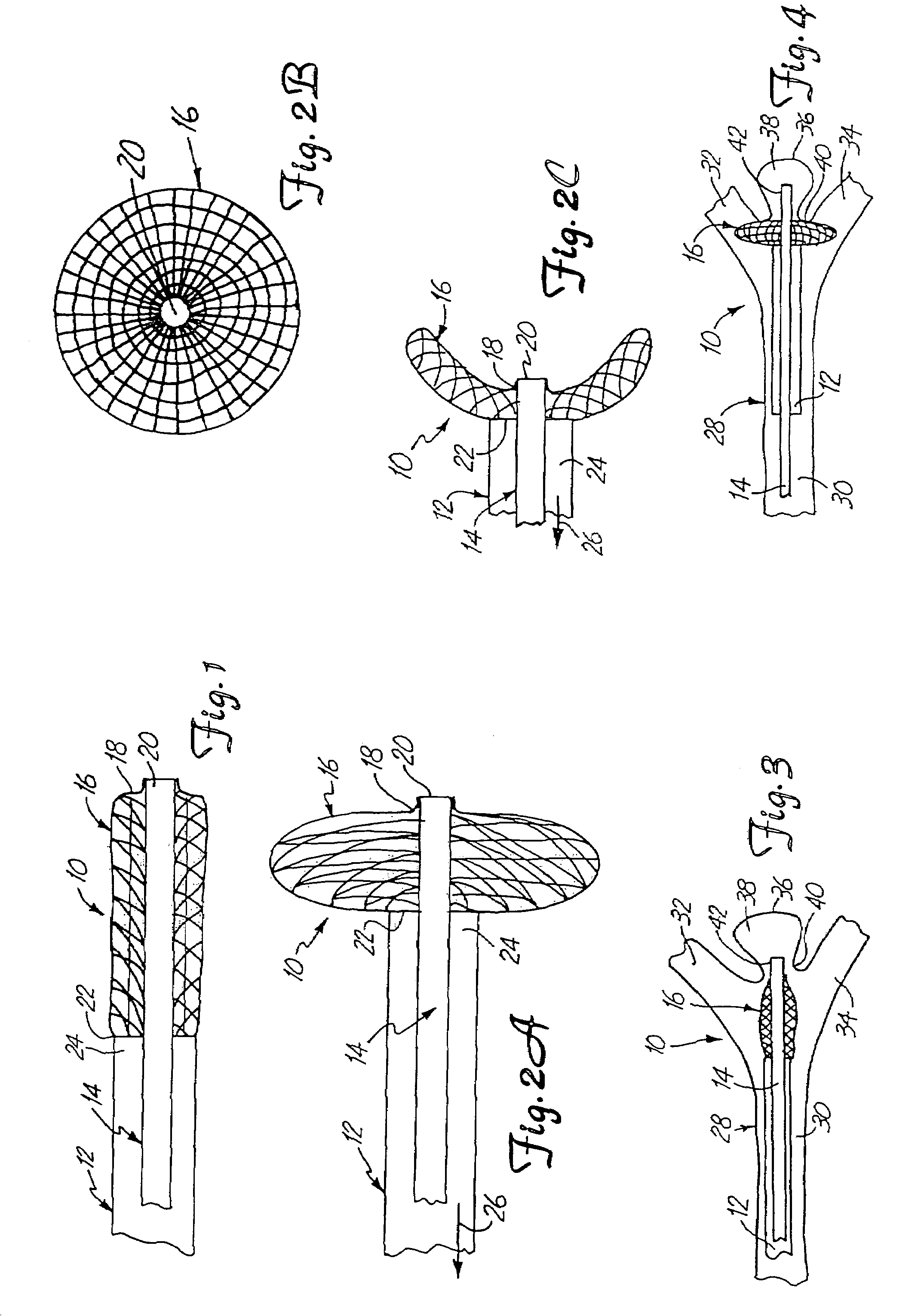
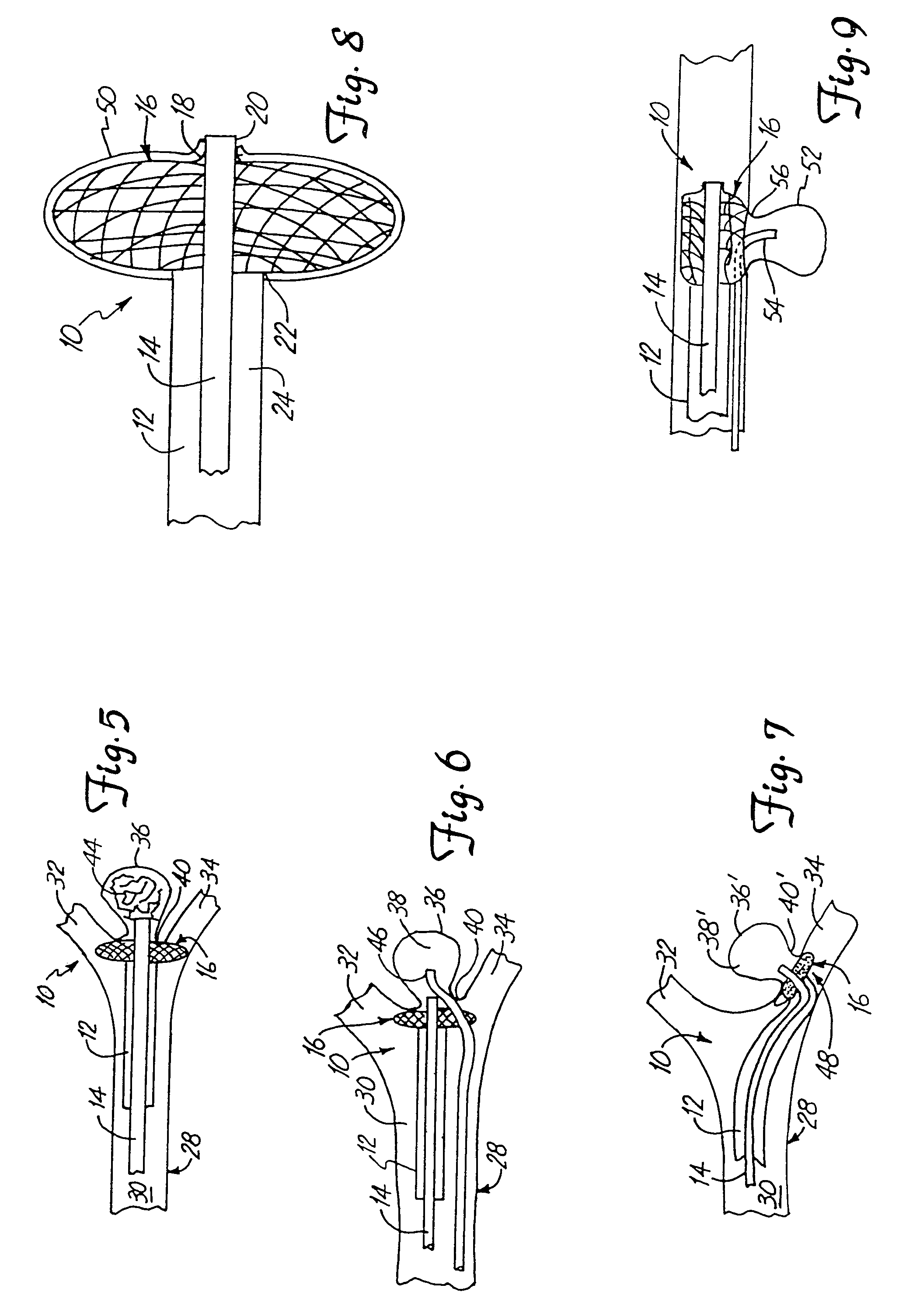
