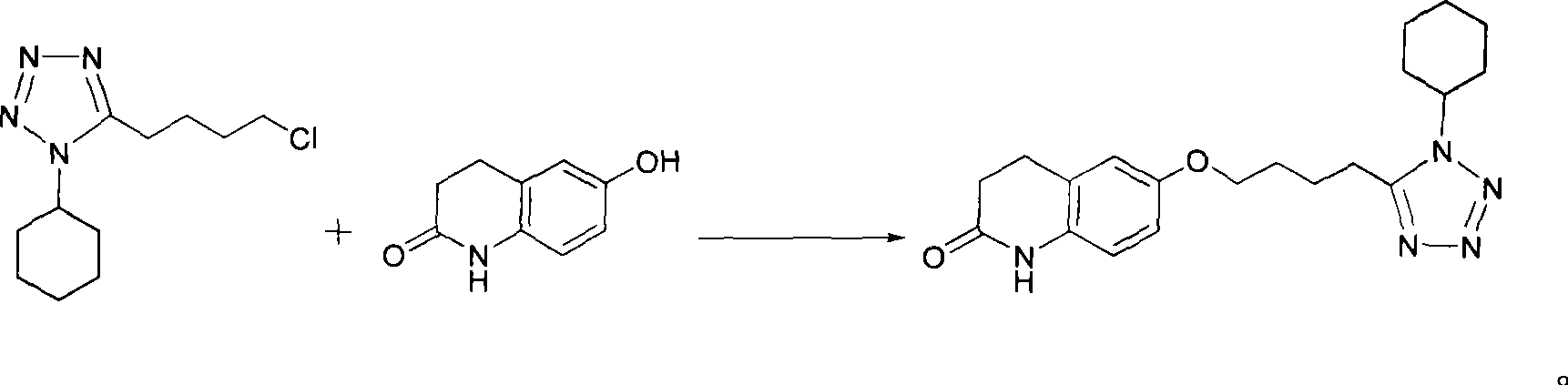
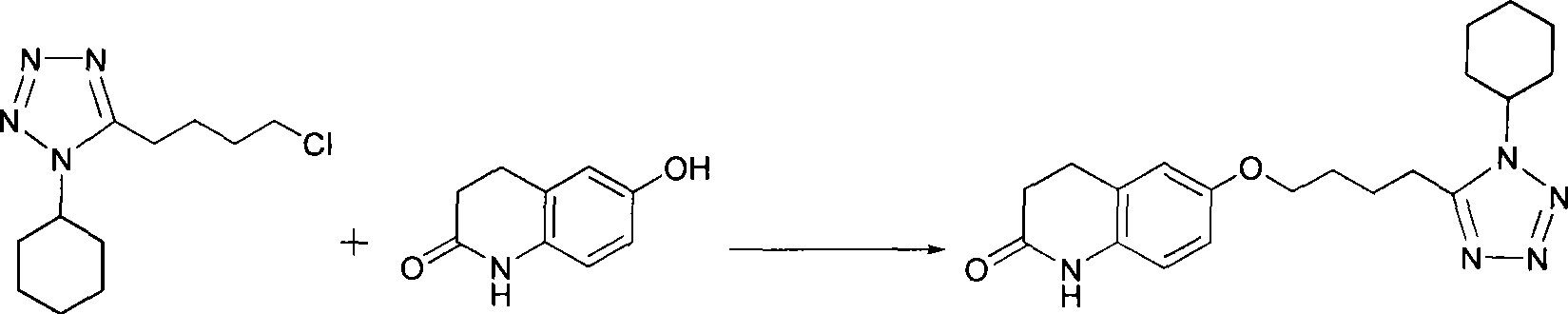
Preparation of cilostazol
A technology for cilostazol and tetrazolium, which is applied in the field of preparation of raw materials, can solve the problems of increased production cost, large environmental pollution, loss of total yield and the like, and achieves low production cost, little environmental pollution, and sufficient reaction. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Embodiment 1, the preparation of cilostazol
[0019] According to the theoretical production amount of cilostazol 161.33g, calculate the amount of raw materials; in a 2000mL three-neck round bottom flask, add 106.0g (0.44mol ), 6-hydroxyl-3,4-dihydroquinolone 71.8g (0.44mol), potassium carbonate 33.2g (0.24mol) and potassium hydroxide 13.5g (0.24mol), then add methanol 800mL, and reflux reaction at a temperature of 80°C For 24 hours, monitor the progress of the reaction with thin-layer chromatography (TLC). After the reaction is complete, distill the reaction solution under reduced pressure to remove about 200 mL of methanol, then cool to a temperature of 0°C, and white crystals are precipitated. Filter the filter cake with methanol After washing, it was vacuum-dried at a temperature of 55° C. to obtain 132.30 g of the product cilostazol with a purity of 99.3% and a yield of 82.0%.
Embodiment 2
[0020] The preparation of embodiment 2, cilostazol
[0021] According to the theoretical production amount of cilostazol 161.33g, calculate the amount of raw materials; in a 2000mL three-neck round bottom flask, add 106.0g (0.44mol ), 6-hydroxy-3,4-dihydroquinolone 79.0g (0.484mol), sodium carbonate 35.0g (0.33mol) and sodium hydroxide 9.6g (0.24mol), then add isopropanol 800mL, at a temperature of 82 ℃ Reflux reaction for 24 hours, monitor the progress of the reaction with TLC method, after the reaction is complete, distill the reaction solution under reduced pressure to remove about 200mL of isopropanol, cool to a temperature of 2°C, white crystals are precipitated, filter with suction, filter the cake with isopropanol After washing with alcohol, it was dried in vacuum at 55° C. to obtain 134.00 g of the product cilostazol with a purity of 99.3% and a yield of 83.1%.
Embodiment 3
[0022] Embodiment 3, the preparation of cilostazol
[0023] According to the theoretical production amount of cilostazol 161.33g, calculate the amount of raw materials; in a 2000mL three-neck round bottom flask, add 106.0g (0.44mol ), 6-hydroxy-3,4-dihydroquinolone 86.5g (0.53mol), potassium carbonate 45.0g (0.33mol) and potassium hydroxide 13.5g (0.24mol), then add isopropanol 800mL, at a temperature of 84 ℃ Reflux reaction for 24 hours, monitor the progress of the reaction with TLC method, after the reaction is complete, distill the reaction solution under reduced pressure to remove about 200mL of isopropanol, then cool to 0°C, white crystals are precipitated, filter with suction, filter the cake with isopropanol After washing with alcohol, the product was vacuum-dried at 55° C. to obtain 137.13 g of the product cilostazol with a purity of 99.8% and a yield of 85.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com