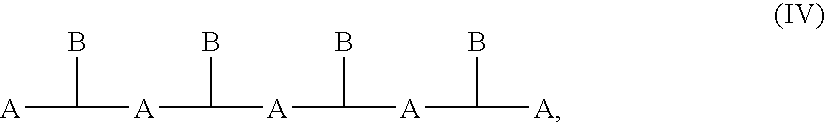
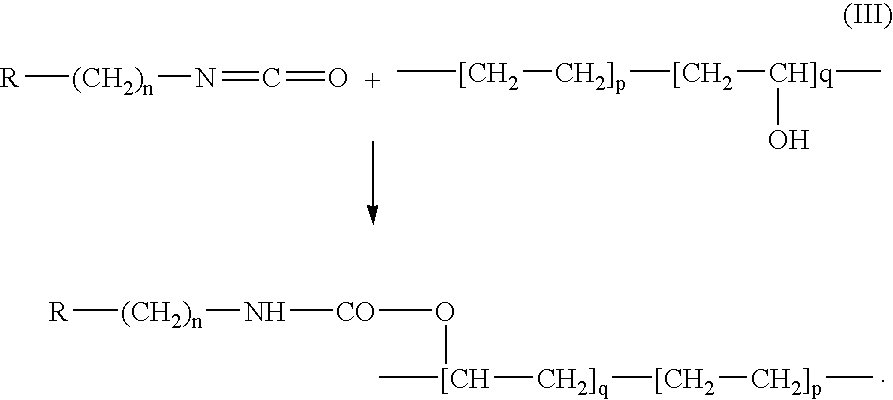
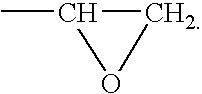
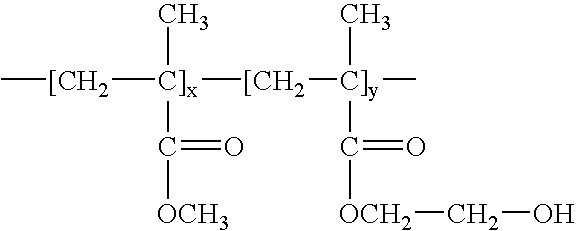Patents
Literature
245 results about "Drug-eluting stent" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
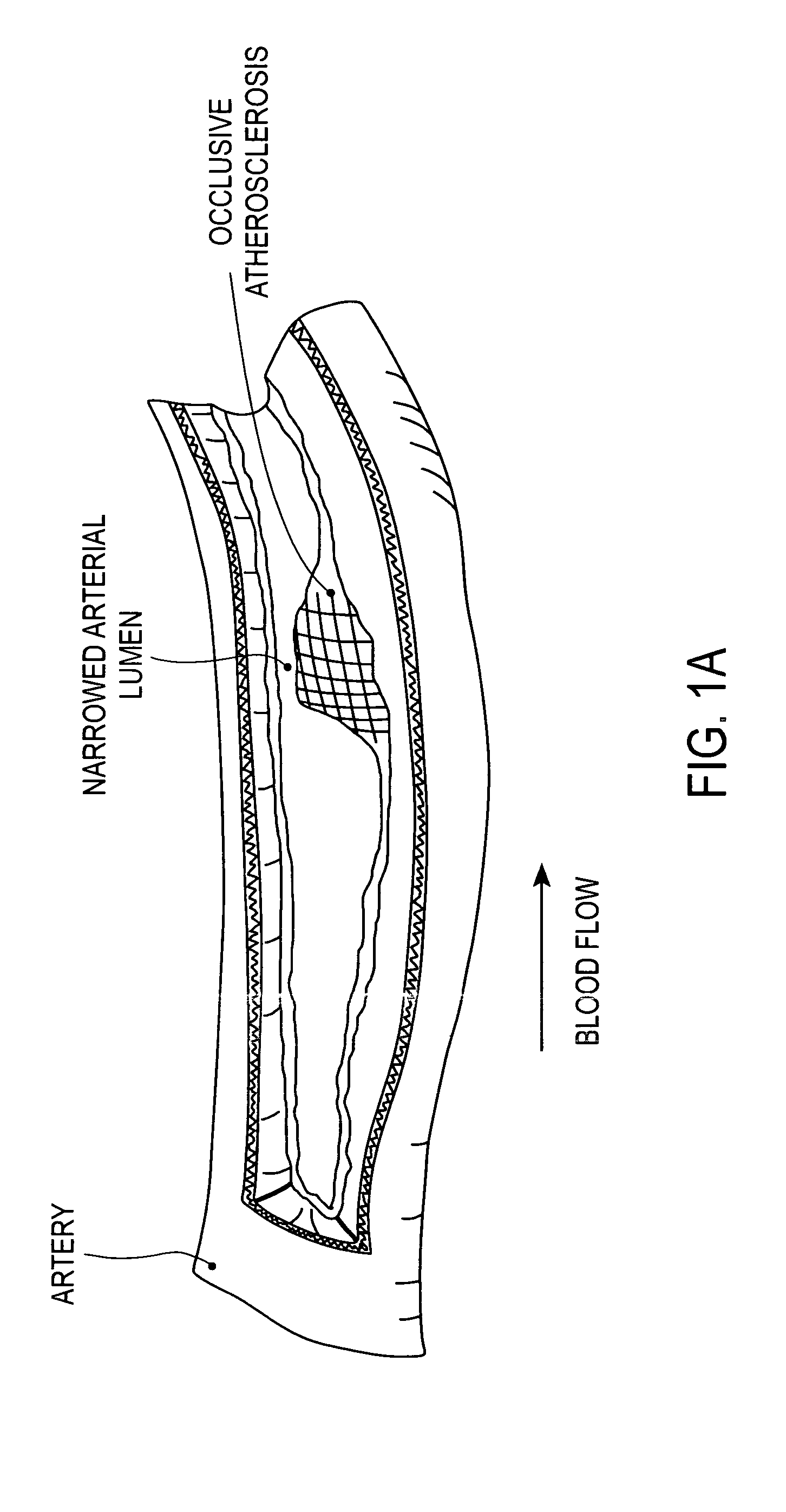
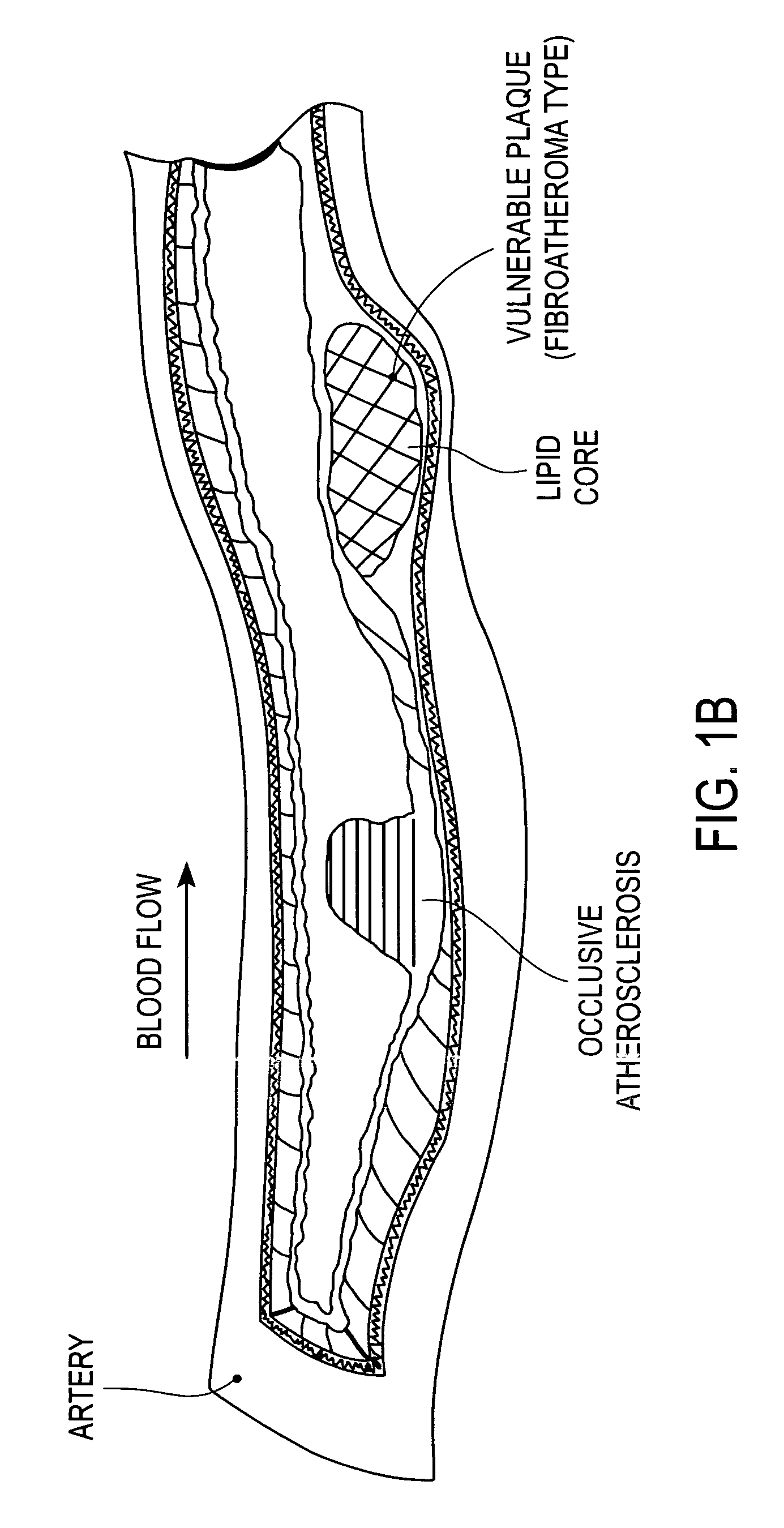
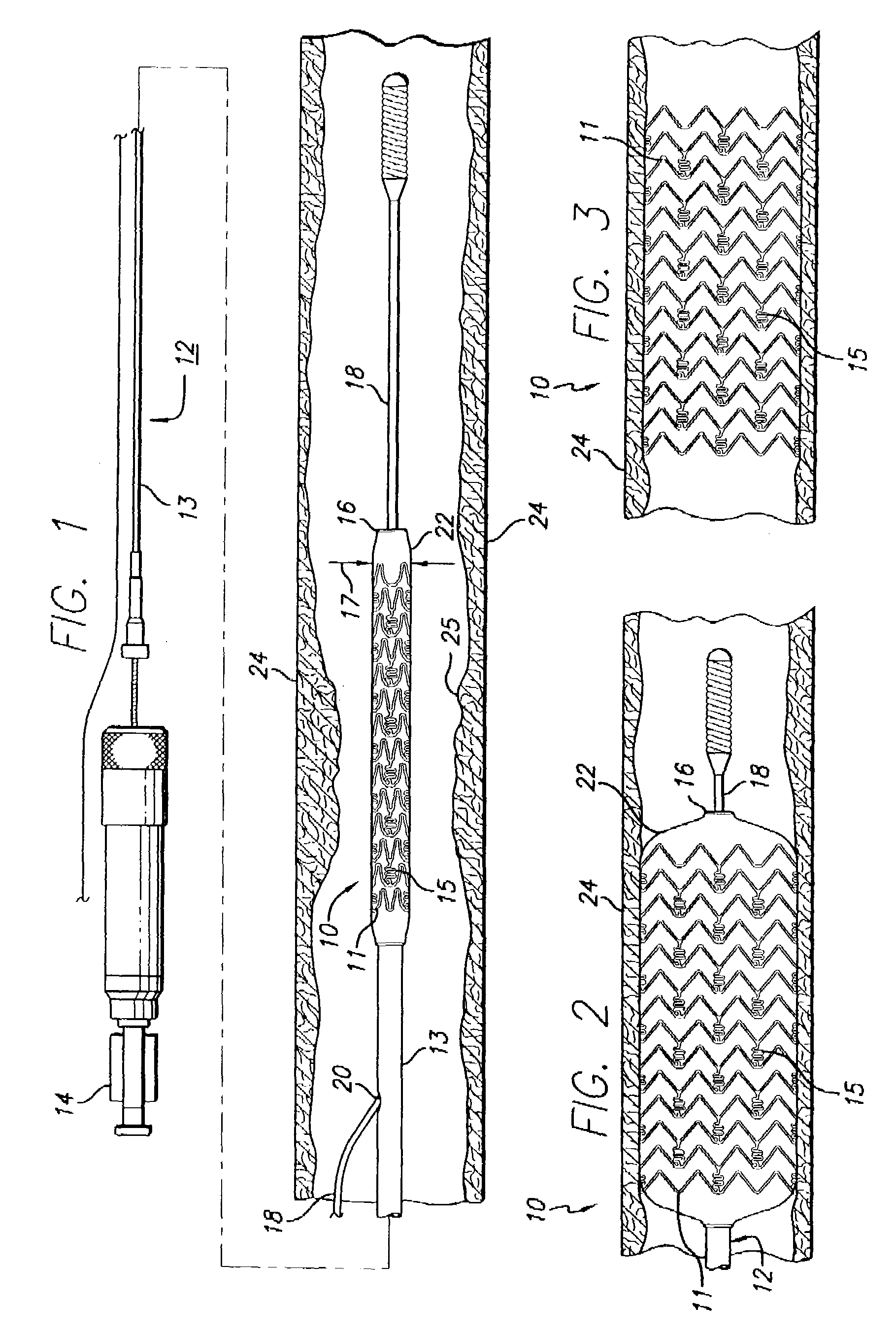
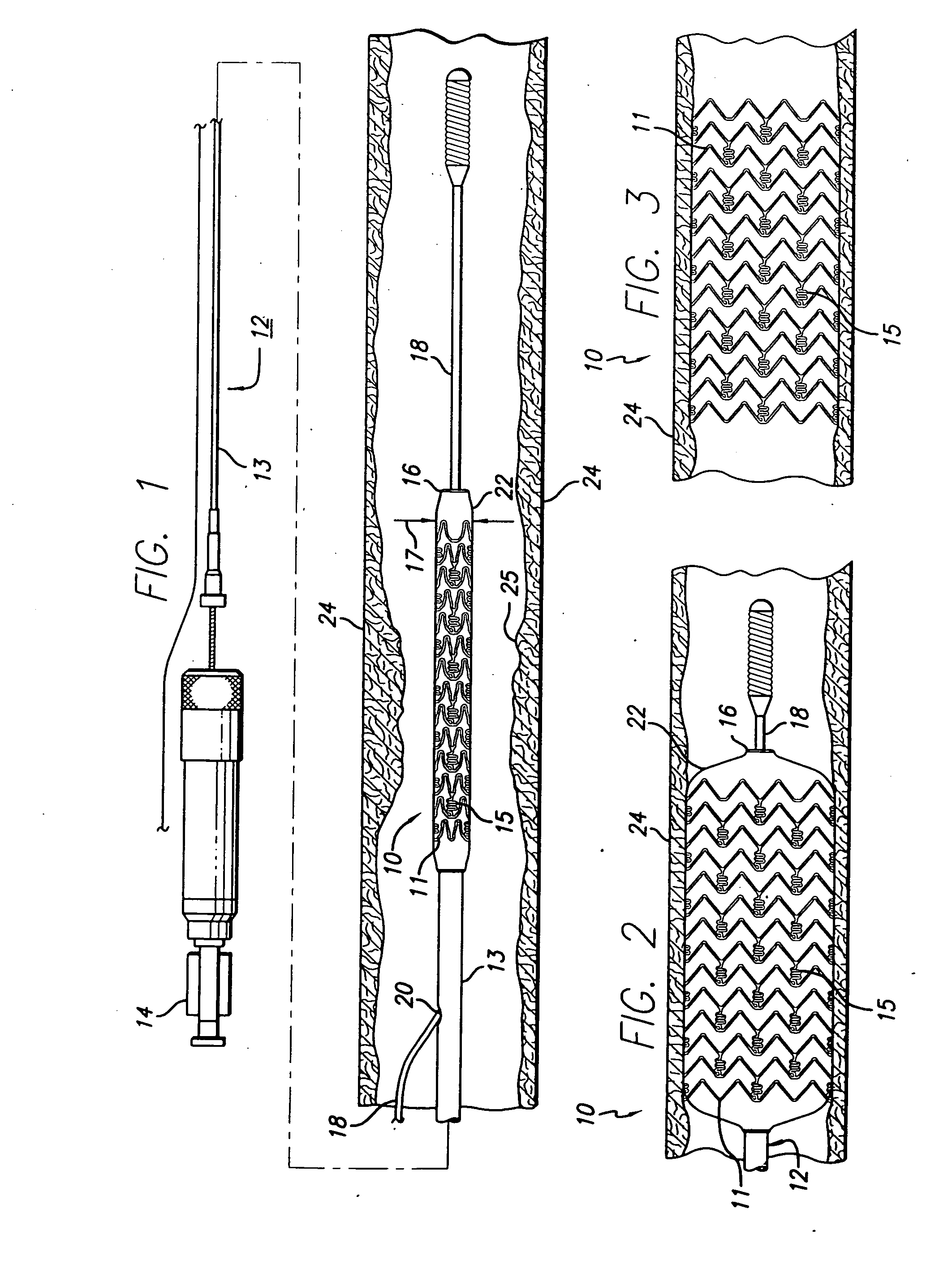
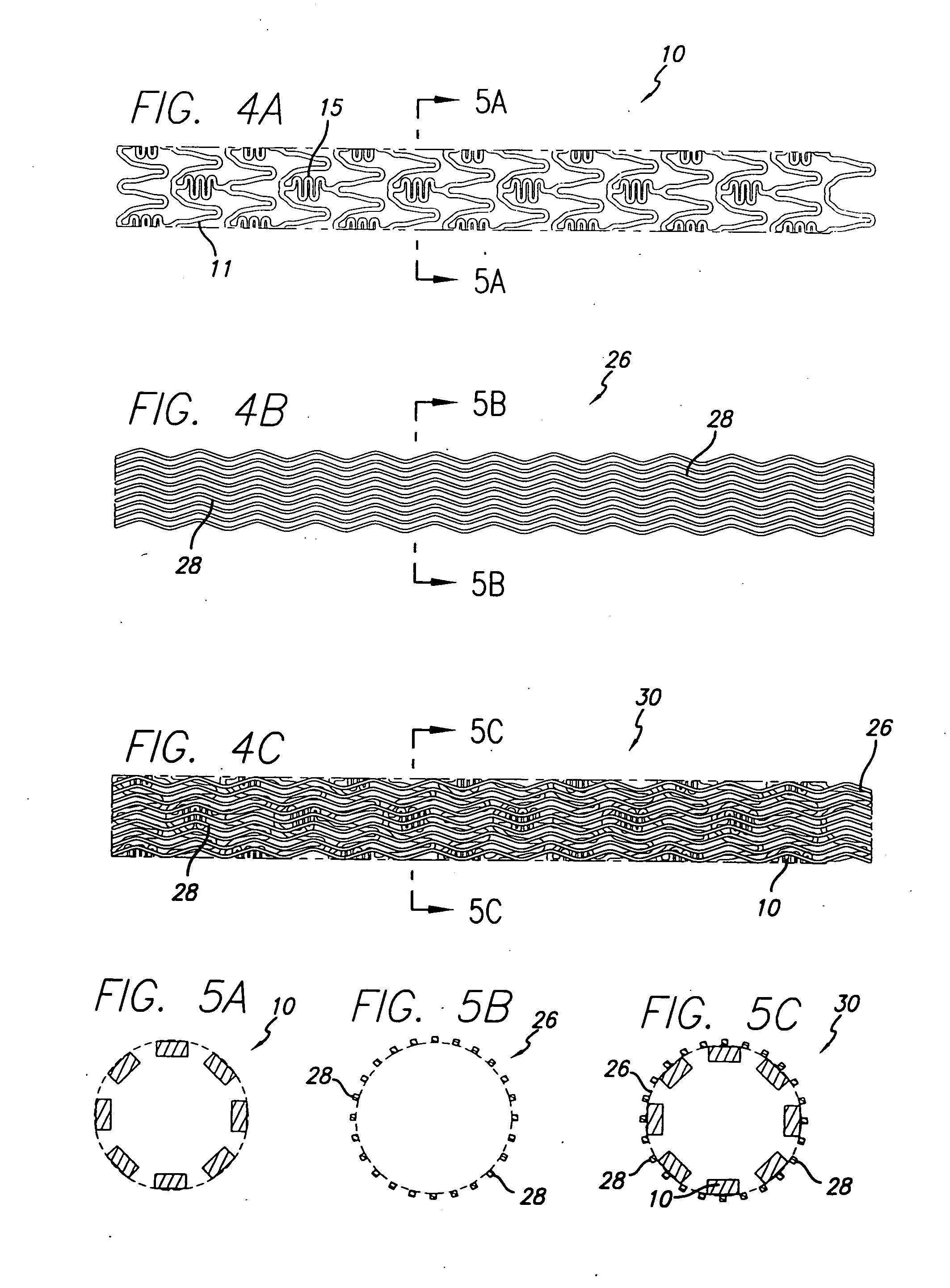
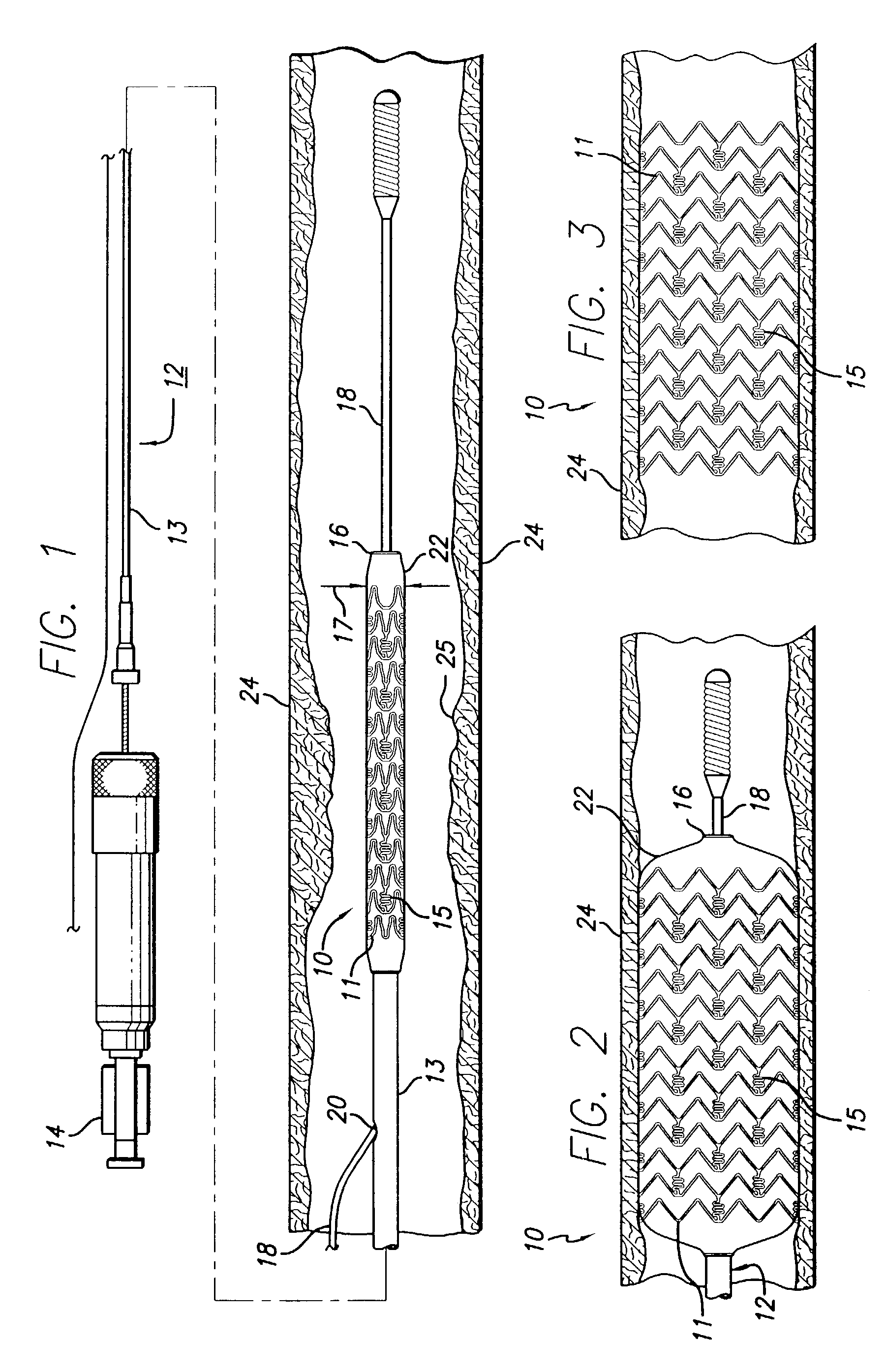
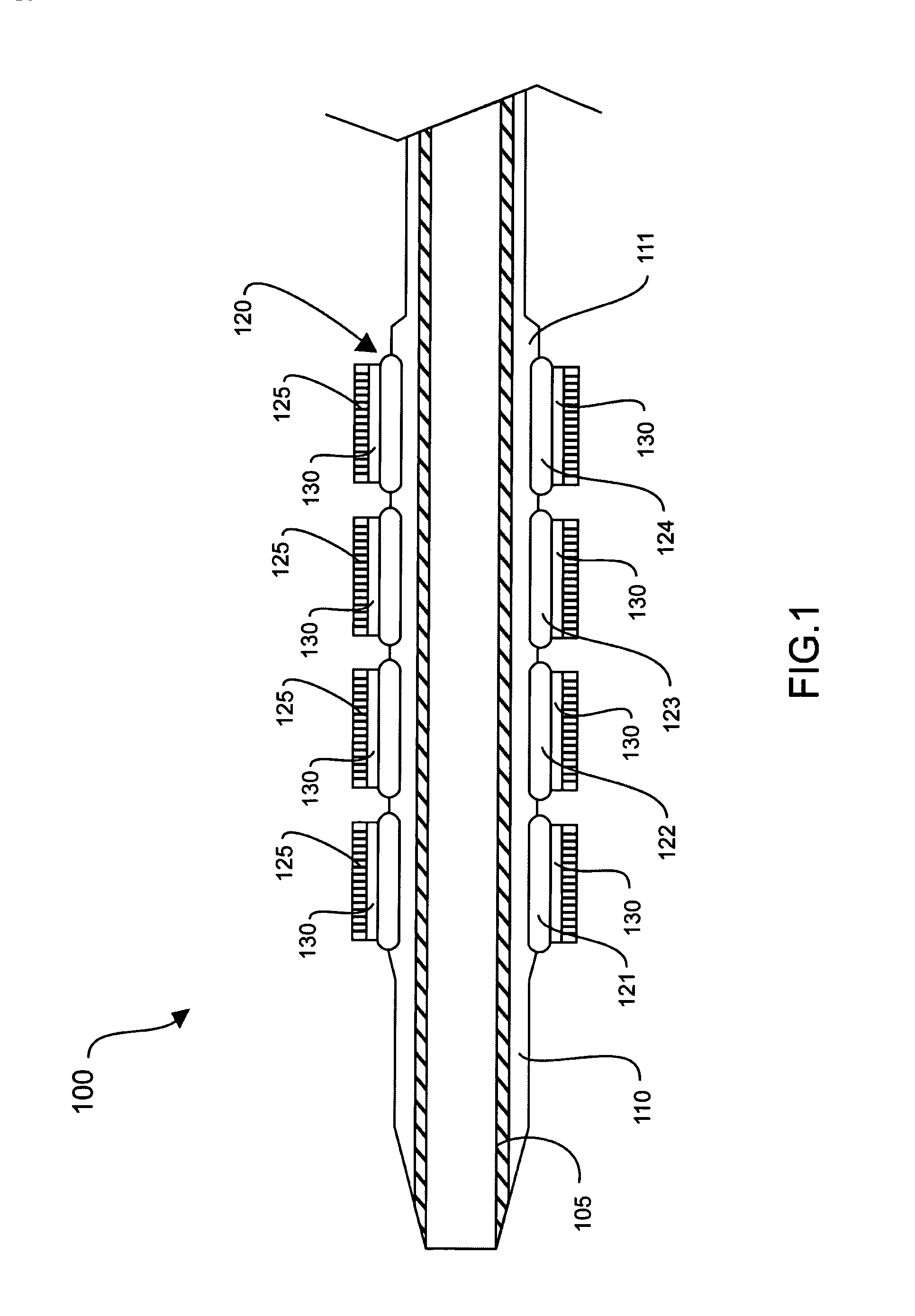
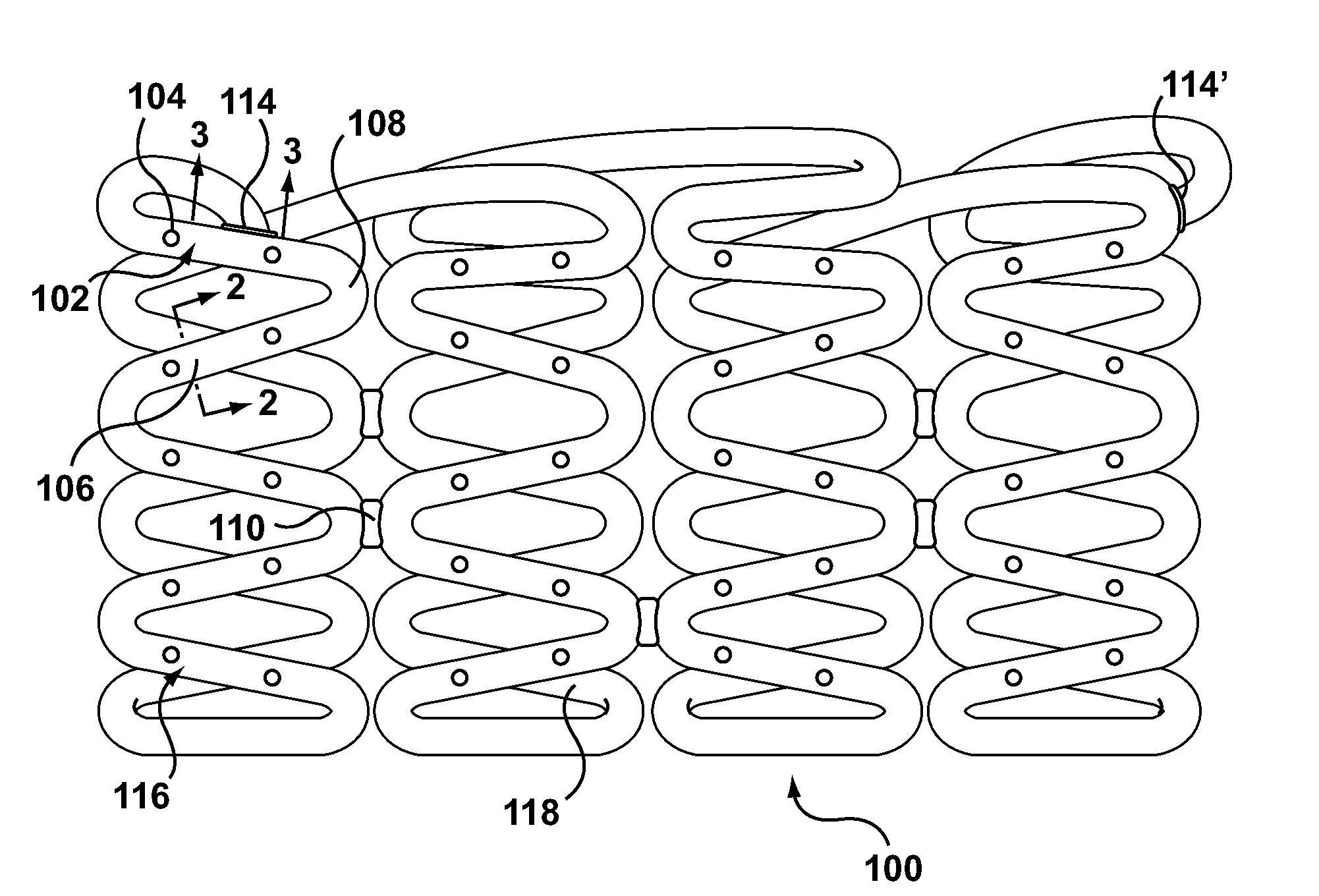
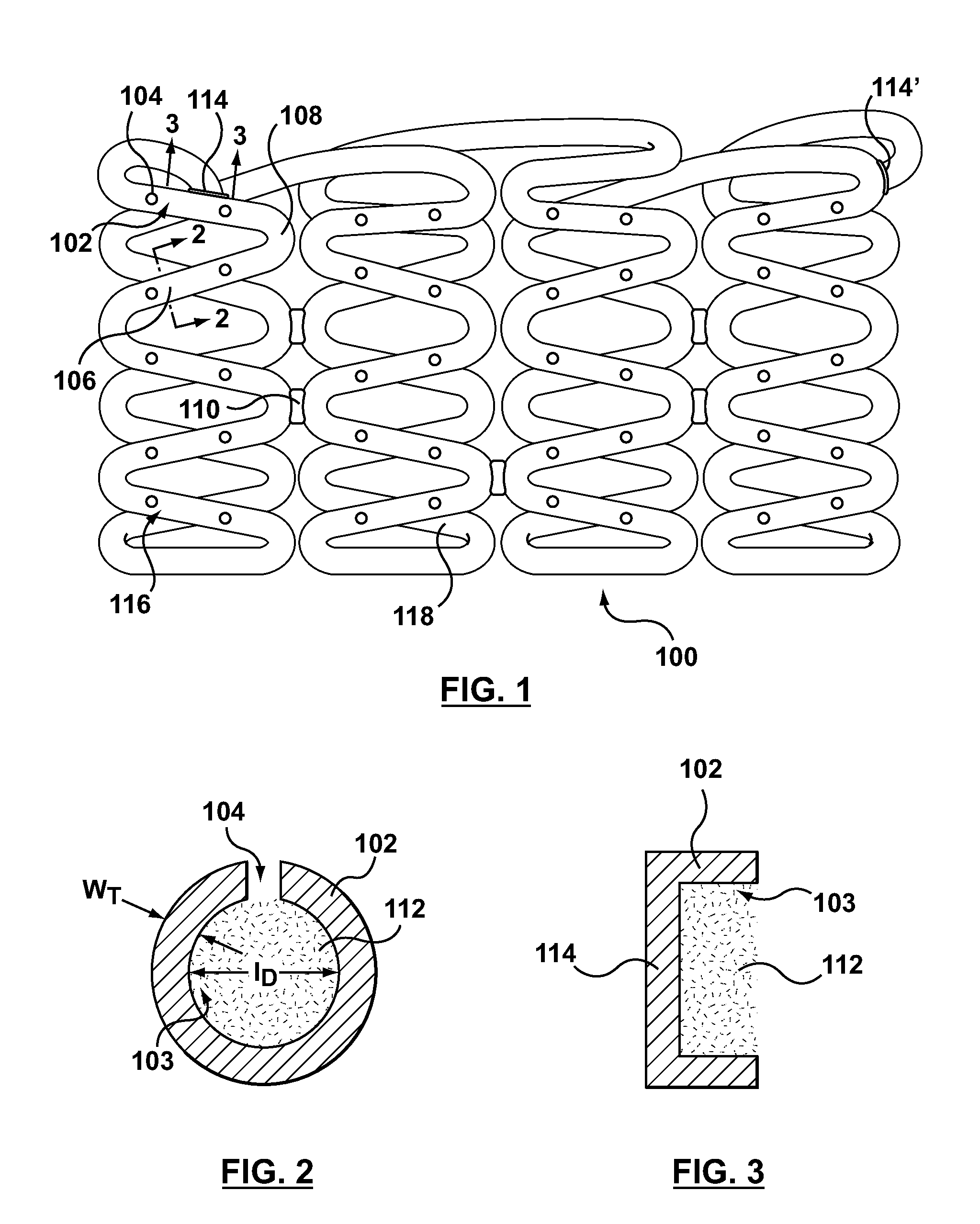
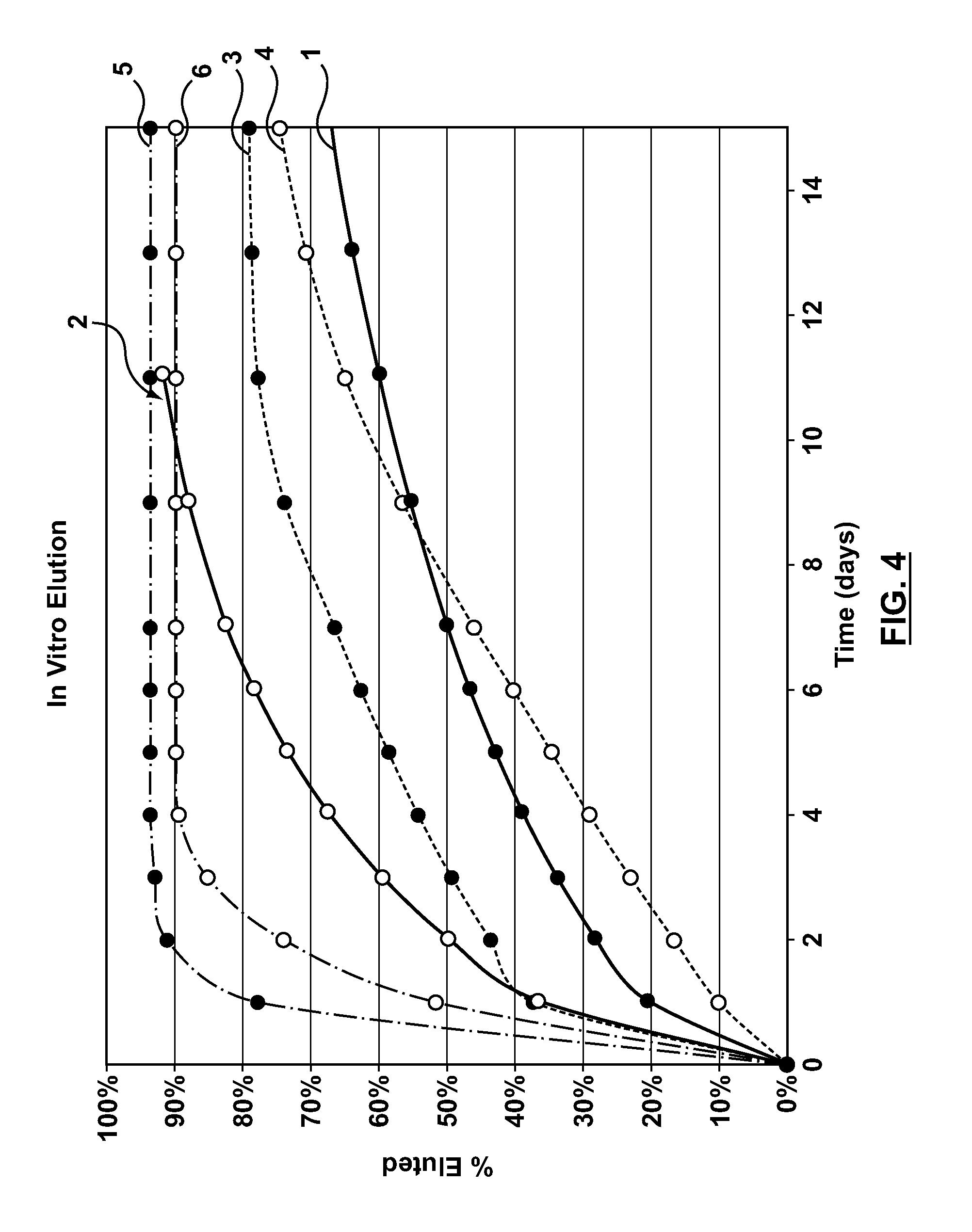
A drug-eluting stent (DES) is a peripheral or coronary stent (a scaffold) placed into narrowed, diseased peripheral or coronary arteries that slowly releases a drug to block cell proliferation. This prevents fibrosis that, together with clots (thrombi), could otherwise block the stented artery, a process called restenosis. The stent is usually placed within the peripheral or coronary artery by an interventional cardiologist or interventional radiologist during an angioplasty procedure.
