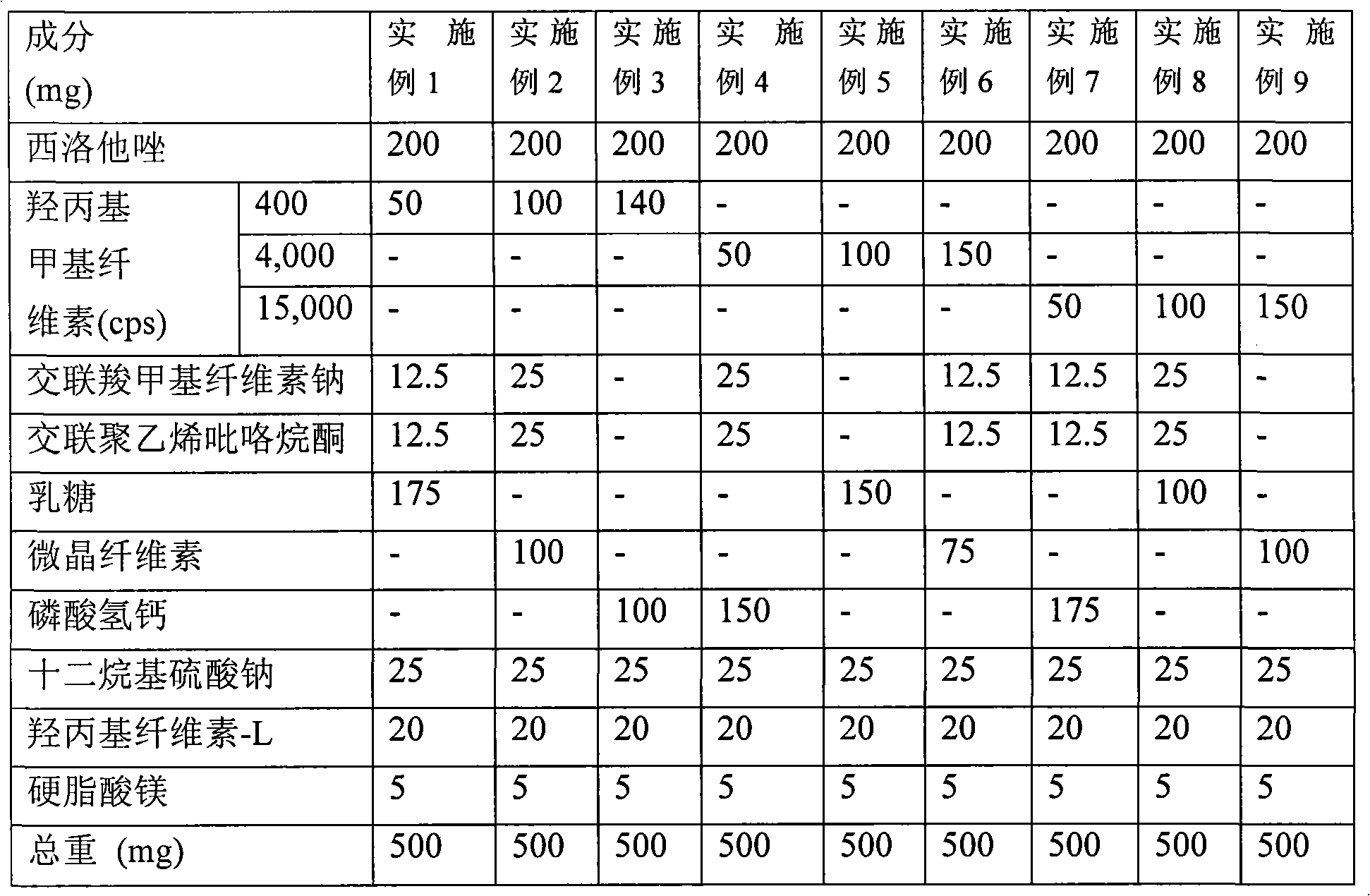
[0055] Examples 1 to 9: Preparation of Tablets of Cilostazol-(1)
[0056] Put cilostazol in a high-speed planetary swing mixer, and slowly add sodium lauryl sulfate and hydroxypropylcellulose-L dissolved in ethanol to cilostazol undergoing high-speed rotation to obtain granules . Subsequently, the granules thus obtained are mixed with hydroxypropylmethylcellulose, croscarmellose sodium or crospovidone, lactose, microcrystalline cellulose and calcium hydrogen phosphate, and further added to the mixture Add hydroxypropylcellulose-L dissolved in ethanol. The resulting mixture was washed and passed through a 14-mesh sieve to obtain granules, which were dried, further filtered through a 18-mesh sieve, and a magnesium stearate lubricant was added thereto. Then, the resulting mixture is compressed to obtain tablets. The amount of each component used is shown in Table 1.