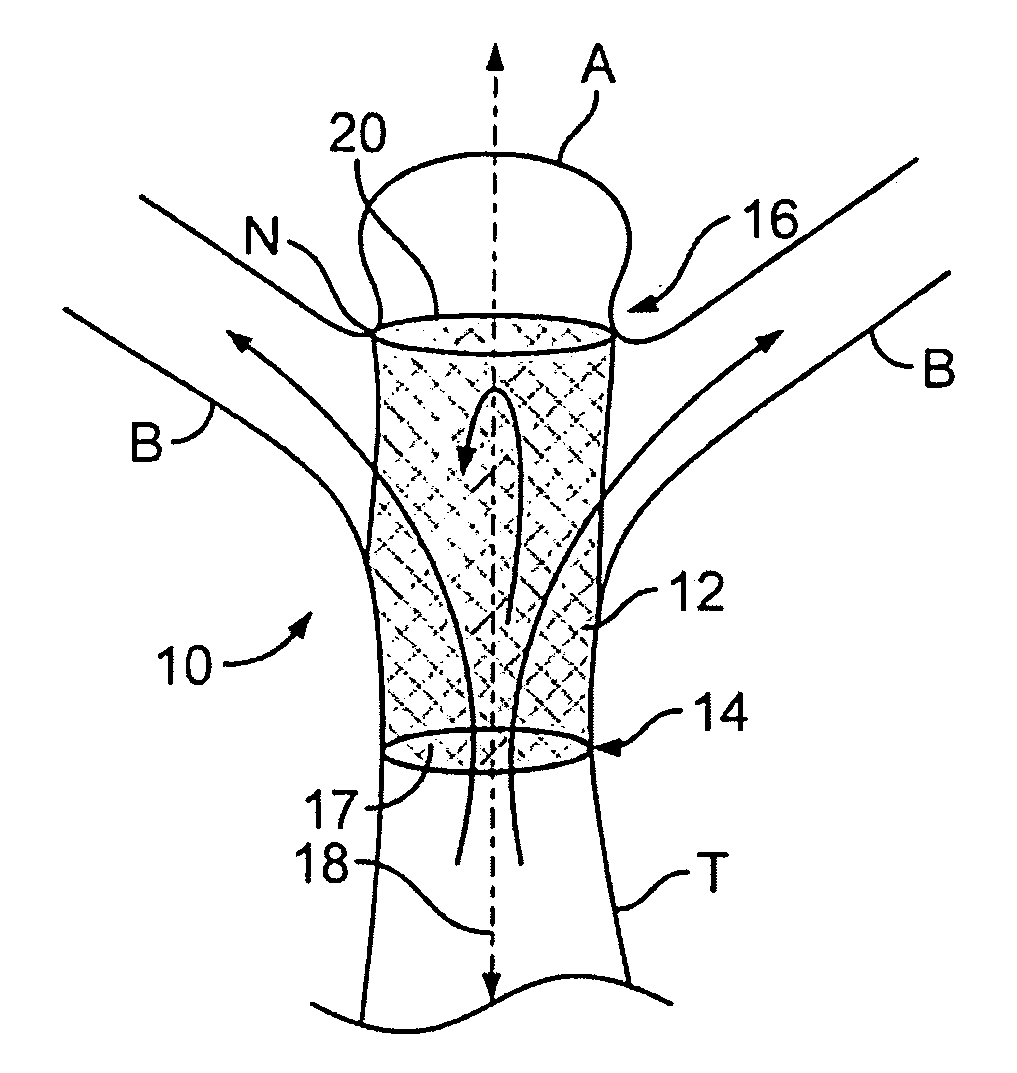
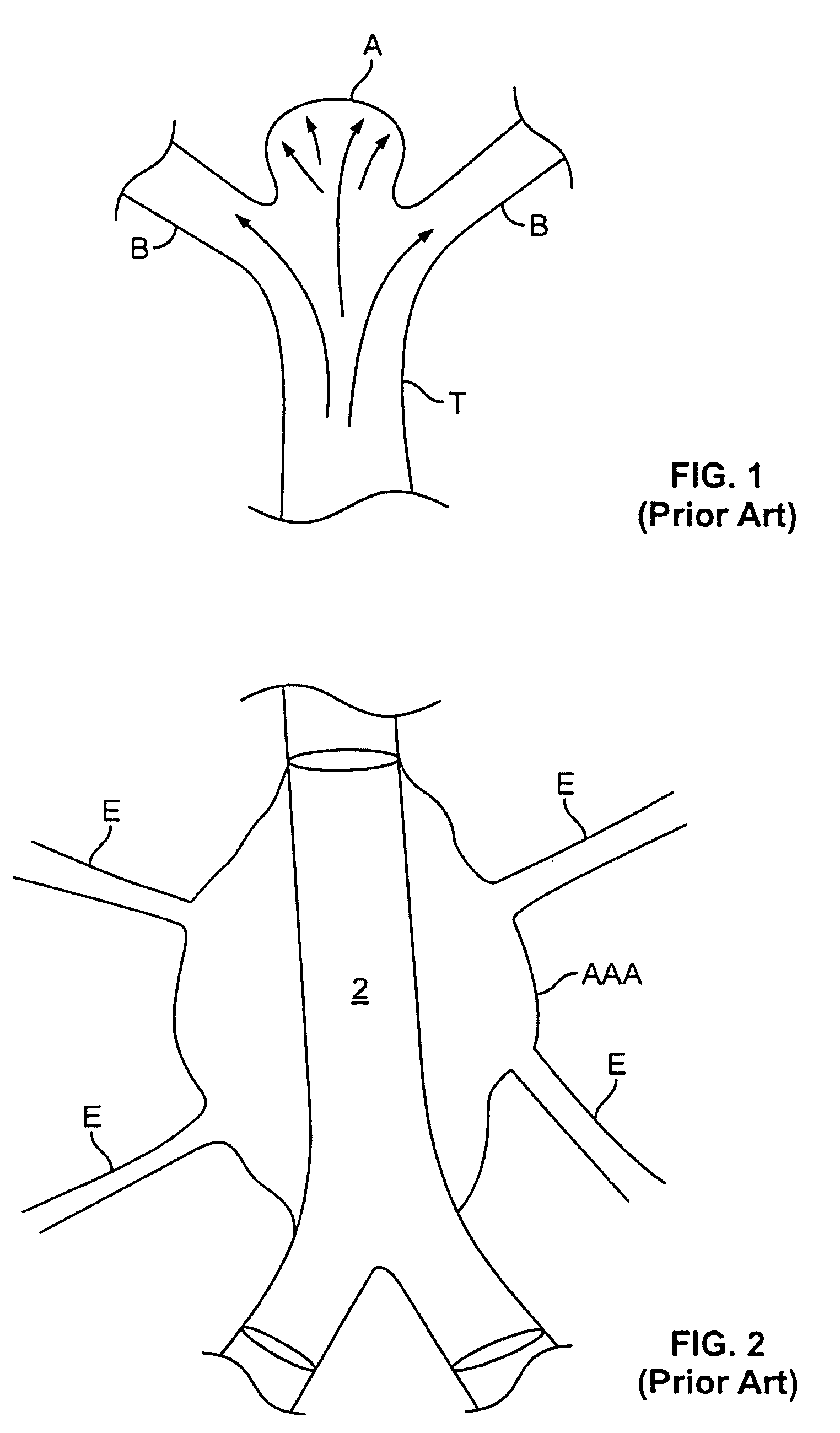
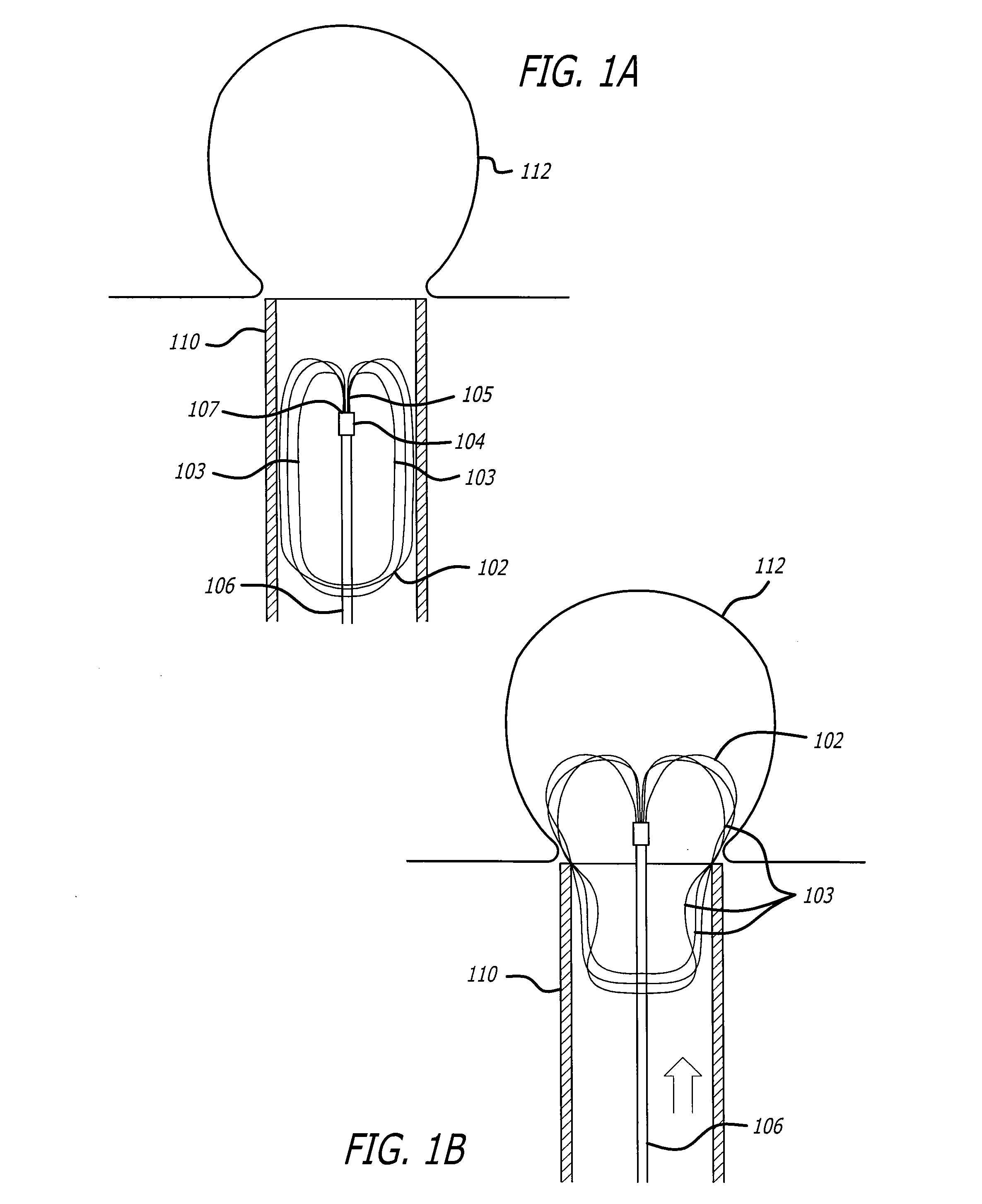