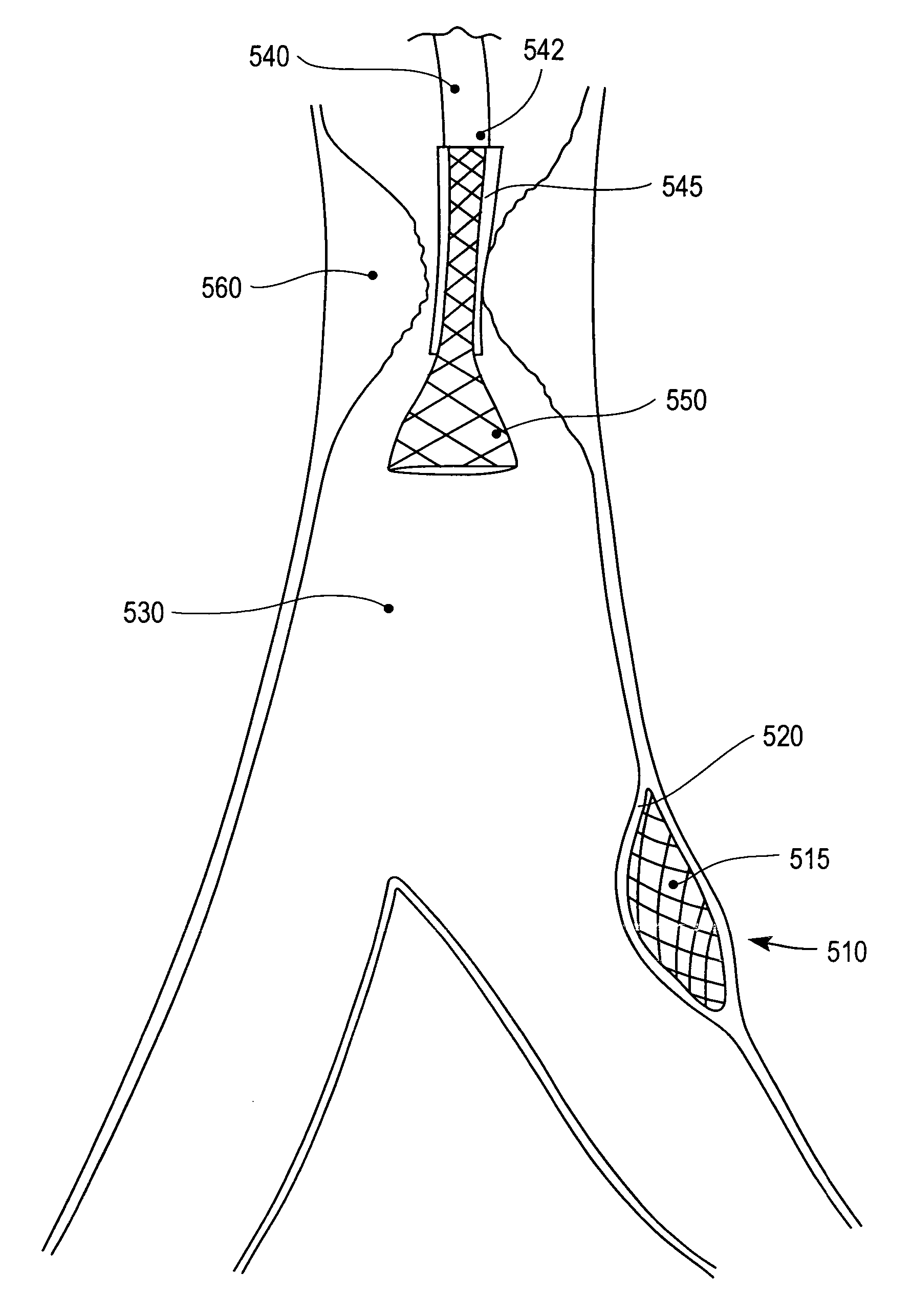
Means and method for the treatment of cerebral aneurysms
Disclosed is a system for the treatment of cerebral aneurysms using a stent and a aneurysm pocket fill structure delivery system. One embodiment of the present invention uses a highly radiopaque, drug eluting stent that is deployed with its sidewall over the ostium of the aneurysm pocket. A fill structure delivery catheter is then advanced through the patient's vascular system until the catheter's distal end is situated within the aneurysm pocket. Compressed aneurysm pocket filling structures are then pushed through the fill structure delivery catheter. As the aneurysm pocket filling structures emerge from an opening in the catheter's distal end, they promptly expand so that their minimum dimension is sufficiently large so that they cannot pass through the spaces between the struts of the stent that cover the ostium of the aneurysm pocket.