[0057] Table 1
[0058]
Embodiment 10
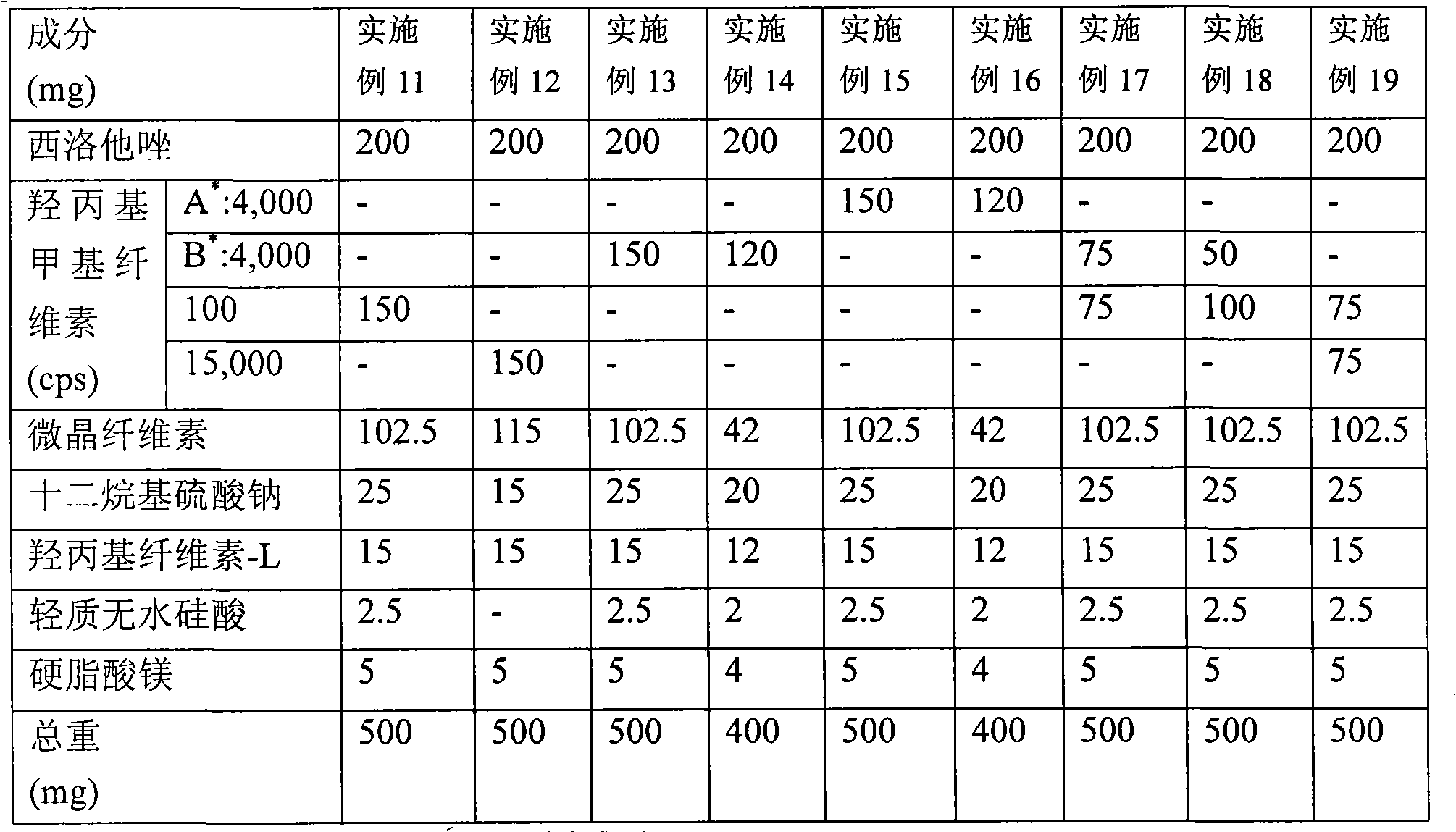
[0066] Example 10: Preparation of tablets containing cilostazol-(3)
[0067] Cilostazol was mixed with sodium lauryl sulfate dissolved in ethanol, and the mixture was dried to form a solid mass. The material was milled and passed through a 20 mesh screen to obtain granules. Subsequently, the particles were mixed with microcrystalline cellulose and propylene glycol alginate, and hydroxypropylcellulose-L dissolved in ethanol was further added to the mixture. The resulting mixture was washed and passed through a 14-mesh sieve to obtain granules, which were dried, further filtered through a 18-mesh sieve, and a magnesium stearate lubricant was added thereto. Then, the resulting mixture is compressed to obtain tablets. The amount of each component used is shown in Table 3.
[0068] table 3
[0069] Composition (mg)
PUM
| Property | Measurement | Unit |
|---|---|---|
| water solubility | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com