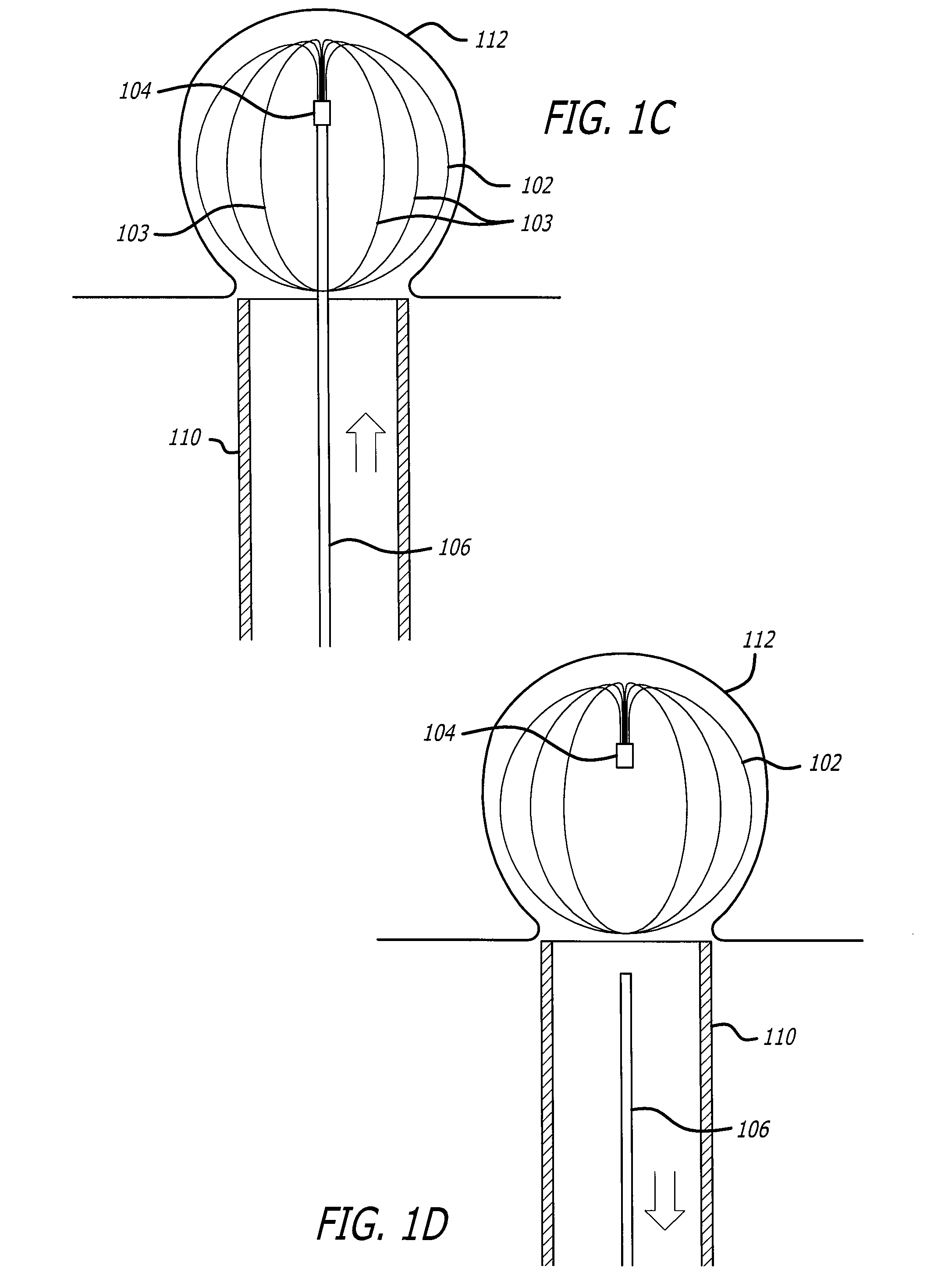
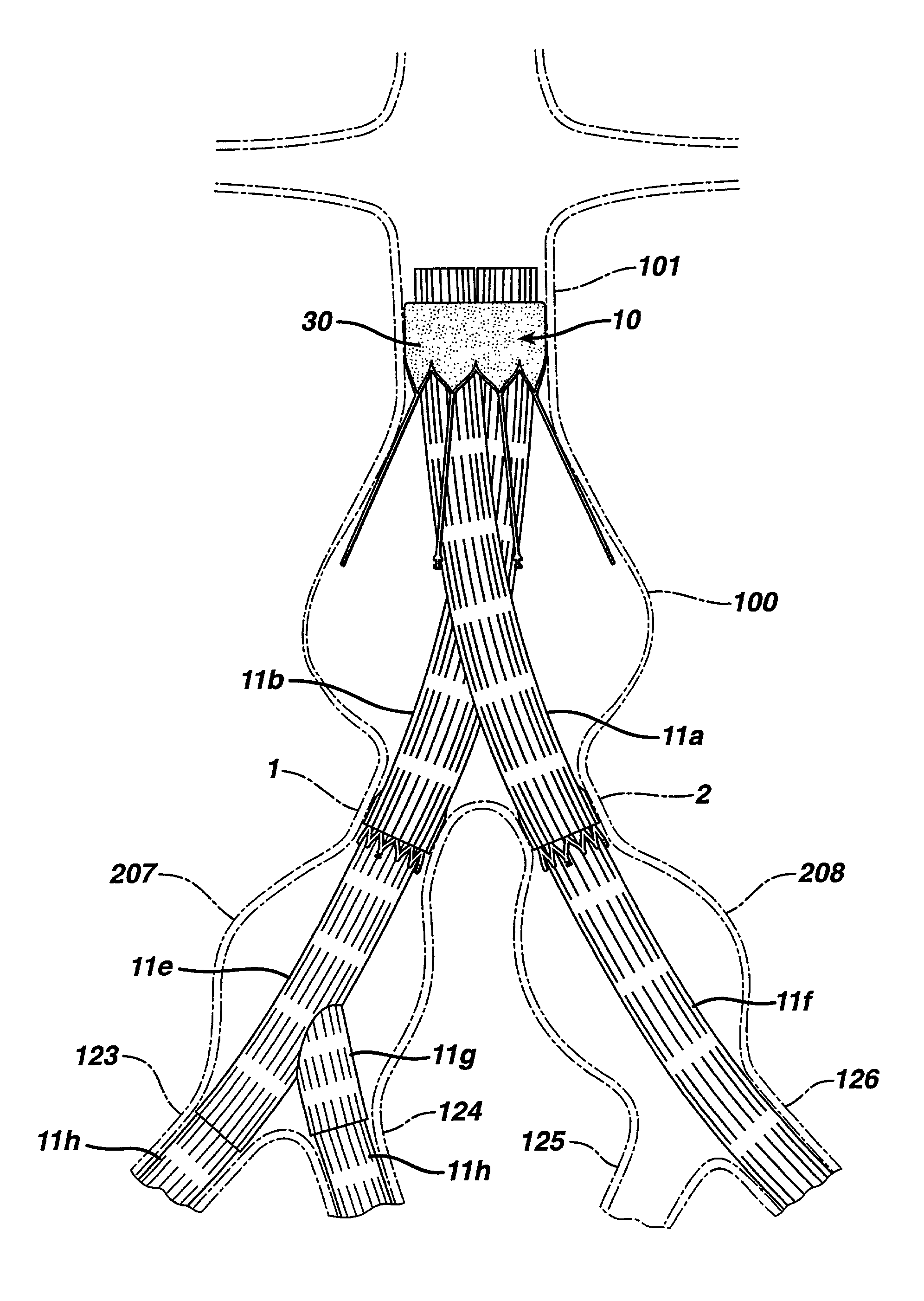