Owner:FISCHELL ROBERT E +1
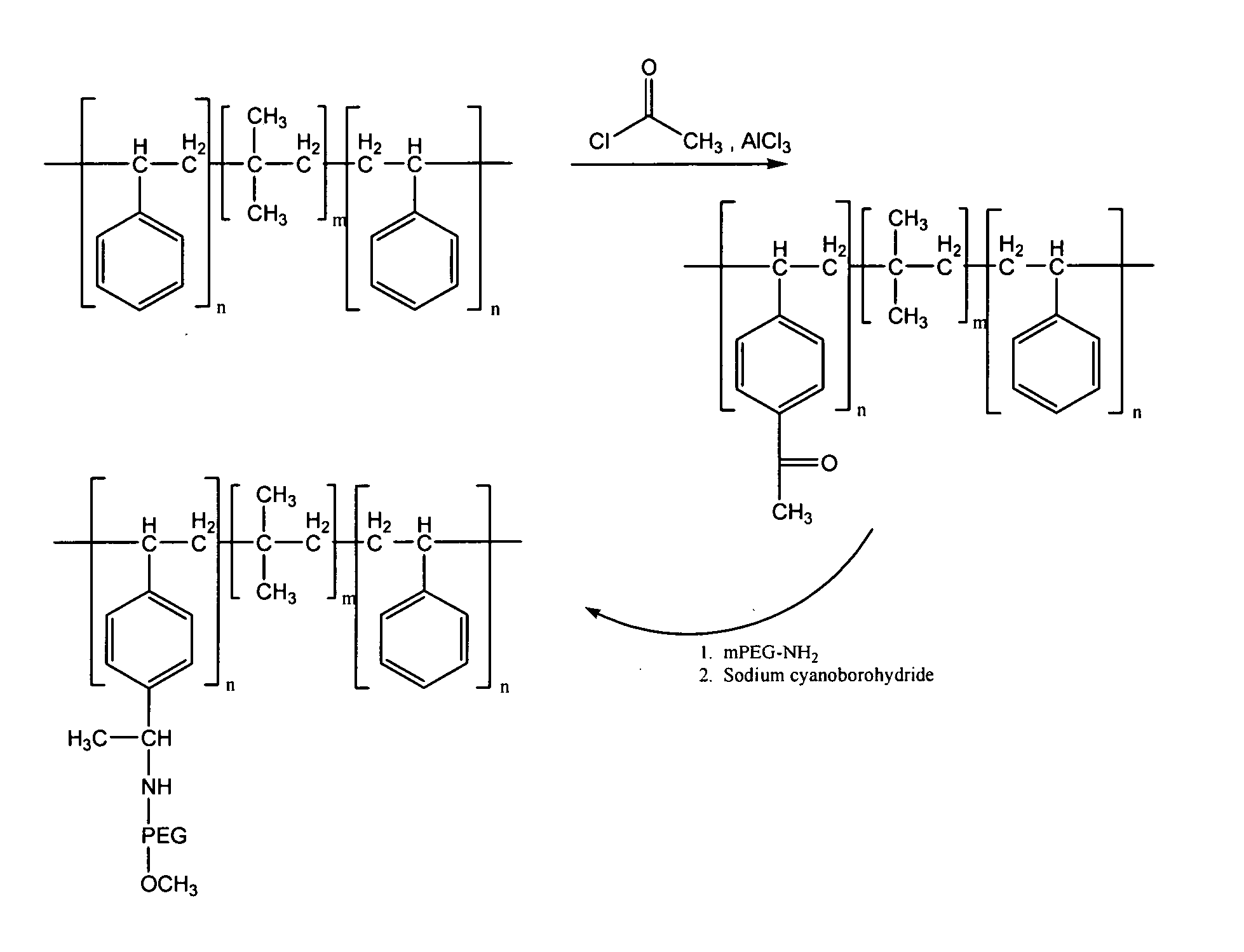
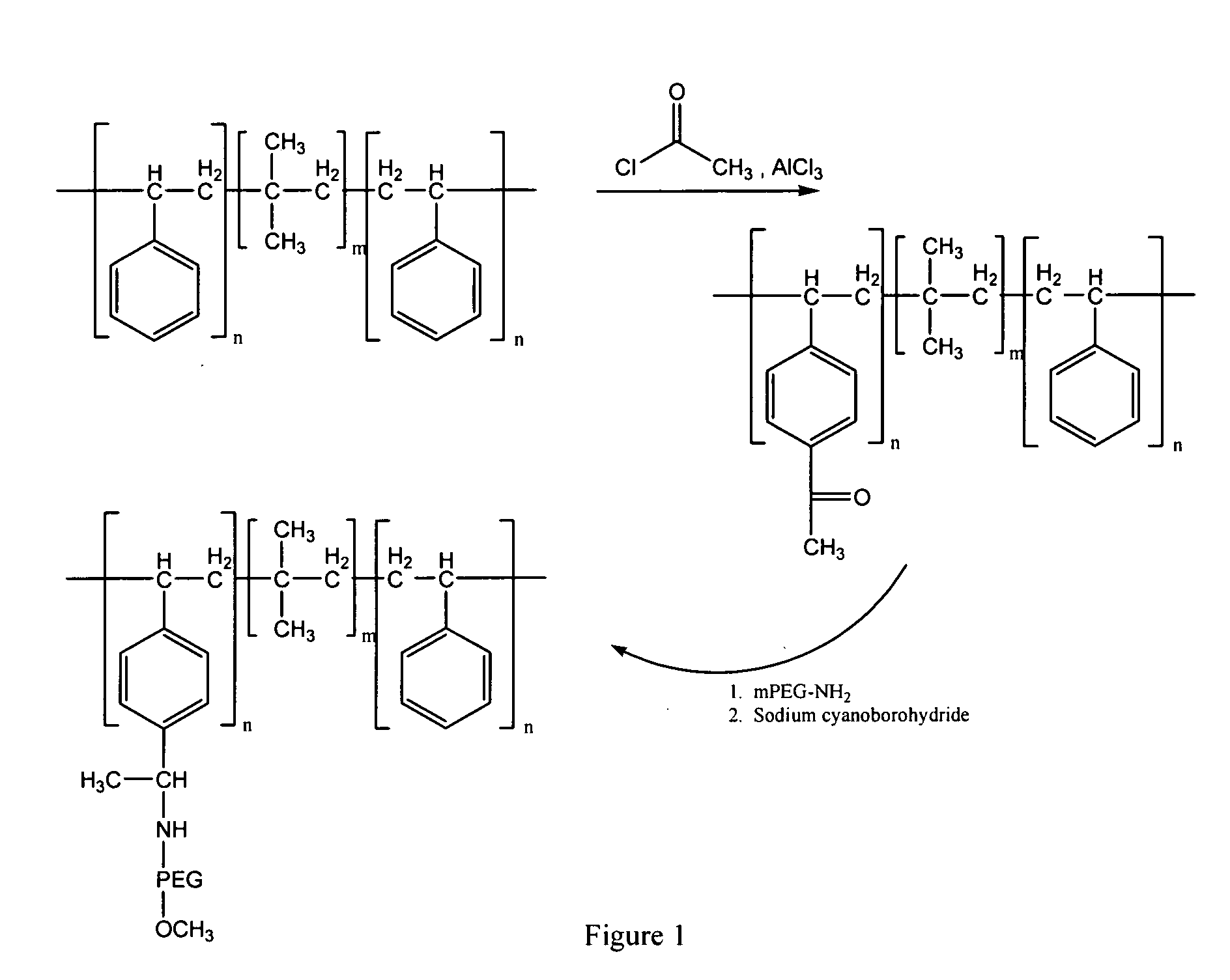
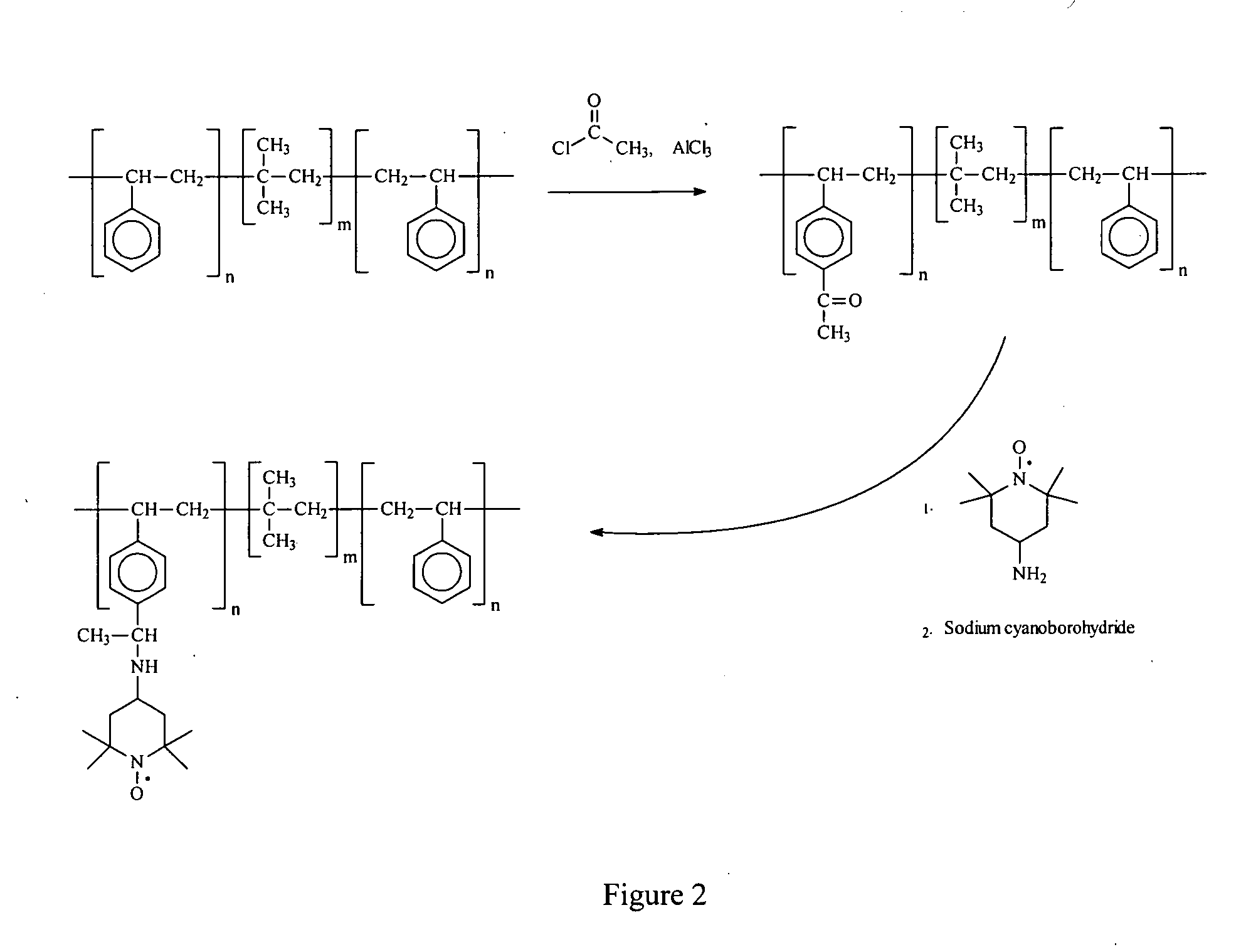
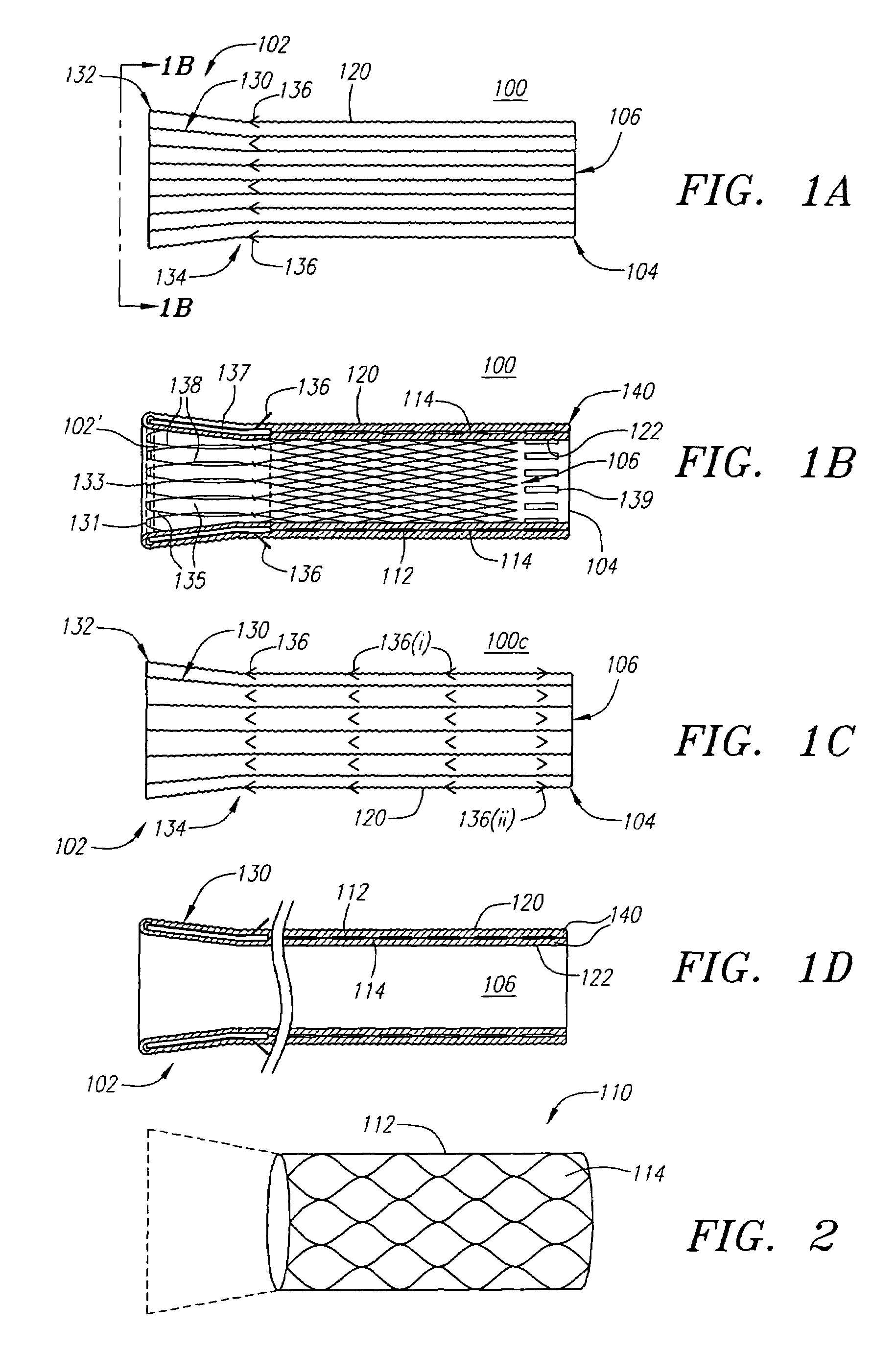
Drug-eluting stent for controlled drug delivery
The present invention provides a stent for delivering drugs to a vessel in a body, including a stent framework with a plurality of reservoirs formed therein, a drug polymer positioned in the reservoirs, and a polymer layer positioned on the drug polymer. The present invention also provides a method of manufacturing a drug-polymer stent and a method of treating a vascular condition using the drug-polymer stent.
Owner:MEDTRONIC VASCULAR INC
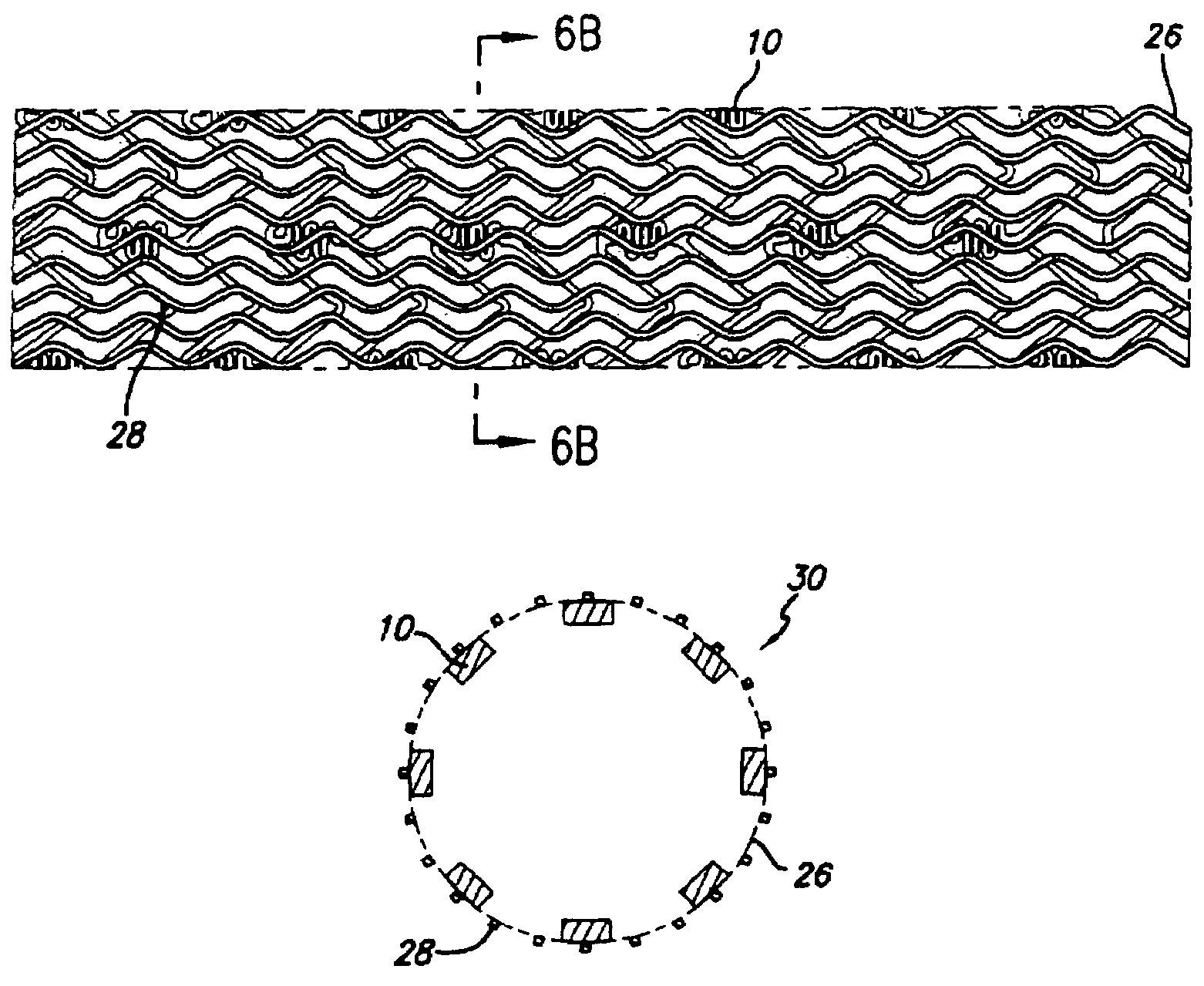
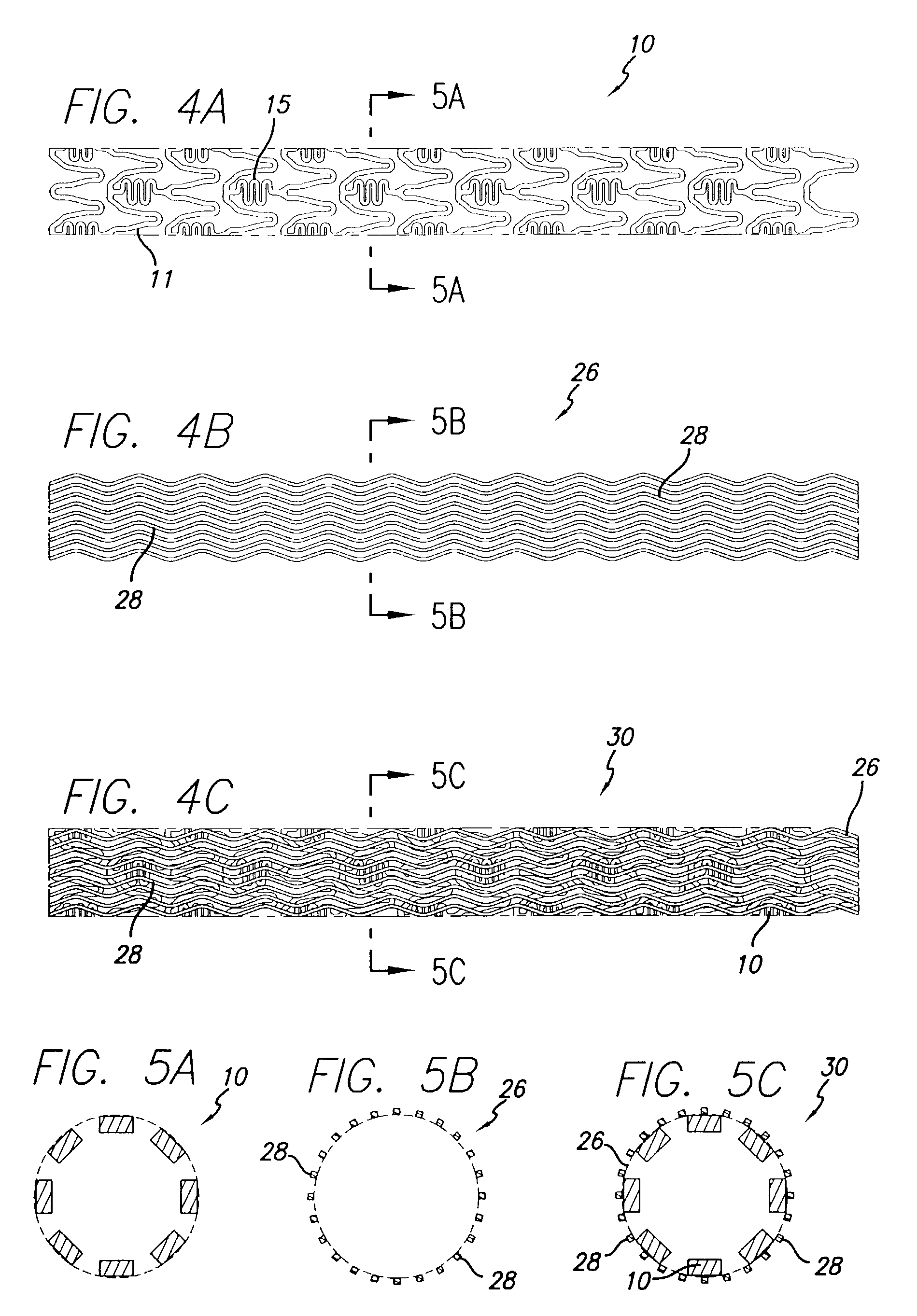
Drug-eluting stent cover and method of use
An intravascular stent includes an eluting sheath fabricated from a mesh for controlled release of therapeutic drugs and for delivery of the therapeutic drugs in localized drug therapy in a blood vessel. The eluting sheath is attached to at least a portion of an outside surface area of the stent structure and is fabricated from a mesh designed to neck down in response to a radially outward directed force resulting in the uniform expansion of the stent. The eluting sheath can be loaded with at least one therapeutic drug for the release thereof at a treatment site to facilitate repair of a damaged vessel. The stent has a high degree of flexibility in the longitudinal direction, yet has adequate vessel wall coverage and radial strength sufficient to hold open an artery or other body lumen.
Owner:ABBOTT CARDIOVASCULAR
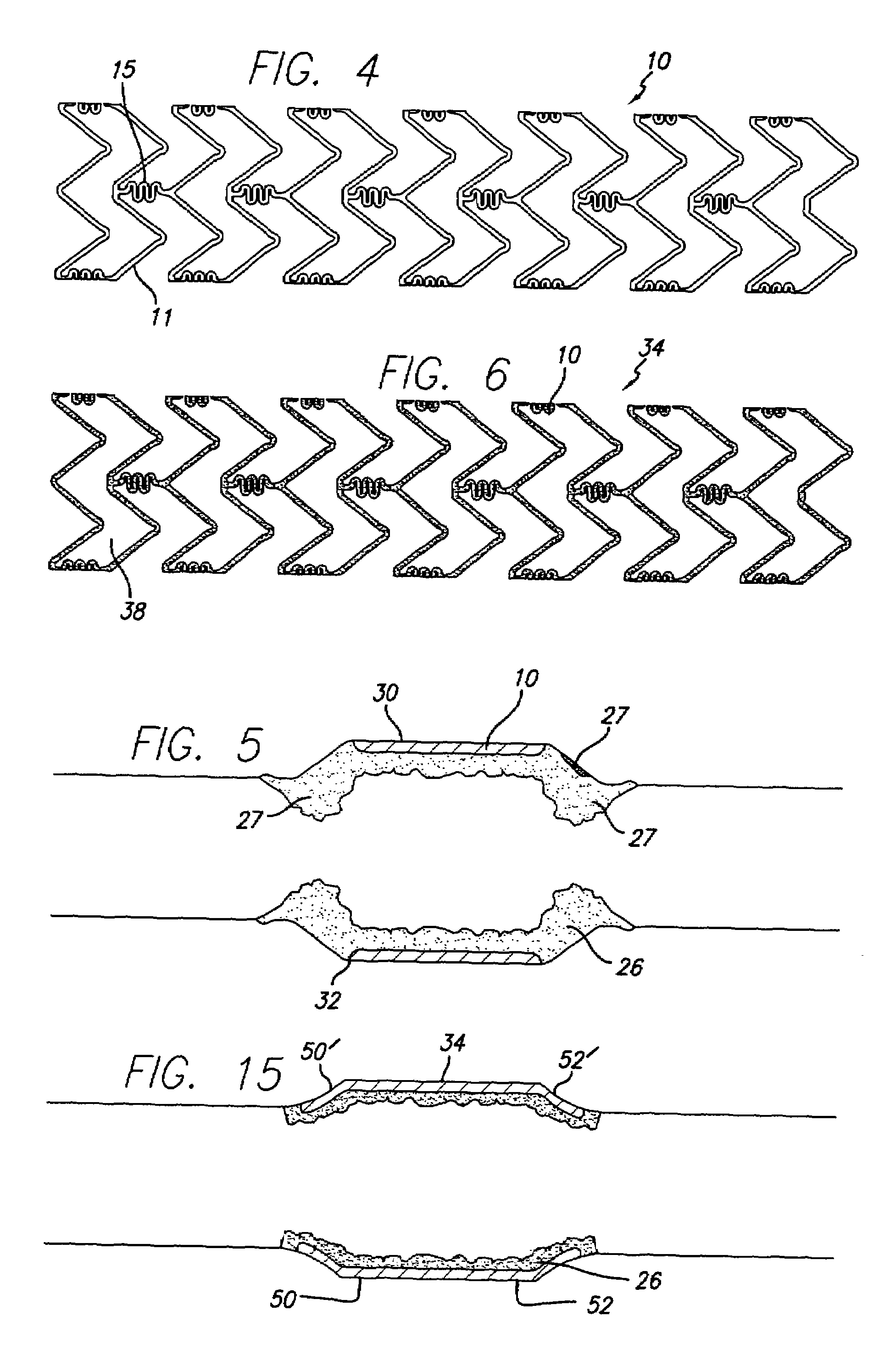
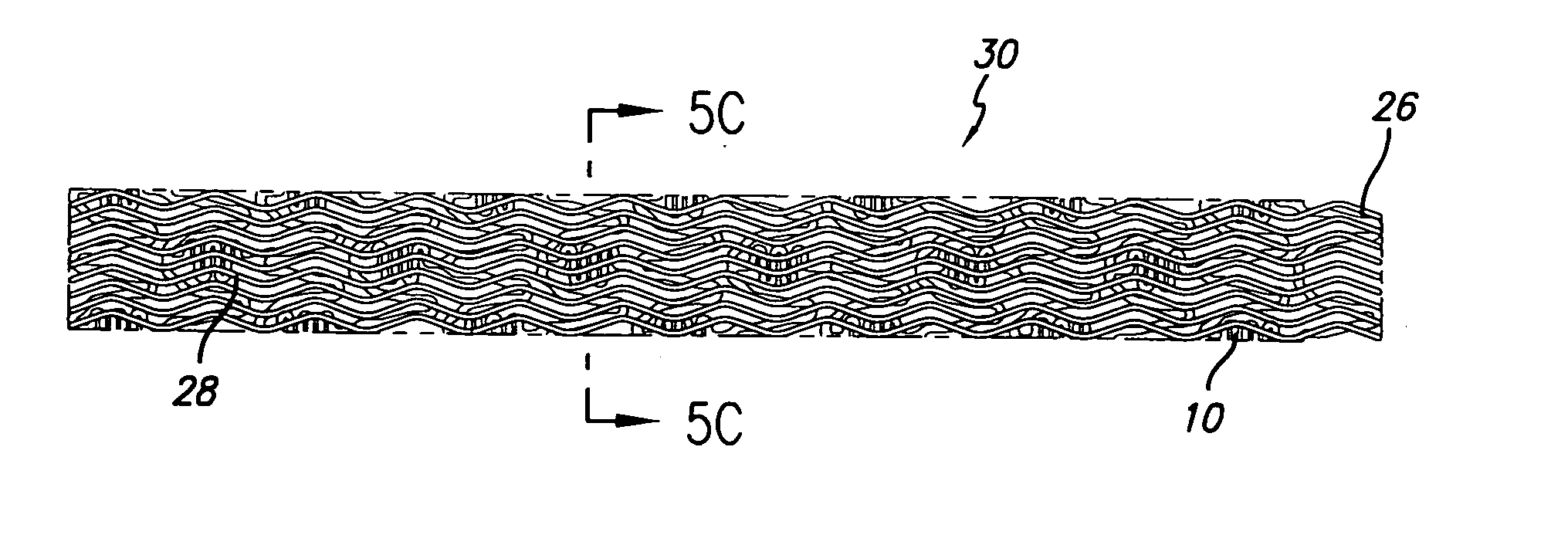
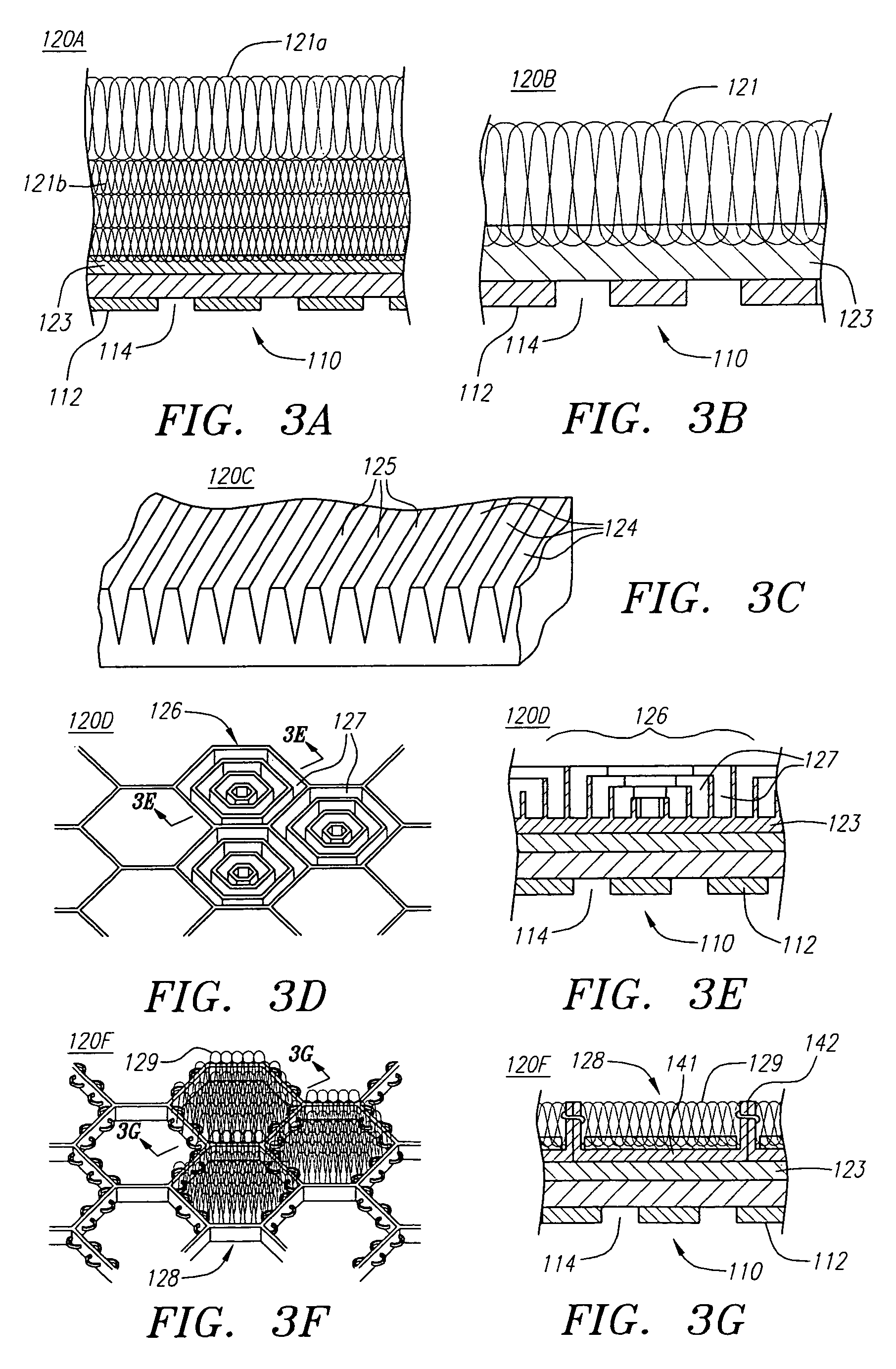
Stent with improved drug loading capacity
The present invention provides a method of forming a drug eluting stent, the method comprising coupling a stent framework to a mandrel, inserting the mandrel with stent framework into an open die, the die including a forming surface including a plurality of raised indention forming portions; closing the die against the stent framework; pressing the raised indention portions into the stent framework to form indentions in the stent framework; and inserting at least one drug polymer into the indentions formed in the stent framework.
Owner:MEDTRONIC VASCULAR INC
Polyacrylates coatings for implantable medical devices
A coating for a medical device, particularly for a drug eluting stent, is described. The coating can include a polyacrylate, a blend of polyacrylates, or a blend of the polyacrylate with other polymers, for example, poly(ethylene-co-vinyl alcohol).
Owner:ABBOTT CARDIOVASCULAR
Drug eluting structurally variable stent
The present invention provides a stent including a tubular body having a plurality of reservoirs disposed therein and a therapeutic agent located in the reservoirs or located in the reservoirs and on a surface portion of the tubular body, wherein the stent is free of polymeric material. The invention also provides a drug-eluting stent made from the process of providing a polymer-free stent body having a plurality of reservoirs disposed therein, diluting a therapeutic agent in a polymer-free solvent to form an agent-solvent mixture, coating the stent with the agent-solvent mixture, and allowing the solvent to dissipate from the stent thereby leaving the agent disposed on the stent.
Owner:VASCULAR CONCEPTS HLDG
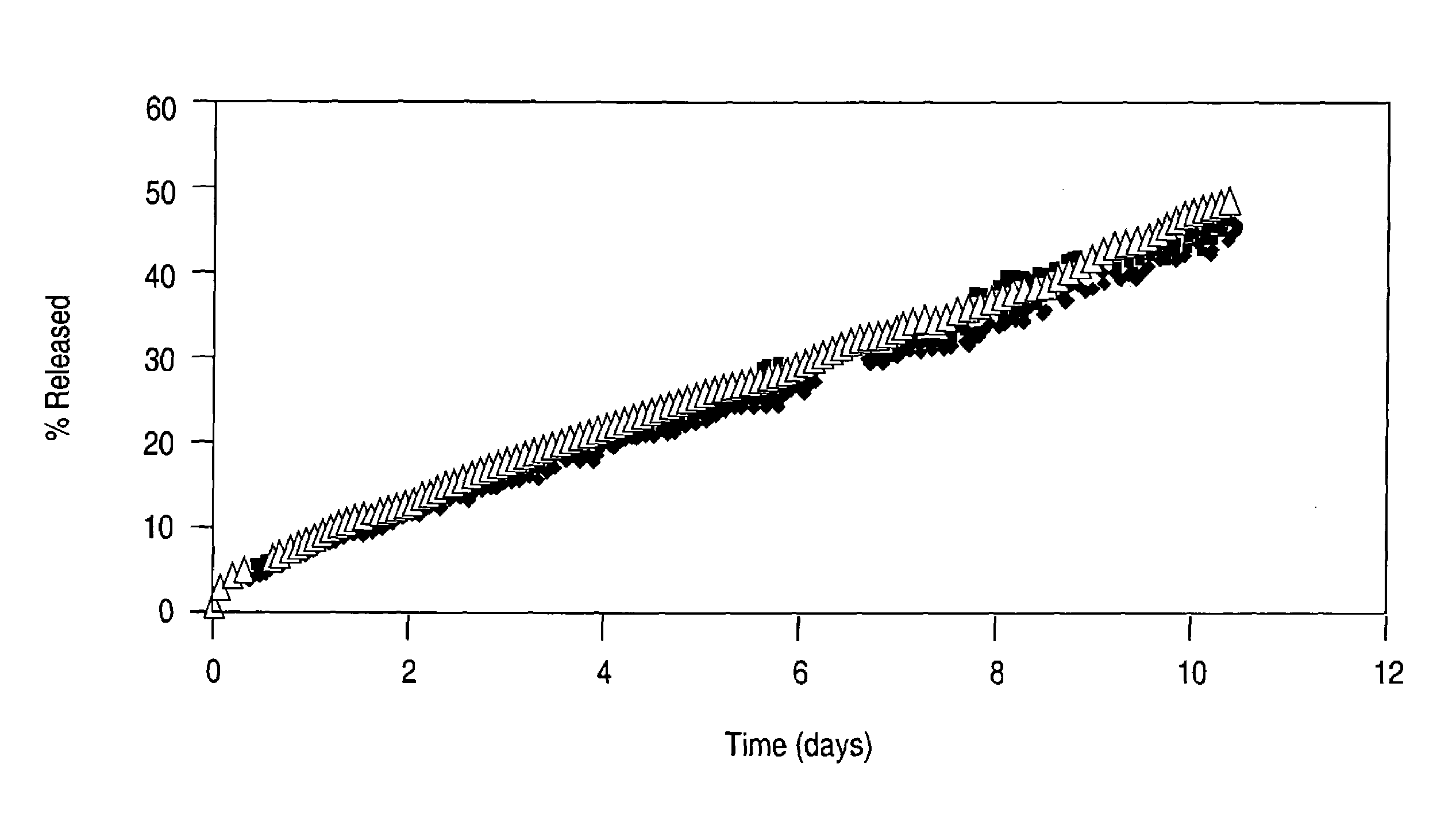
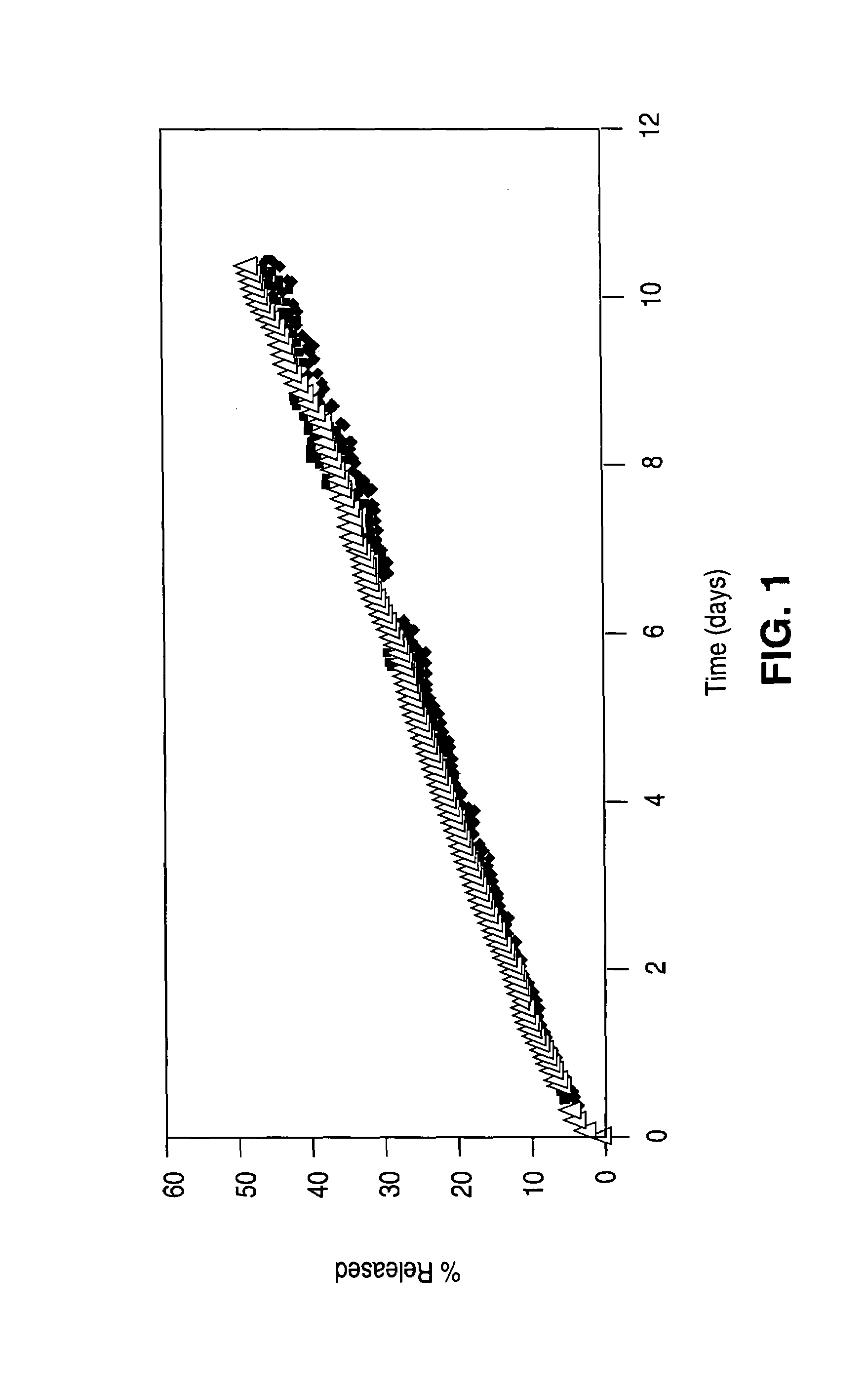
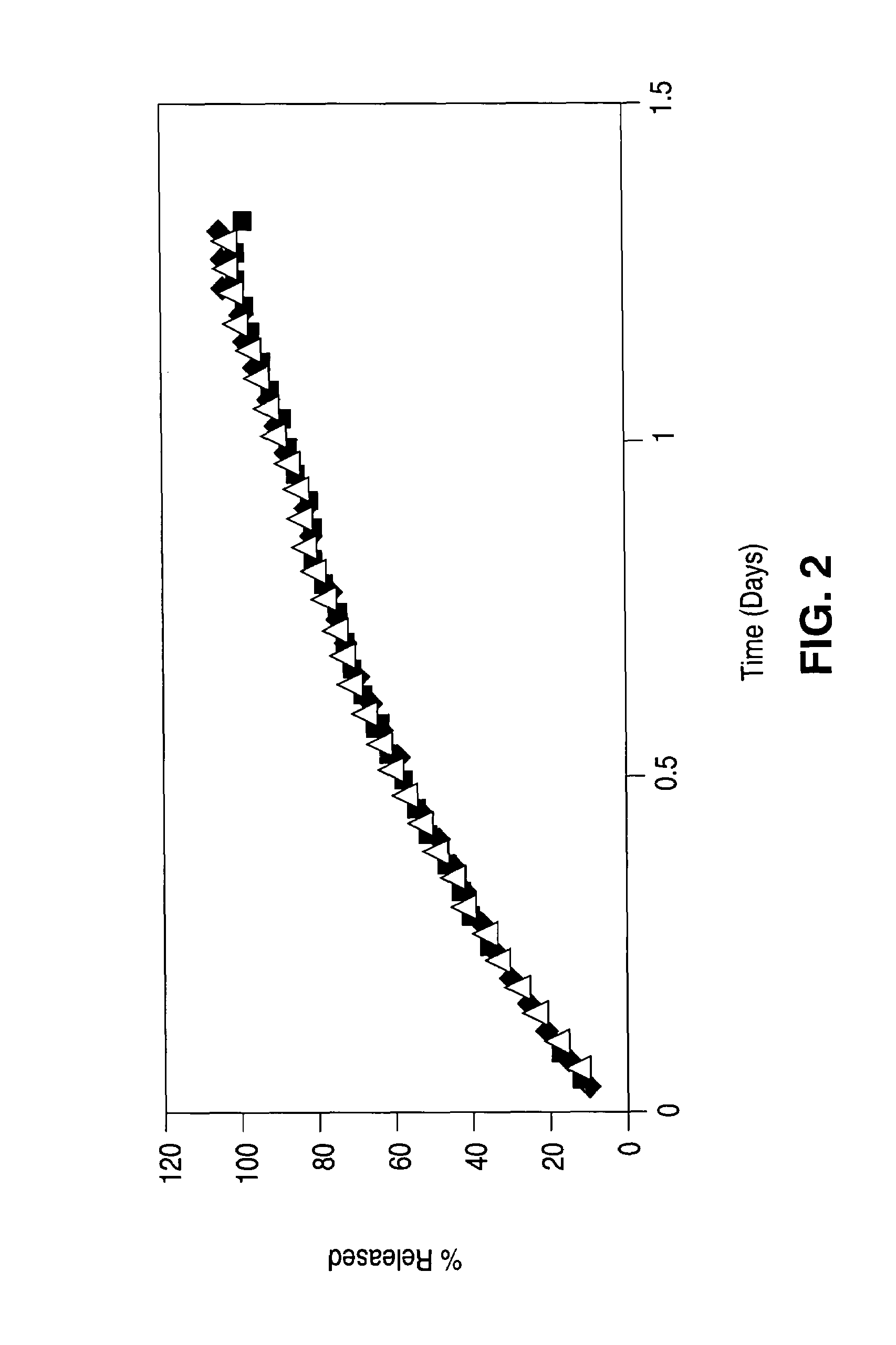
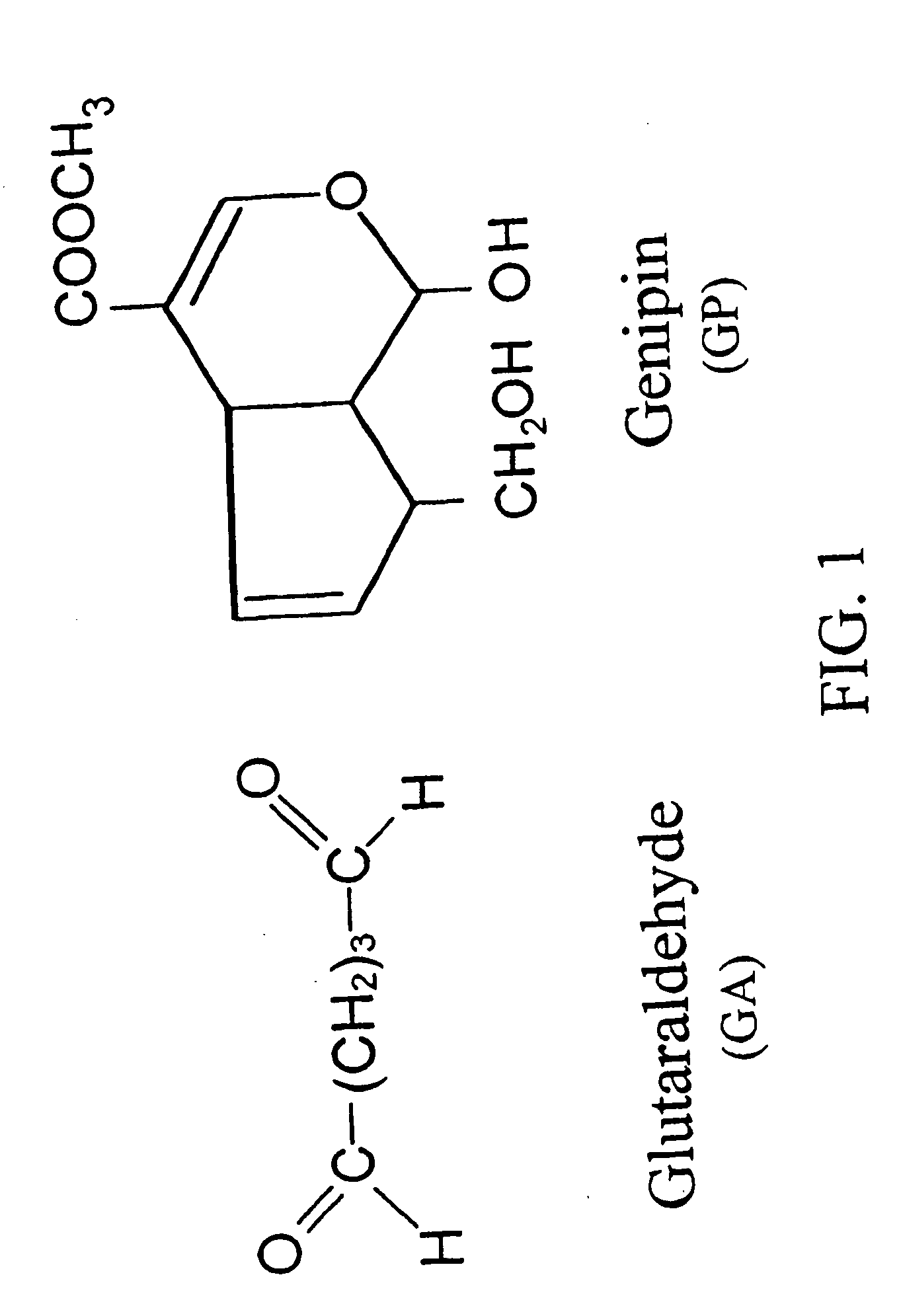
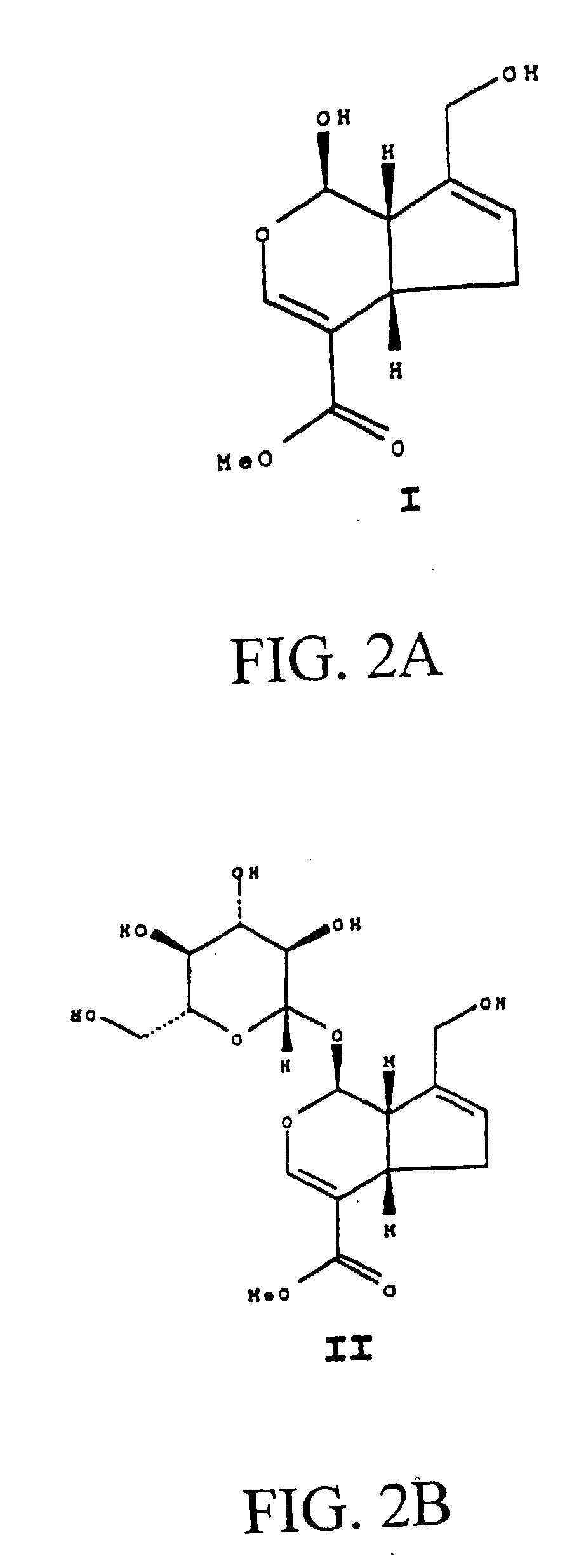
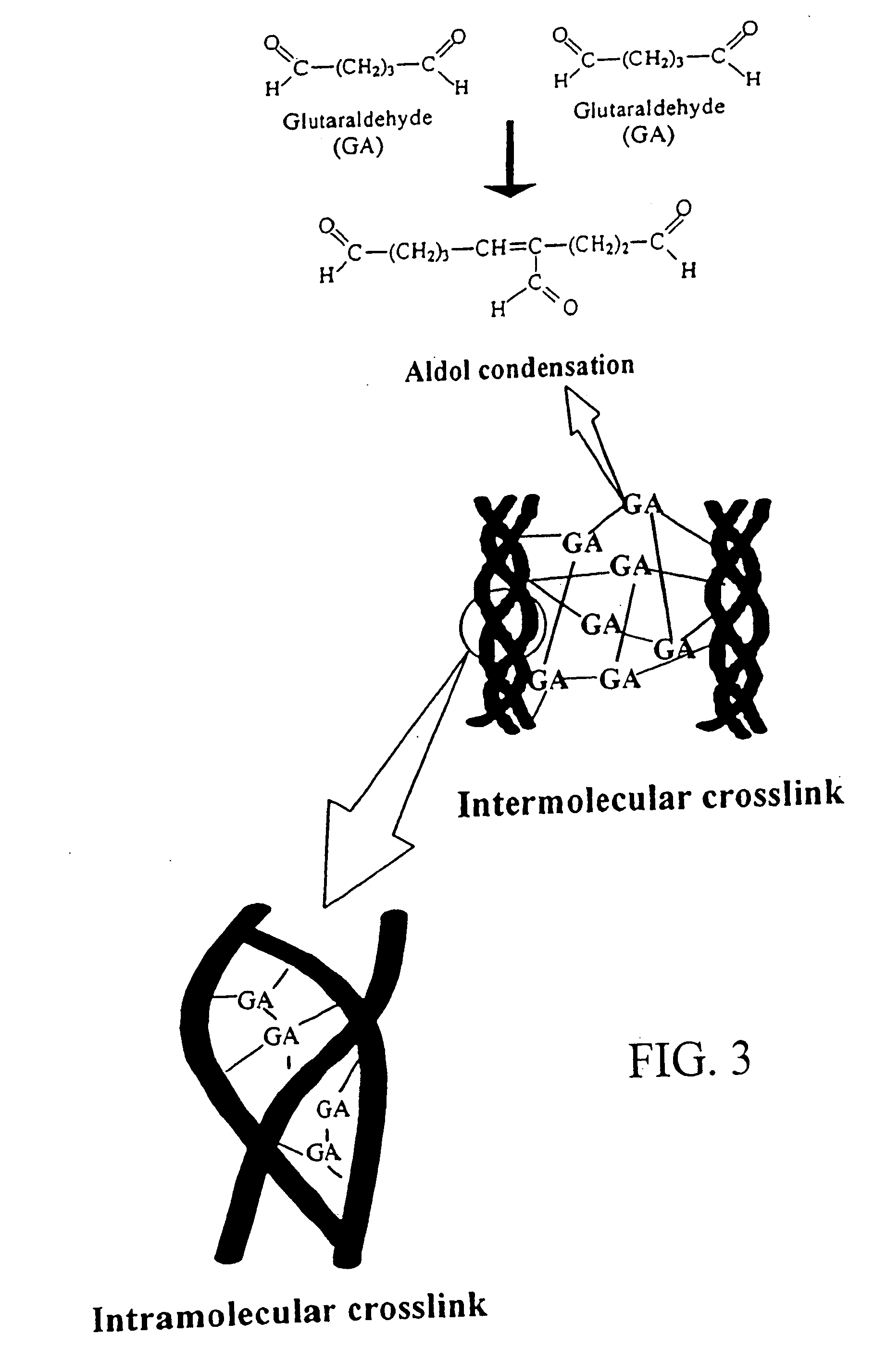
Drug-eluting stent having collagen drug carrier chemically treated with genipin
InactiveUS20050123582A1Reduce releaseAntibacterial agentsOrganic active ingredientsVulnerable plaqueInsertion stent
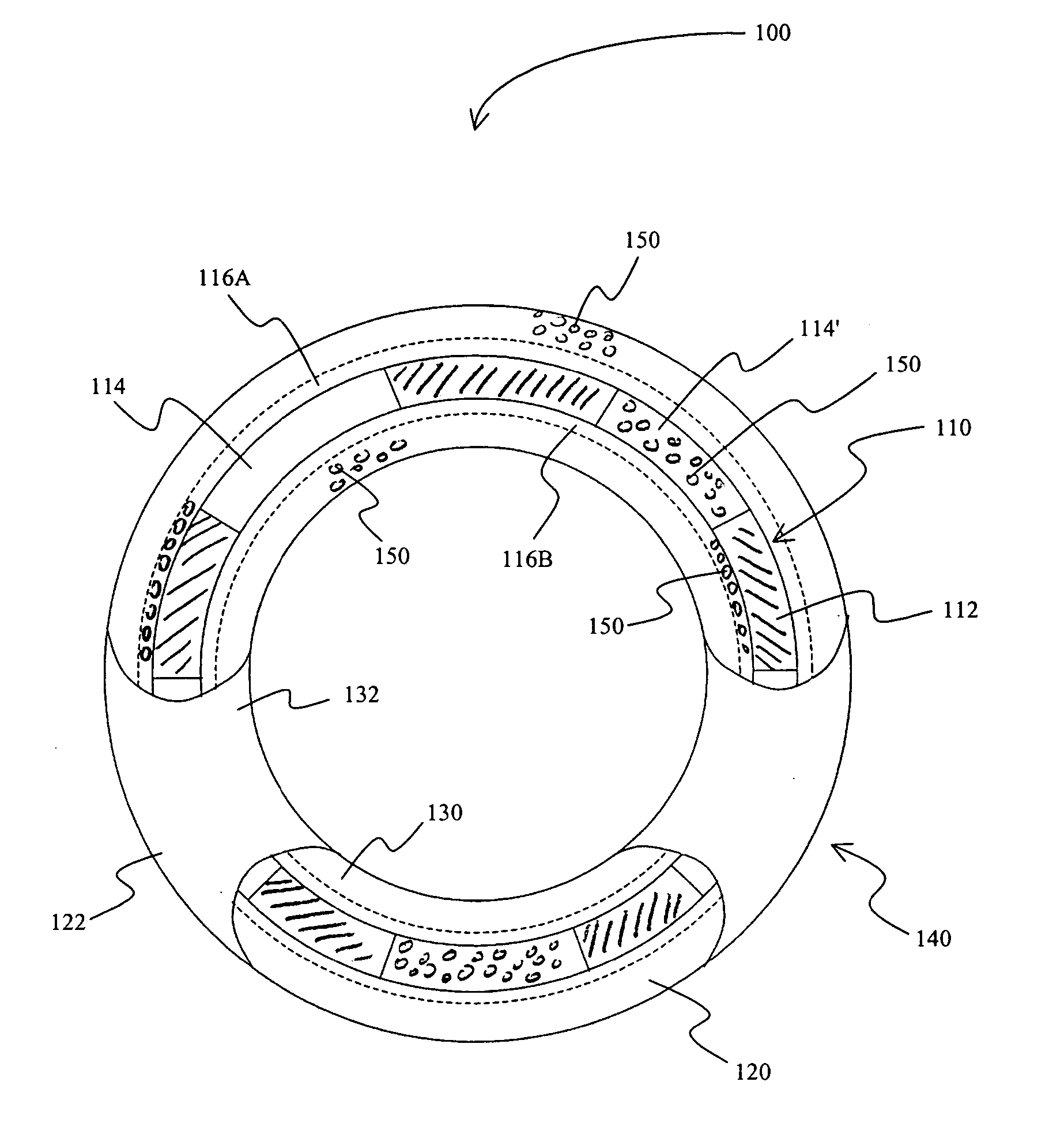
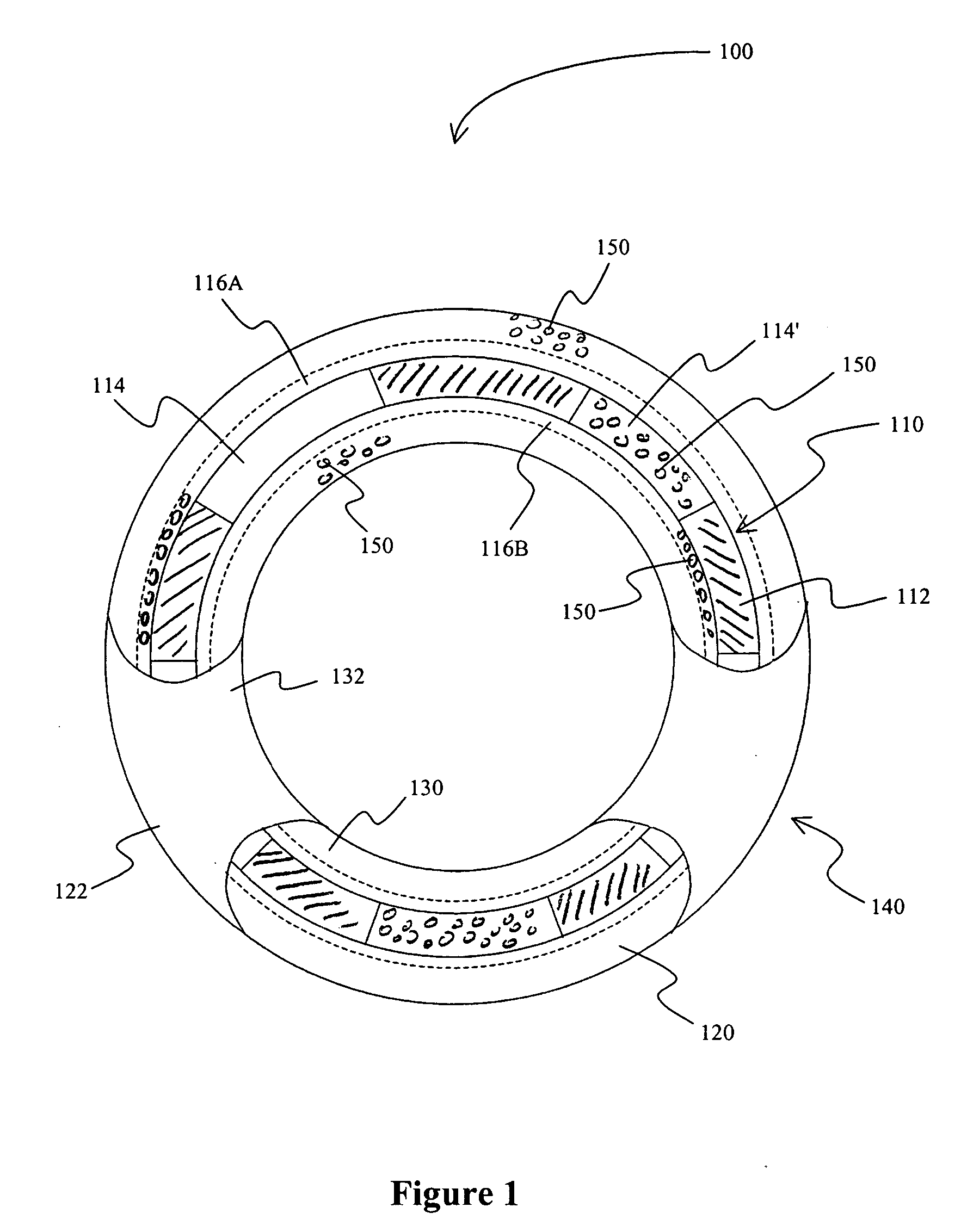
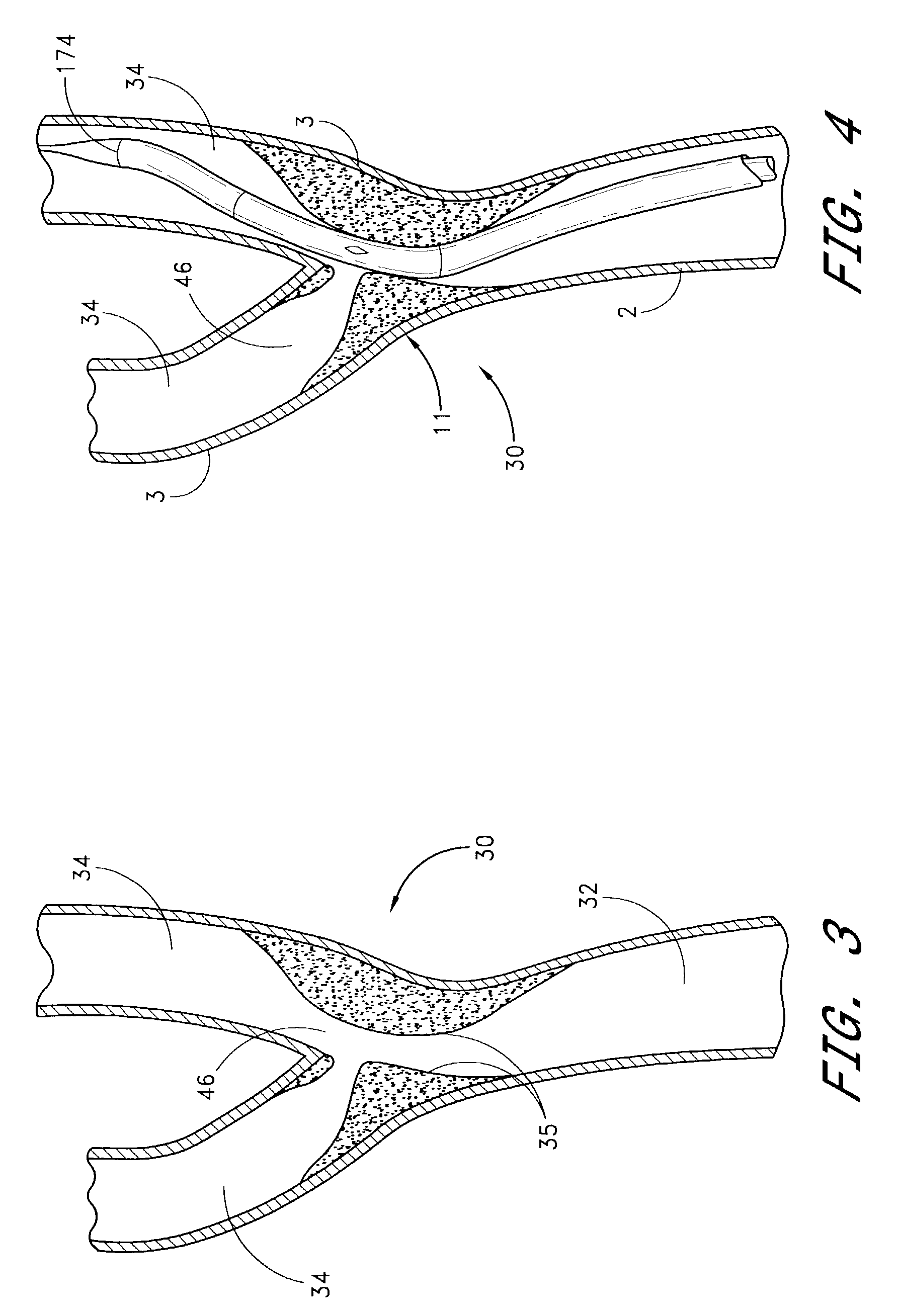
A method for treating vulnerable plaques of a patient, comprising: providing a biodegradable stent comprising a first supporting zone made of a first biodegradable material, wherein the supporting zone comprises at least a portion of continuous circumference of the stent; and a second therapeutic zone made of a second biodegradable material, wherein the therapeutic zone comprises at least one bioactive agent; delivering the biodegradable stent to the vulnerable plaques; orienting the therapeutic zone at about the luminal surface of the vulnerable plaque; and releasing the at least one bioactive agent for treating the vulnerable plaques.
Owner:GP MEDICAL
Poly(ester amide) coating composition for implantable devices
InactiveUS20050271700A1Improve mechanical propertiesImprove surface propertiesSurgeryPharmaceutical containersPercent Diameter StenosisImplanted device
Owner:ABBOTT CARDIOVASCULAR
Stent coatings containing self-assembled monolayers
InactiveUS7232573B1Material nanotechnologyOrganic active ingredientsSelf-assembled monolayerMedical device
Owner:ABBOTT CARDIOVASCULAR
Biobeneficial coating compostions and methods of making and using thereof
Owner:ABBOTT CARDIOVASCULAR
Method and apparatus for treating vulnerable plaque
Various apparatuses and methods are described to treat vulnerable plaque with the use of combination drug therapy. In one embodiment, the apparatus has an elongated catheter body adapted for insertion in a body lumen, with a drug delivery device attached near a distal portion of the elongated body. The drug delivery device is configured to deliver a therapeutic or biologically active agent to stabilize a vulnerable plaque. In another embodiment, a drug eluting stent is delivered and deployed in conjunction with a second apparatus that delivers drug in pressurized retrograde perfusion. Still another embodiment uses a uniquely shaped balloon to deploy a stent while utilizing a drug infusion needle to inject drug into the vessel wall through a hollow guide wire.
Owner:ABBOTT CARDIOVASCULAR
Polymeric, degradable drug-eluting stents and coatings
ActiveUS7618448B2Improve performanceImprove propertiesSuture equipmentsPharmaceutical containersAbsorbable polymersEthylene Homopolymers
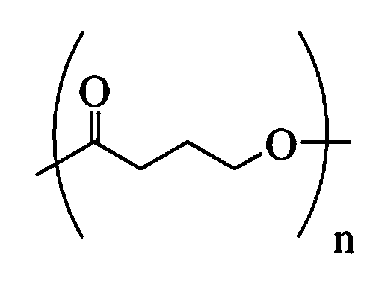
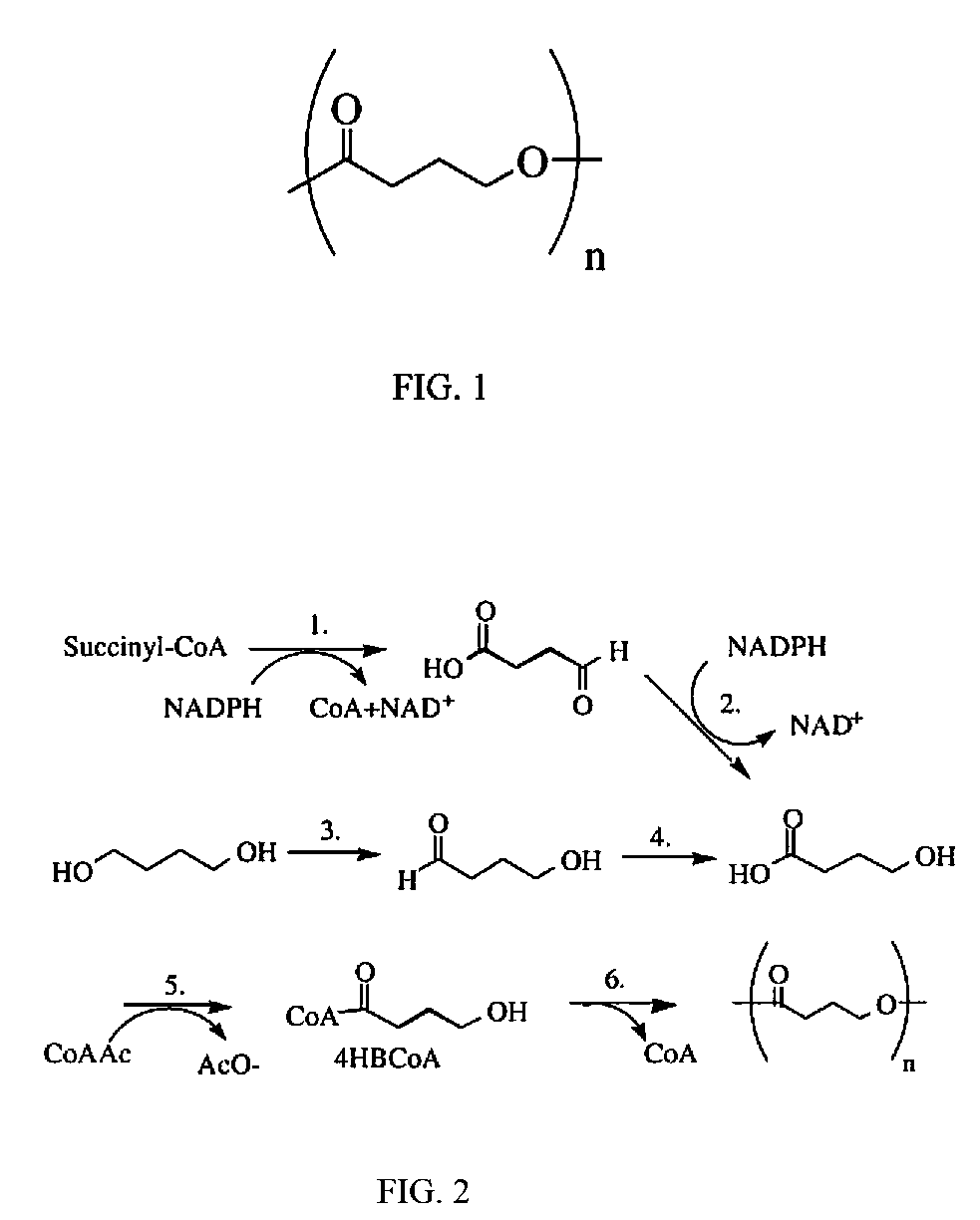
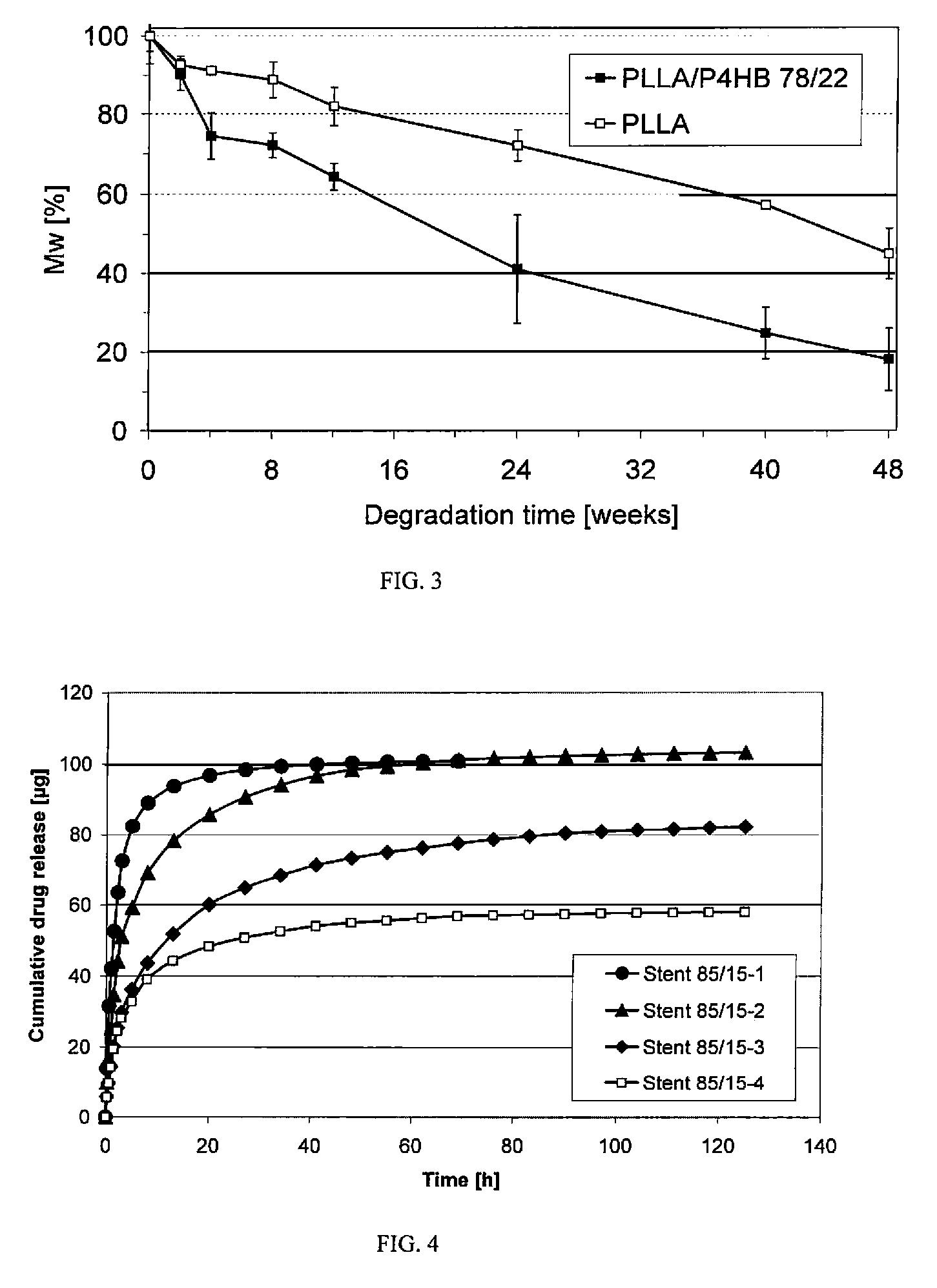
Absorbable stents and absorbable stent coatings have been developed with improved properties. These devices preferably comprise biocompatible copolymers or homopolymers of 4-hydroxybutyrate, and optionally poly-L-lactic acid and other absorbable polymers and additives. Compositions of these materials can be used to make absorbable stents that provide advantageous radial strengths, resistance to recoil and creep, can be plastically expanded on a balloon catheter, and can be deployed rapidly in vivo. Stent coatings derived from these materials provide biocompatible, uniform coatings that are ductile, and can be expanded without the coating cracking and / or delaminating and can be used as a coating matrix for drug incorporation.
Owner:TEPHA INC
Thin-film nitinol based drug eluting stent
InactiveUS20070173787A1Reduce drug toxicityGood curative effectStentsPharmaceutical delivery mechanismDiseaseBlood vessel
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. In addition, these therapeutic drugs, agents and / or compounds may be utilized to promote healing, including the formation of blood clots. The drugs, agents, and / or compounds may also be utilized to treat specific diseases, including vulnerable plaque. Therapeutic agents may also be delivered to the region of a disease site. In regional delivery, liquid formulations may be desirable to increase the efficacy and deliverability of the particular drug. Also, the devices may be modified to promote endothelialization. Various materials and coating methodologies may be utilized to maintain the drugs, agents or compounds on the medical device until delivered and positioned. In addition, the devices utilized to deliver the implantable medical devices may be modified to reduce the potential for damaging the implantable medical device during deployment. Medical devices include stents, grafts, anastomotic devices, perivascular wraps, sutures and staples. These devices may also comprise thin films that perform a number of functions. In addition, various polymer combinations may be utilized to control the elution rates of the therapeutic drugs, agents and / or compounds from the implantable medical devices.
Owner:CORDIS CORP
Dual-Layer Medical Balloon
A dual-layer dilatation balloon includes an inner layer that includes a polymer selected from the group consisting of a polyester, polyether, polyamide and copolymers thereof, and an outer layer that includes a polyamide. The dual-layer balloon optionally further includes a stent disposed on the balloon. The stent is optionally a drug-eluting stent. A process for forming a dual-layer dilatation balloon includes forming a dual-layer extrudate having an outer layer that includes a polyamide and an inner layer that includes a polymer selected from the group consisting of a polyester, polyether, polyamide and copolymers thereof. The process also includes forming the dual-layer balloon from the dual-layer extrudate in a balloon forming machine, wherein the balloon has a hoop strength of about 10,000 to about 60,000 p.s.i.
Owner:MEDTRONIC VASCULAR INC
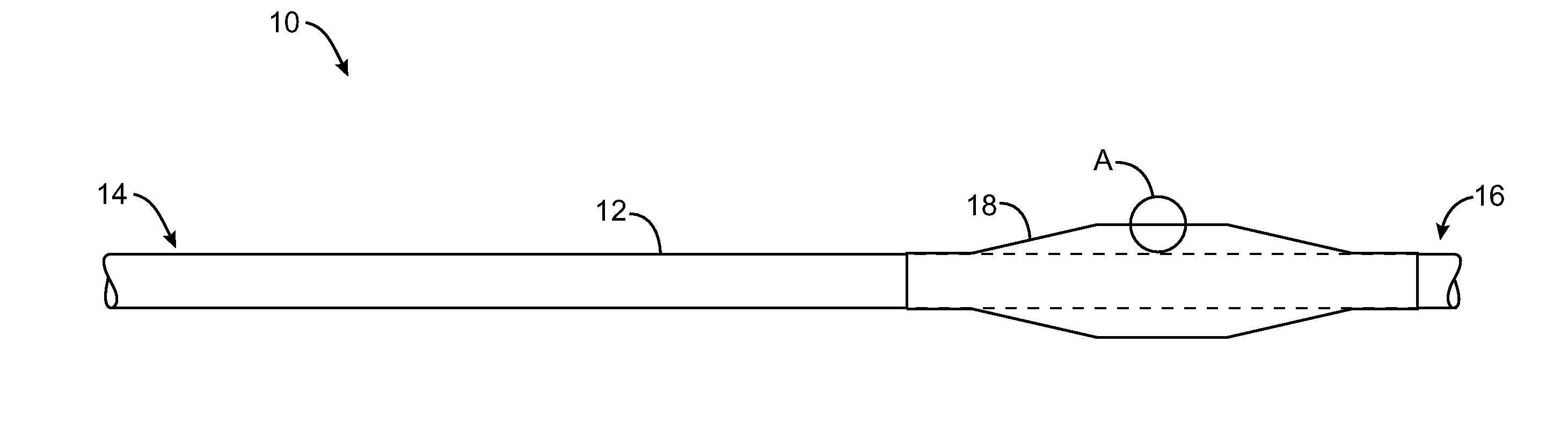
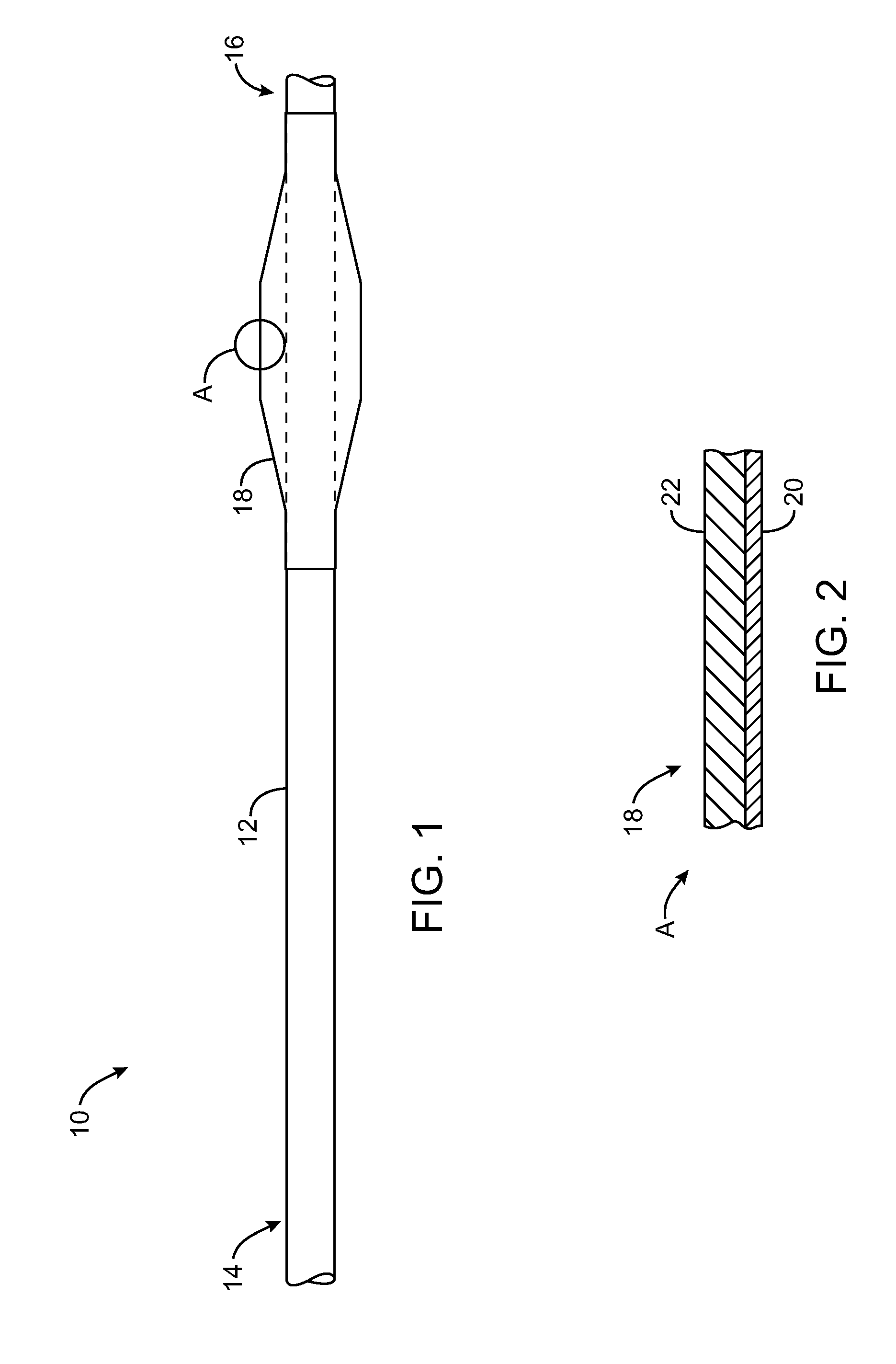
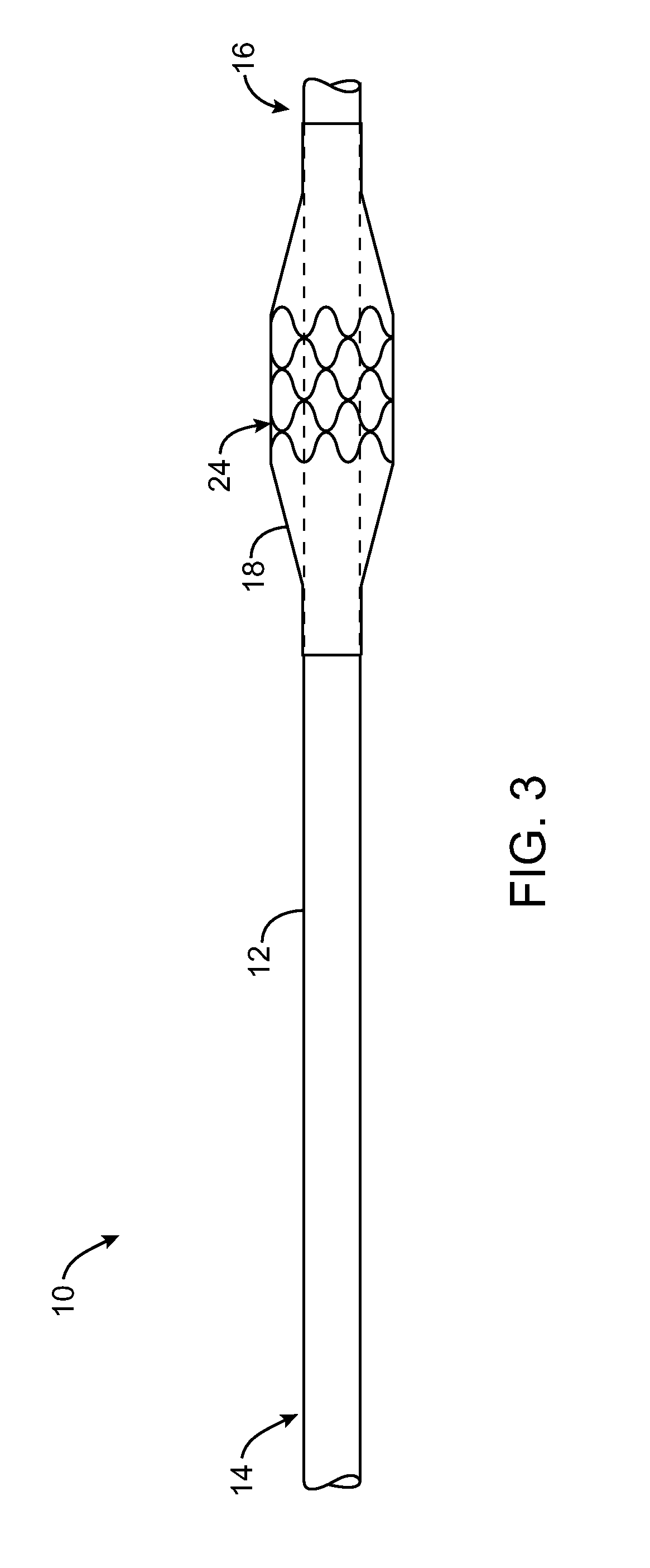
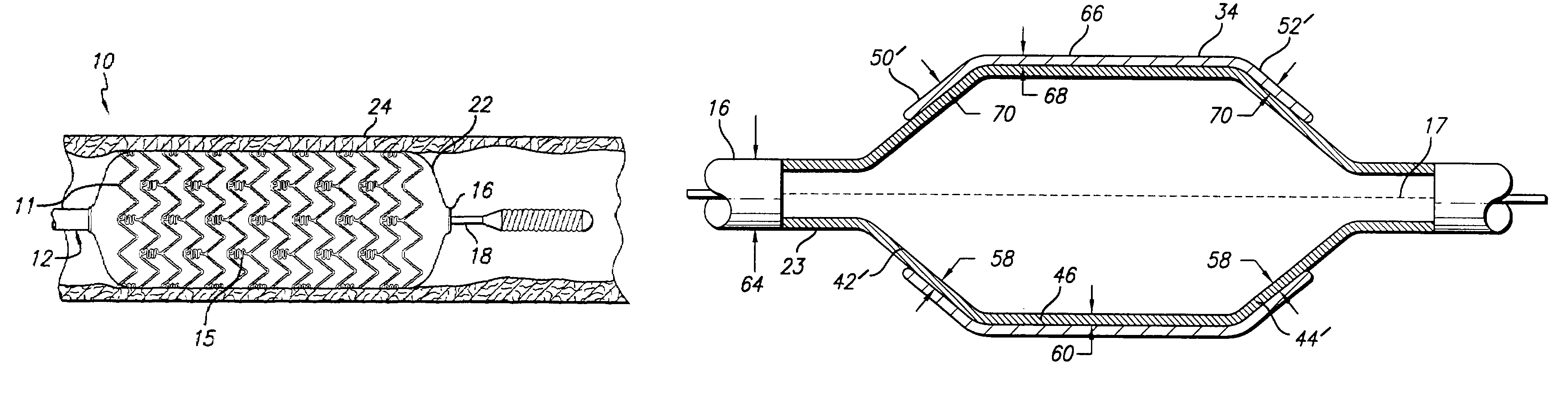
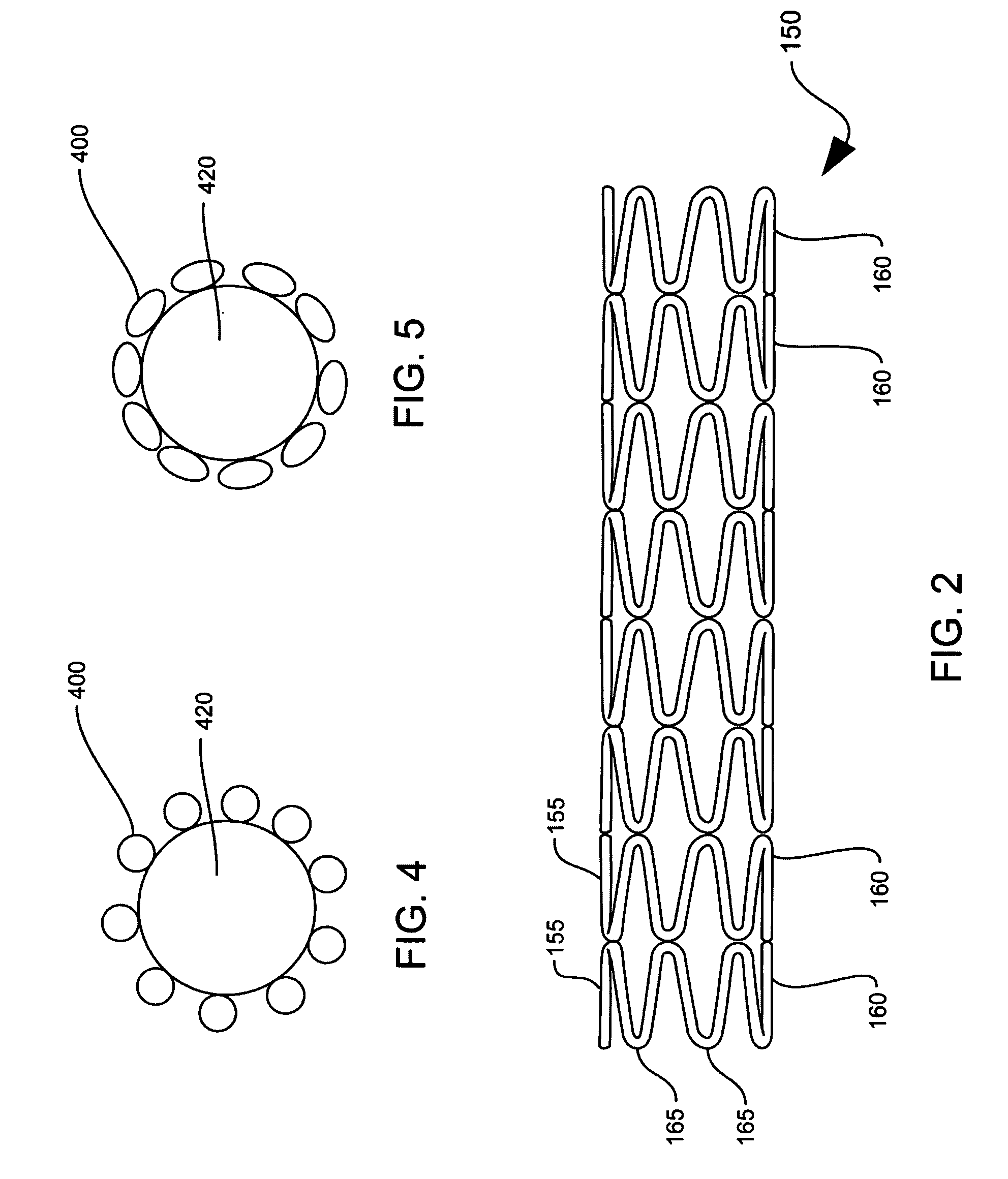
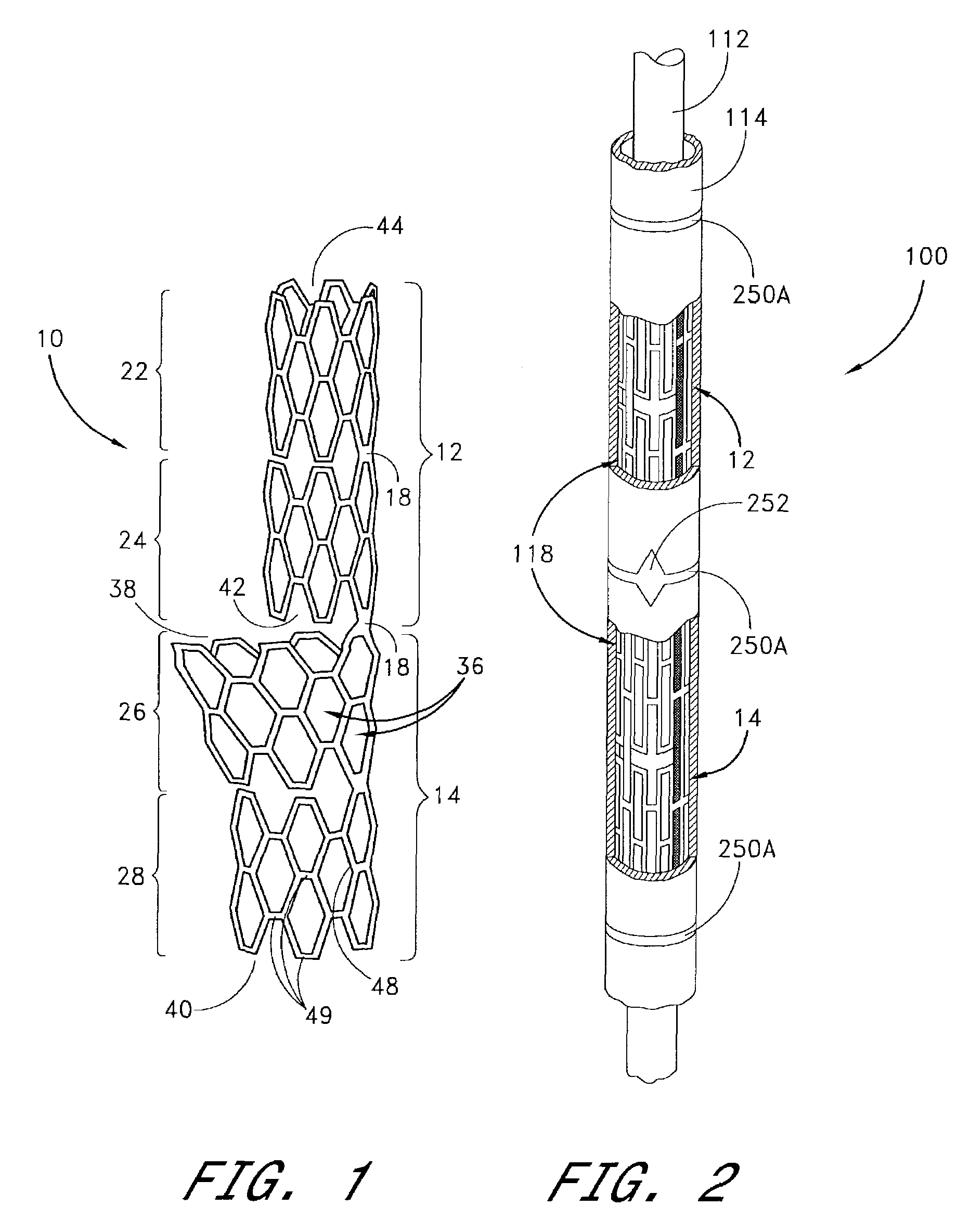
Drug-eluting stent and delivery system with tapered stent in shoulder region
A drug-eluting stent delivery system for the treatment of edge restenosis in a blood vessel. The drug-eluting stent delivery system has a balloon disposed about at least a portion of a catheter, the balloon having a first end and a second end and a working length therebetween, the first end and the second end each including a tapered portion, each tapered portion being attached to the catheter, the balloon being inflatable from a collapsed configuration to an inflated configuration. A drug-eluting stent contacts a wall of the blood vessel to maintain the patency of the vessel. The drug-eluting stent has a first end and a second end, the first end and the second end each including a tapered portion, wherein the drug-eluting stent is disposed over the balloon such that at least a portion of the first end and the second end of the balloon are covered by the tapered drug-eluting stent. A method for making the same is also disclosed herein.
Owner:ABBOTT CARDIOVASCULAR
Drug-eluting stent and methods of making the same
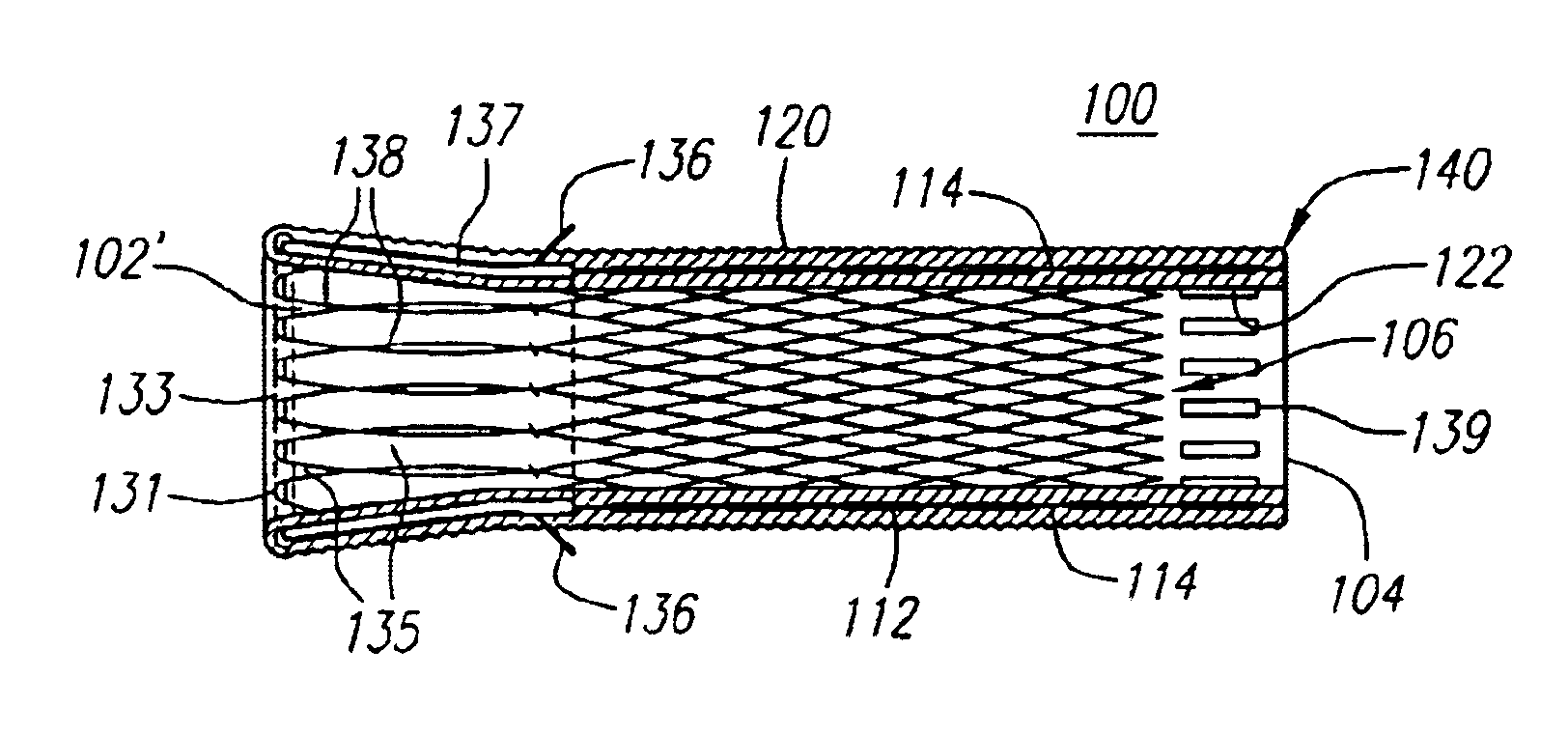
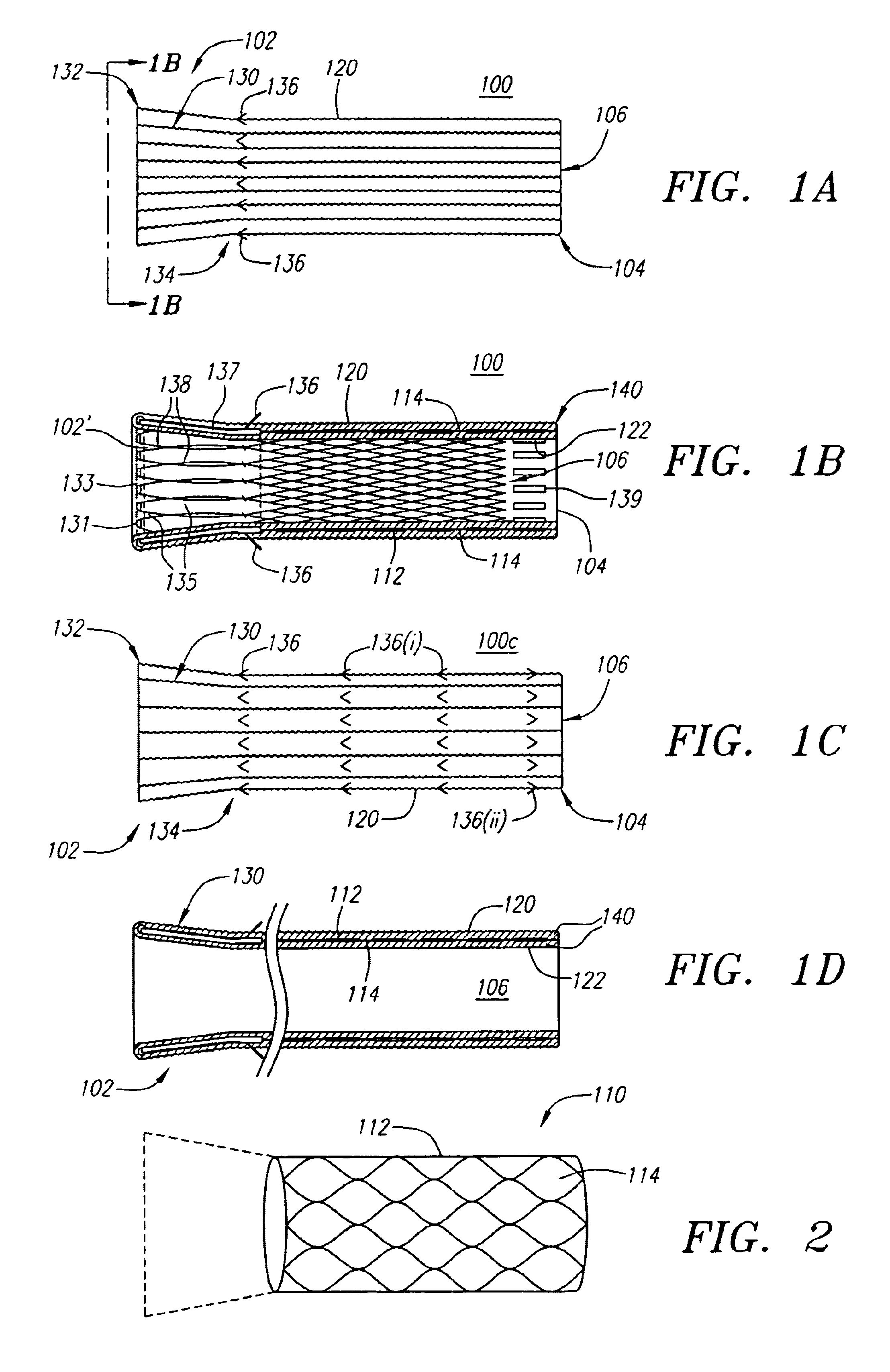
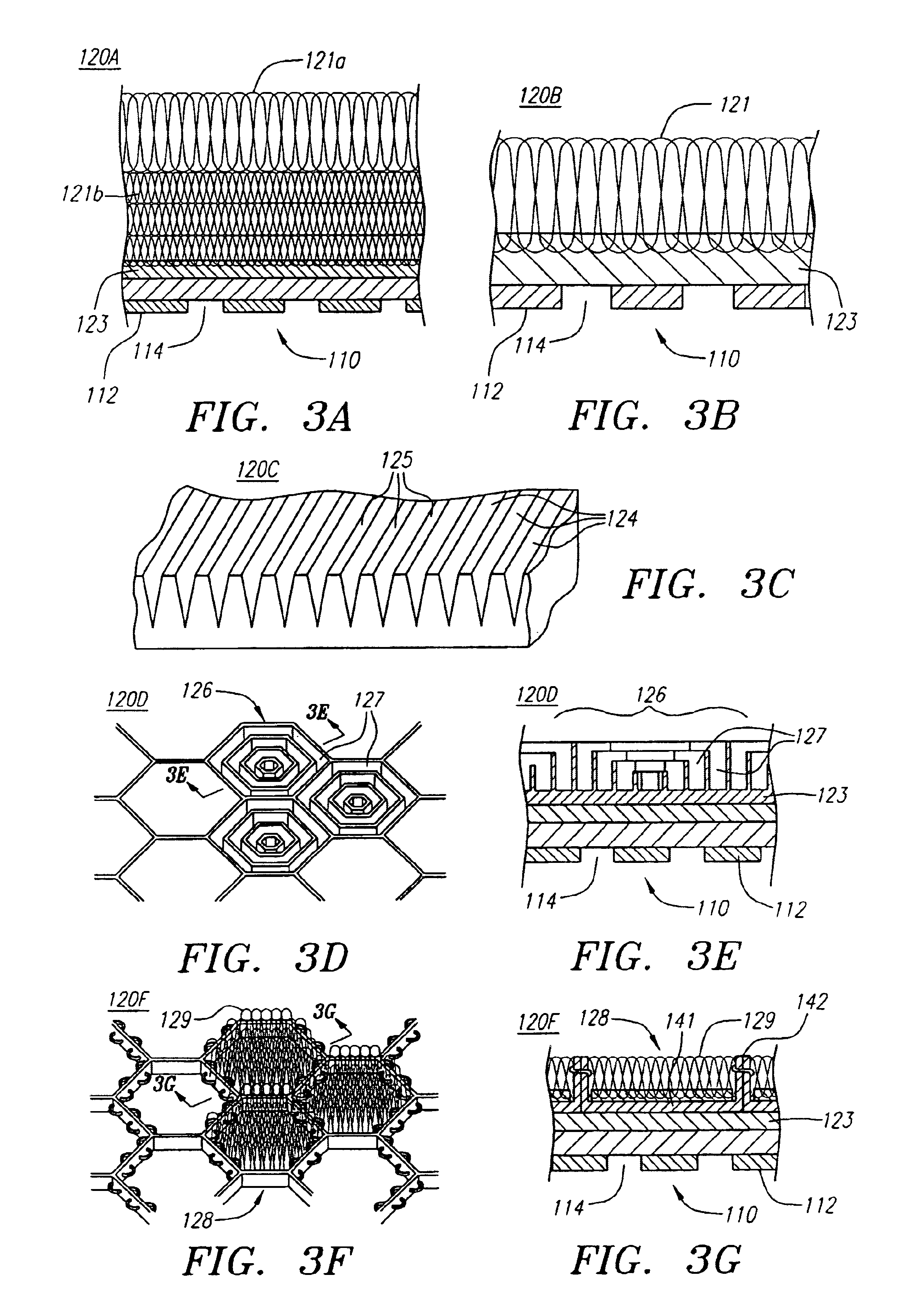
An intravascular stent having a prefabricated, patterned polymeric sleeve for controlled release of therapeutic drugs and for delivery of the therapeutic drugs in localized drug therapy in a blood vessel is disclosed. The polymeric sleeve is attached to at least a portion of an outside surface area of the stent structure. Alternatively, a plurality of individual microfilament strands are longitudinally attached to an outer surface of a stent structure in a spaced apart orientation and loaded with at least one therapeutic drug for the release thereof at a treatment site. The stent has a high degree of flexibility in the longitudinal direction, yet has adequate vessel wall coverage and radial strength sufficient to hold open an artery or other body lumen. Methods for making the same are also disclosed.
Owner:ABBOTT CARDIOVASCULAR
Drug-eluting stent and methods of making the same
Owner:ABBOTT CARDIOVASCULAR
Micromantled drug-eluting stent
InactiveUS20060195142A1Avoid complicationsEliminate direct impactSuture equipmentsStentsElectrospinningMedicine
Pharmacologically active, easy-to-deploy, biomechanically compatible, inflatable endovascular, drug-eluting stent are formed of a primary expandable polymeric or metallic construct, intimately mantled with a biomechanically compatible, polymeric microporous, microfibrous, compliant, stretchable fabric formed by direct electrospinning onto the outside surface of the primary construct using at least one polymer solution containing at least one active compound, selected from those expected to control key biological events leading to in-stent restenosis.
Owner:POLY MED
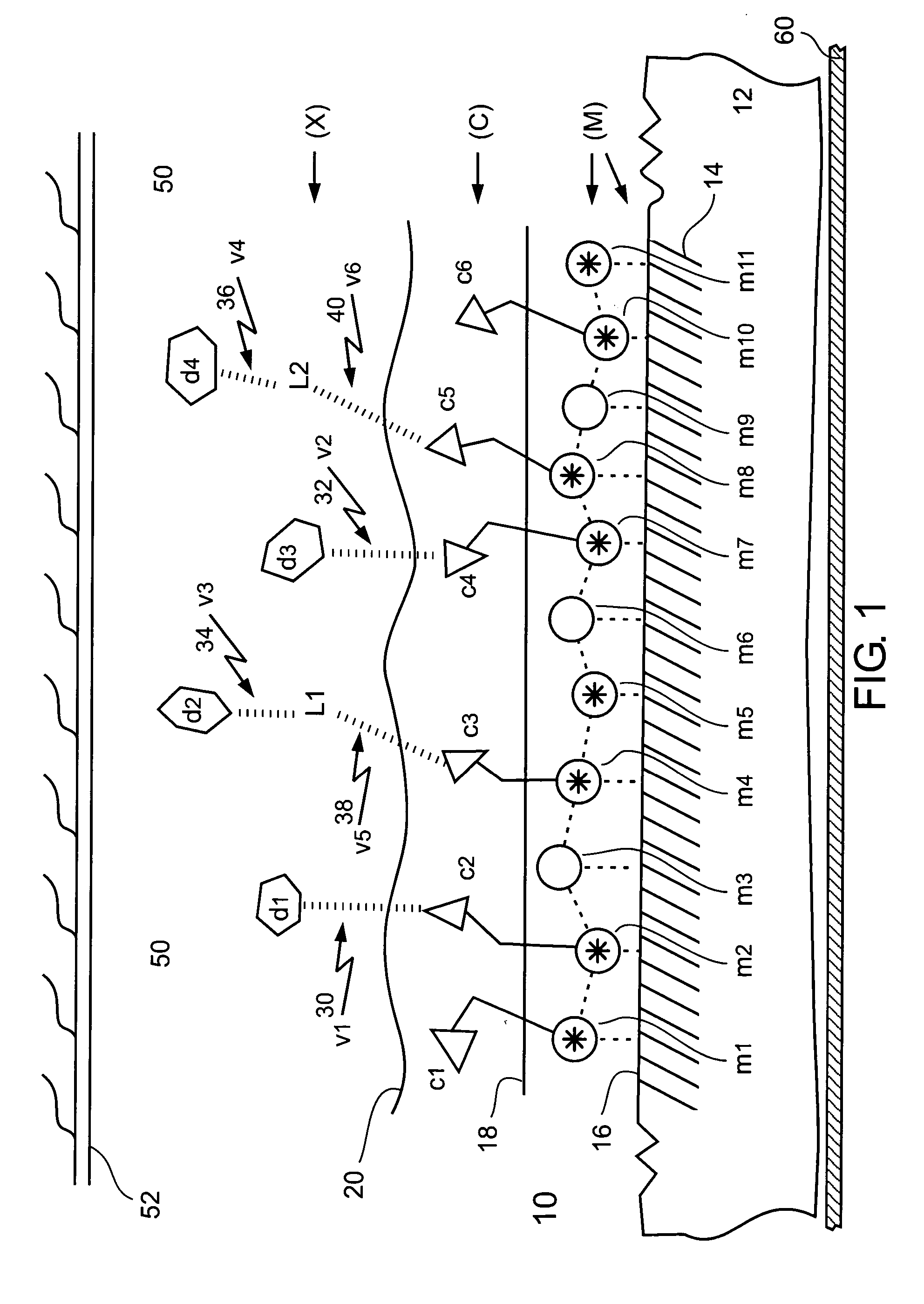
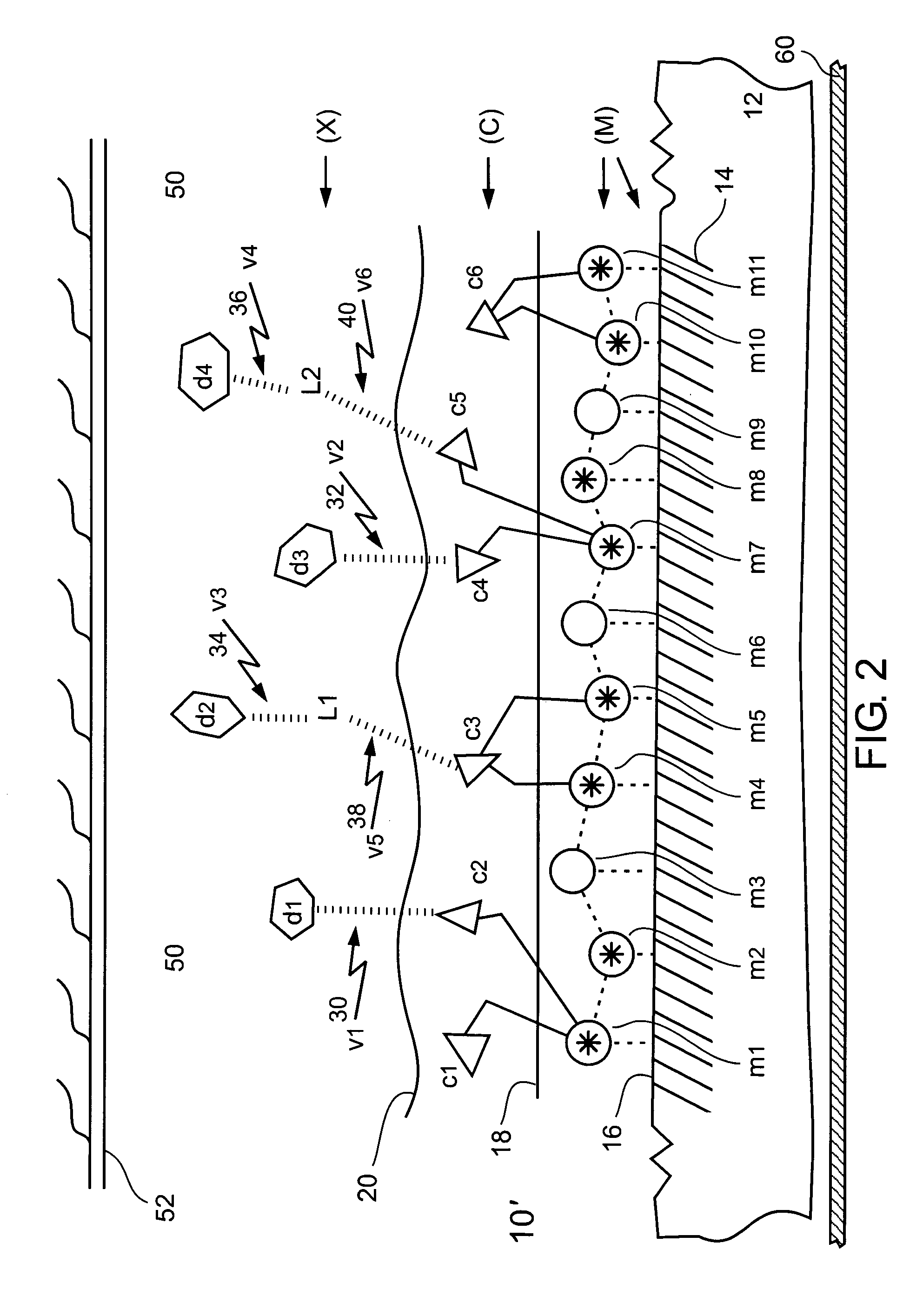
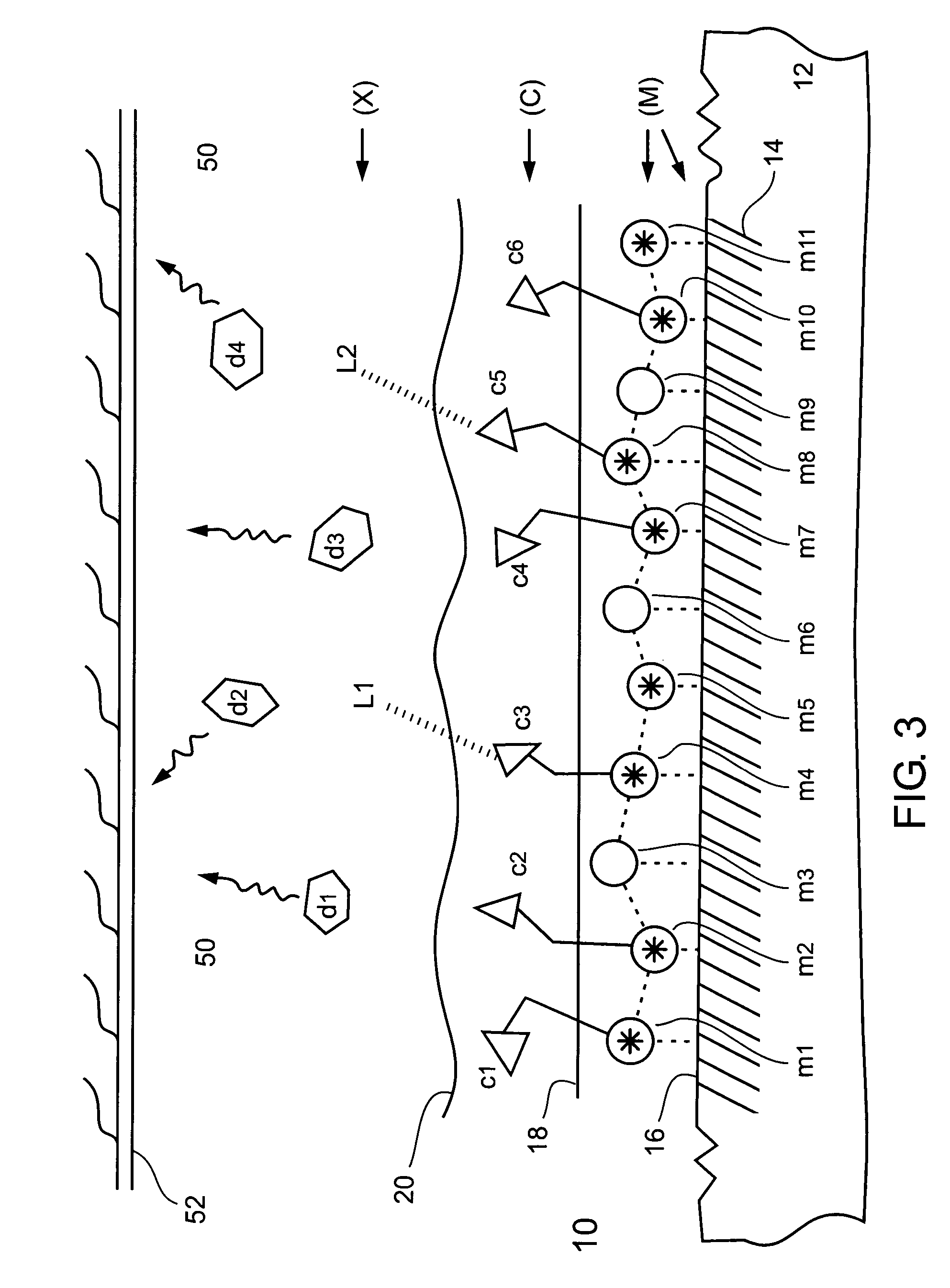
Chelating and binding chemicals to a medical implant, medical device formed, and therapeutic applications
Chelating and binding chemicals to a medical implant, and therapeutic applications. Implantable metal chelated surface and chemical coated medical implant device—drug (or biological moiety) coated or drug eluting stent, prosthesis, or other, includes a medical implant component having metal surface (M) with chemical entity (X) bound via chelator (C) chelated to the metal surface in an (M)-(C)-(X) configuration. Chelator or / and chemical entity—drug (or biological moiety), linker bonded to a drug (or biological moiety), other, are bound at surface concentration greater than 100 picograms per cm2. Manufacturing the implantable medical device. Medical implant system including medical implant component and delivery device for delivering and implanting medical implant component in a subject. Implanting the medical device. Preventing or / and treating medical conditions, such as restenosis or / and thrombosis, by implanting the medical device, wherein activity of bound chemical entity exhibits efficacy towards the medical condition.
Owner:STENTOMICS INC
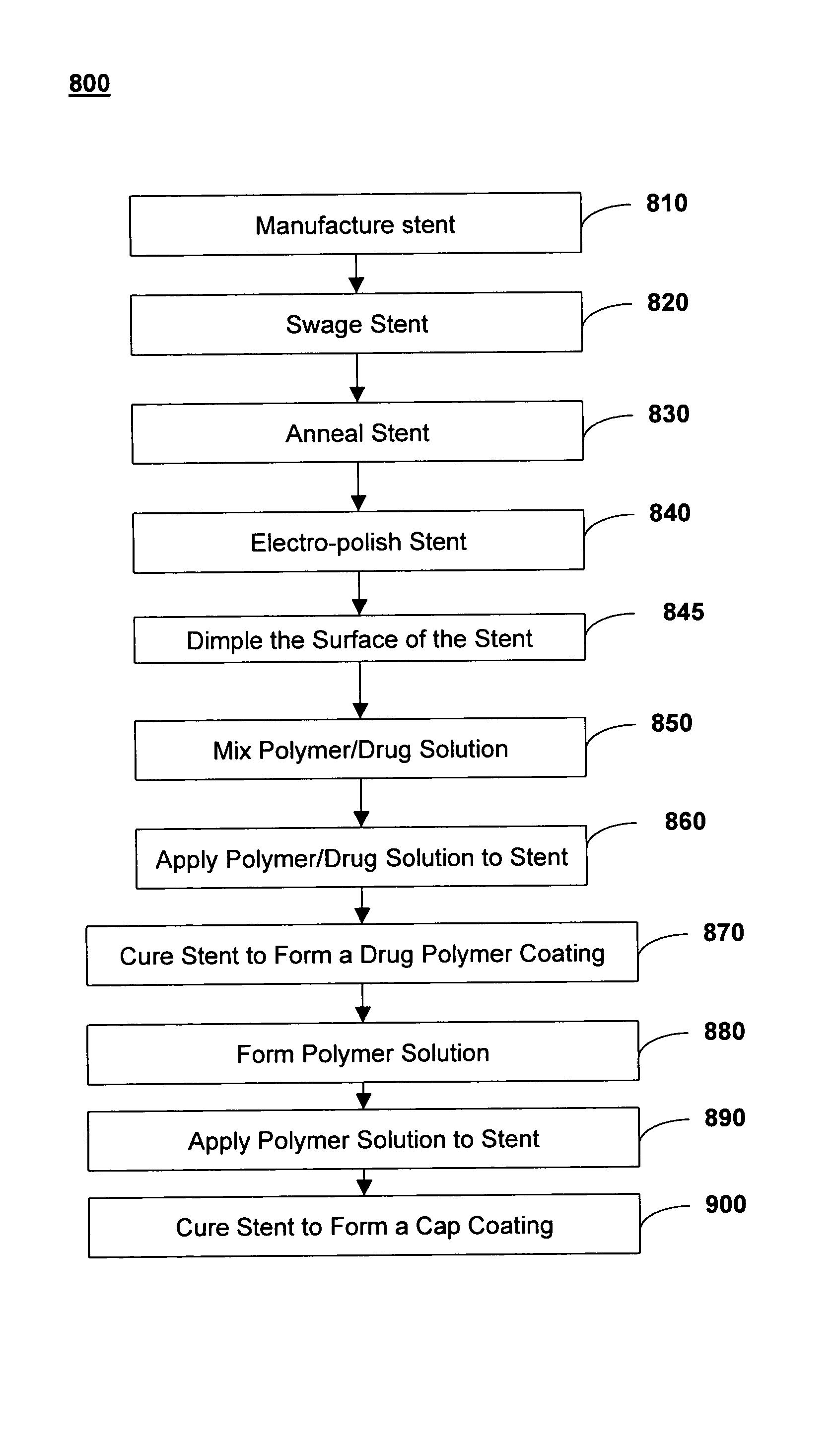
Method of forming a drug eluting stent
The present invention provides a method of forming a drug eluting stent, the method comprising coupling a stent framework to a mandrel, inserting the mandrel with stent framework into an open die, the die including a forming surface including a plurality of raised indention forming portions; closing the die against the stent framework; pressing the raised indention portions into the stent framework to form indentions in the stent framework; and inserting at least one drug polymer into the indentions formed in the stent framework.
Owner:MEDTRONIC VASCULAR INC
Stent covered by a layer having a layer opening
InactiveUS20050228482A1Modulate vascular toneEnhance macrophage-mediated microbial killingStentsBlood vesselsThree vesselsBiomedical engineering
A drug eluting stent has a frame with at least one frame opening, and the frame is covered with an outer continuous layer and / or an inner continuous layer. The outer and / or inner layers further include a layer opening that at least partially overlaps with the frame opening to form a continuous opening having a size that allows endothelialization when the stent is implanted into a blood vessel.
Owner:HERZOG WILLIAM +2
Apparatus and Methods for Loading a Drug Eluting Medical Device
Methods and apparatus are disclosed for loading a therapeutic substance or drug within a lumenal space of a hollow wire having a plurality of side openings along a length thereof that forms a hollow drug-eluting stent with a plurality of side drug delivery openings. Loading a drug within the lumenal space of the hollow stent includes a drug filling step, in which the drug is mixed with a solvent or dispersion medium. The lumenal space may be filled with the drug solution or suspension in a reverse fill process and / or a forward fill process. After the drug filling step, a solvent or dispersion medium extracting step is performed to extract the solvent or dispersion medium from within the lumenal space such that only the drug remains within the hollow stent. A stent cleaning step may be performed to an exterior surface of the hollow stent.
Owner:MEDTRONIC VASCULAR INC
Textured and drug eluting coronary artery stent
InactiveUS7041127B2Enhances the atraumatic character of the proximal end of the collarImprove abilitiesStentsBlood vesselsSurface layerStent grafting
The present invention provides for reinforced and drug eluting stent-grafts and related methods of implanting and manufacturing the stent-grafts. A stent-graft of the present invention may include a tubular stent, a biocompatible covering surrounding the stent, and a supporting collar coupled to the proximal end of the stent-graft. A drug agent may be applied to a textured external surface layer of the biocompatible covering, or alternatively to a space between the textured external surface layer and a smooth luminal surface layer of the biocompatible covering, and allowed to elute over time into a wall of a body lumen after the stent-graft is deployed. The collar of the stent-graft absorbs pressure exerted on the stent-graft by fluid flow within the body lumen in order to minimize potential damage to the stent-graft, and may also include barbs to further secure the stent-graft to the body lumen.
Owner:LEDERGERBER WALTER J
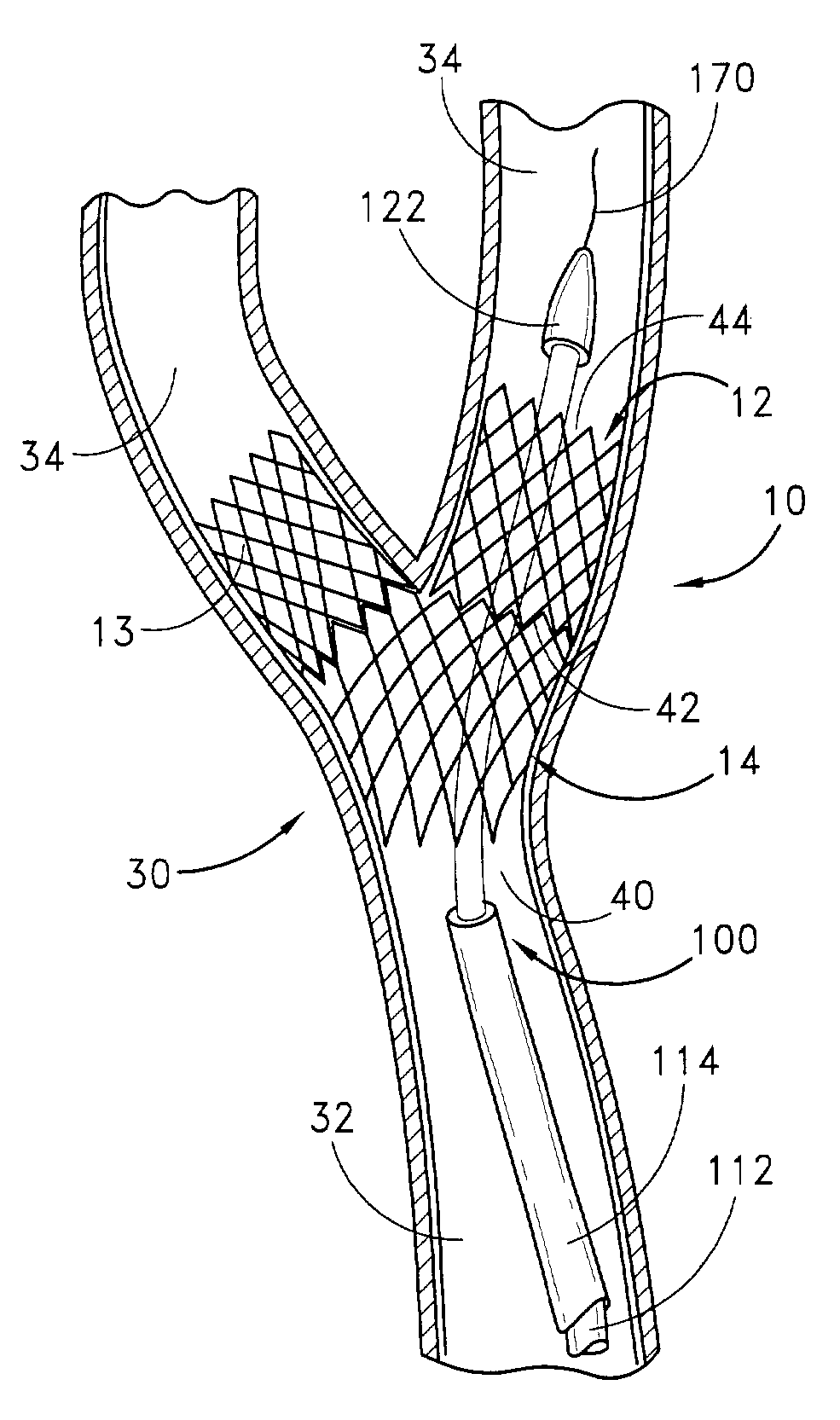
Noncylindrical drug eluting stent for treating vascular bifurcations
A device and method for treating pathological narrowing of fluid-carrying conduits of the human body (such as blood vessels) in an area of a bifurcation is disclosed. In particular, a stent delivery system configured to carry one or more of a pair of dissimilar stents. At least one of the stents is particularly suited for treating a widened portion of a blood vessel immediately proximal to a bifurcation. The stent delivery system can also include a handpiece adapted to selectively deliver the stents.
Owner:BIOSENSORS INT GROUP
Geometry and material for use in high strength, high flexibility, controlled recoil drug eluting stents
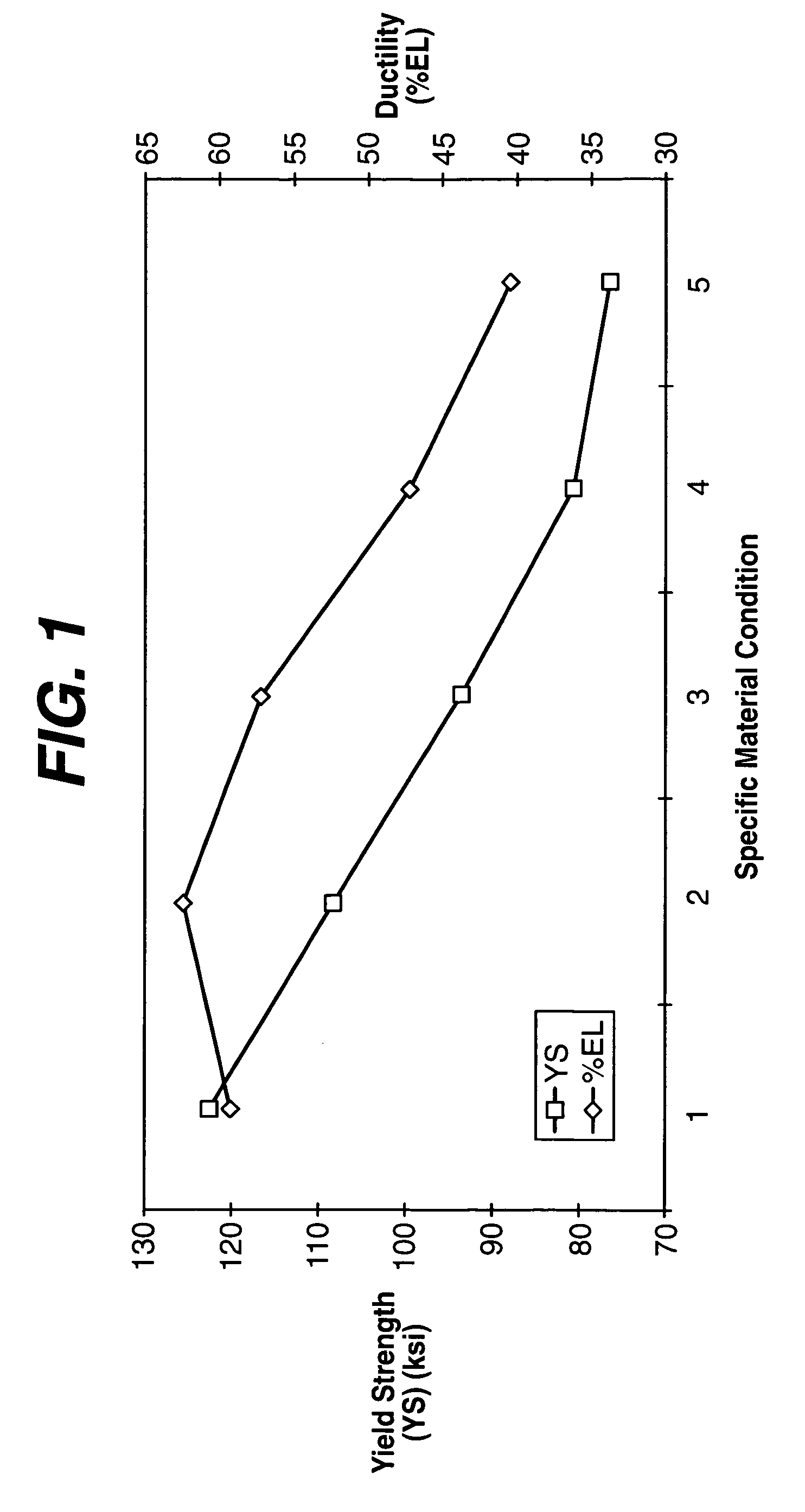
InactiveUS20060200229A1Less brittleImprove ductilityStentsSurgeryMetallic materialsMedical treatment
A biocompatible material may be configured into any number of implantable medical devices including intraluminal stents. The biocompatible material may comprise metallic and non-metallic materials. These materials may be designed with a microstructure that facilitates or enables the design of devices with a wide range of geometries adaptable to various loading conditions. Both the load bearing elements and the substantially non-load bearing elements may utilize these materials. Additionally, therapeutic agents may be incorporated into the microstructure or the bulk material.
Owner:CORDIS CORP
Medical devices and EFAB methods and apparatus for producing them
Various embodiments of the invention present miniature medical devices that may be formed totally or in part using electrochemical fabrication techniques. Sample medical devices include micro-tweezers or forceps, internally expandable stents, bifurcated or side branch stents, drug eluting stents, micro-valves and pumps, rotary ablation devices, electrical ablation devices (e.g. RF devices), micro-staplers, ultrasound catheters, and fluid filters. In some embodiments devices may be made out of a metal material while in other embodiments they may be made from a material (e.g. a polymer) that is molded from an electrochemically fabricated mold. Structural materials may include gold, platinum, silver, stainless steel, titanium or pyrolytic carbon-coated materials such as nickel, copper, and the like.
Owner:MICROFAB
Biodegradable Metal Barrier Layer for a Drug-Eluting Stent
InactiveUS20080243240A1Reduce and prevent cell proliferationReduce and prevent and inflammationStentsSurgeryAnti mitoticMedical treatment
An implantable medical device includes a substrate, a drug-impregnated layer deposited over the substrate, and a barrier layer at least partially covering the drug-impregnated layer. The barrier layer may be a biodegradable metal, biodegradable metal oxide, or biodegradable metal alloy, such as, magnesium, a magnesium oxide or a magnesium alloy. The drug-impregnated layer includes a therapeutic substance, such as, antineoplastic, anti-inflammatory, antiplatelet, anticoagulant, fibrinolytic, thrombin inhibitor, antimitotic, antiallergic, and antiproliferative substances.
Owner:MEDTRONIC VASCULAR INC
Methods for forming and fabricating textured and drug eluting coronary artery stent
InactiveUS7273493B2Enhances the atraumatic character of the proximal end of the collarImprove abilitiesStentsPretreated surfacesSurface layerStent grafting
The present invention provides for reinforced and drug eluting stent-grafts and related methods of implanting and manufacturing the stent-grafts. A stent-graft of the present invention may include a tubular stent, a biocompatible covering surrounding the stent, and a supporting collar coupled to the proximal end of the stent-graft. A drug agent may be applied to a textured external surface layer of the biocompatible covering, or alternatively to a space between the textured external surface layer and a smooth luminal surface layer of the biocompatible covering, and allowed to elute over time into a wall of a body lumen after the stent-graft is deployed. The collar of the stent-graft absorbs pressure exerted on the stent-graft by fluid flow within the body lumen in order to minimize potential damage to the stent-graft, and may also include barbs to further secure the stent-graft to the body lumen.
Owner:LEDERGERBER WALTER J
Micromantled drug-eluting stent
InactiveUS7416559B2Avoid complicationsEliminate direct impactSuture equipmentsStentsElectrospinningMedicine
Owner:POLY MED
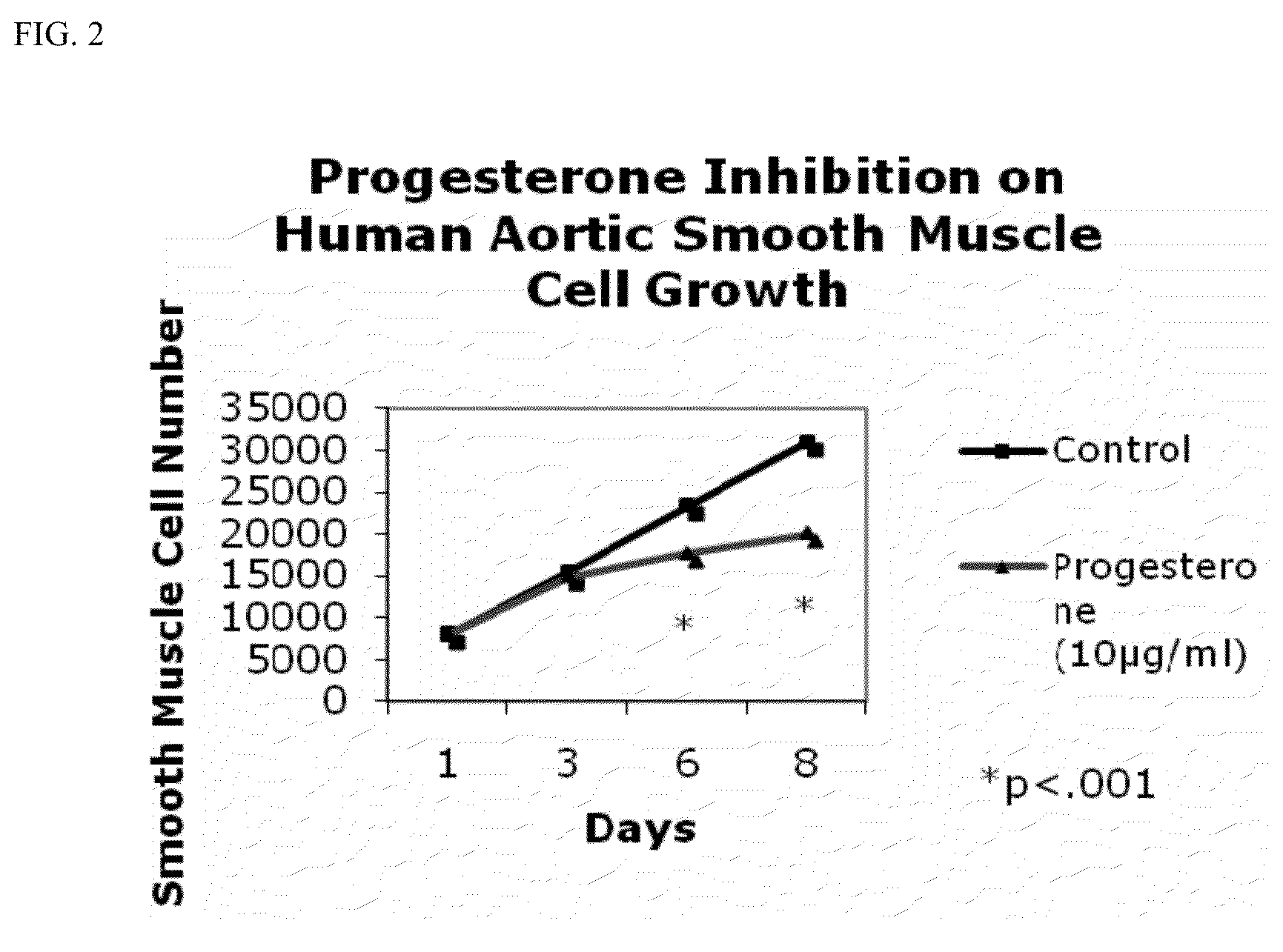
Progesterone-containing compositions and devices
InactiveUS20130245570A1Undesirable growthBiocideOrganic active ingredientsIntimal proliferationPercent Diameter Stenosis
Progesterone-containing compositions and devices that can maintain opening of a body passageway are described. One aspect of the invention provides a therapeutically effective (e.g., relaxative, anti-oxidative, anti-restenotic, anti-angiogenic, anti-neoplastic, anti-cancerous, anti-precancerous and / or anti-thrombotic) composition or formulation containing progesterone and optionally vitamin E and / or conjugated linoleic acid. Another aspect of the invention provides a drug eluting device, such as a drug eluting stent, with at least one coating layer comprising a progesterone composition that can minimize or eliminate inflammation, thrombosis, restenosis, neo-intimal hyperplasia, rupturing of vulnerable plaque, and / or other effects related to device implantation, treatment, or interaction. Other aspects of the invention provide for methods of using such compositions, formulations, and devices.
Owner:JACKSON GREGG A
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com