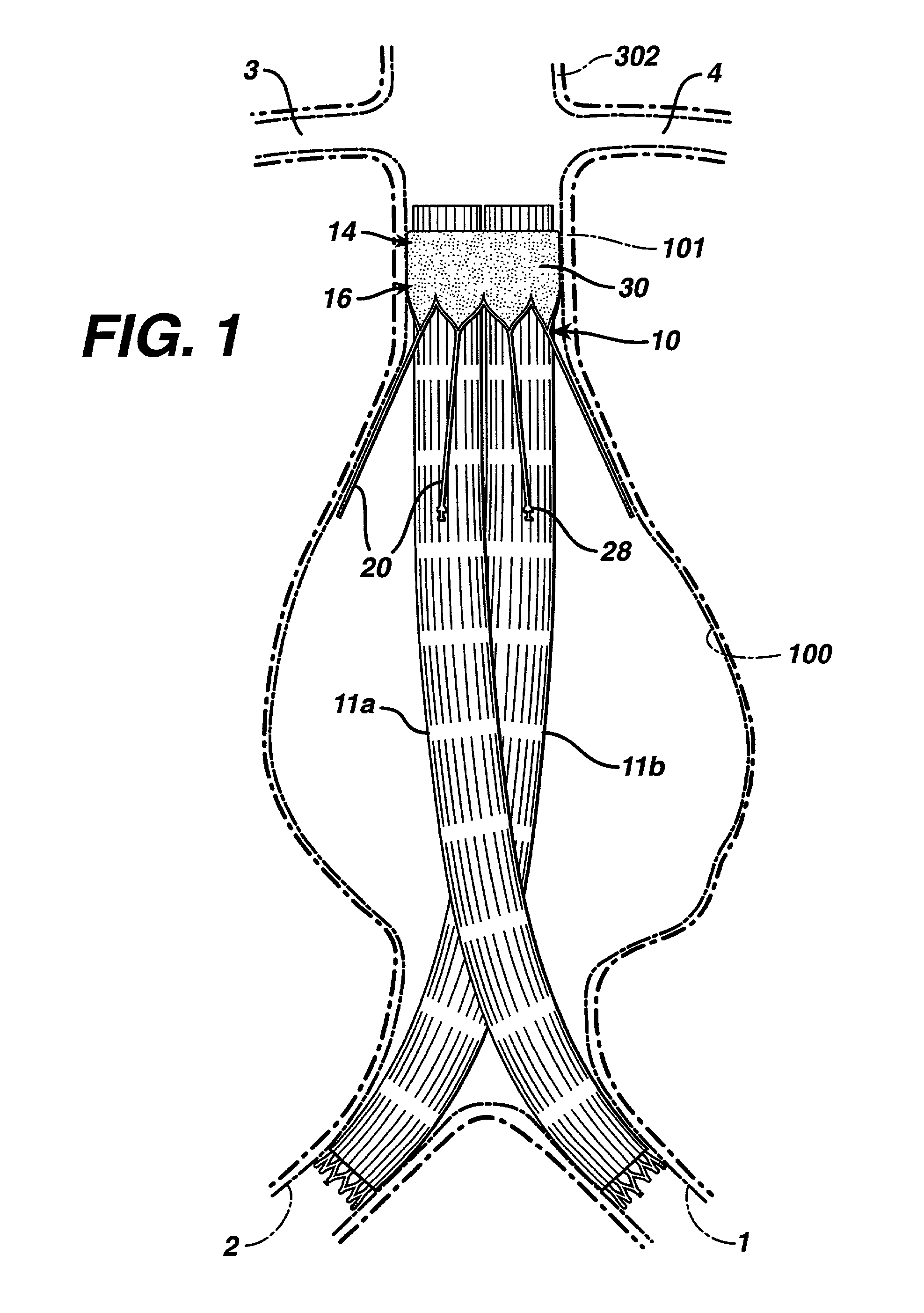
Patents
Literature
713 results about "Anterior Cerebral Artery Aneurysm" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Cerebral aneurysms, also known as intracranial or brain aneurysms, occur most commonly in the anterior cerebral artery, which is part of the circle of Willis. This can cause severe strokes leading to death. The next most common sites of cerebral aneurysm occurrence are in the internal carotid artery.
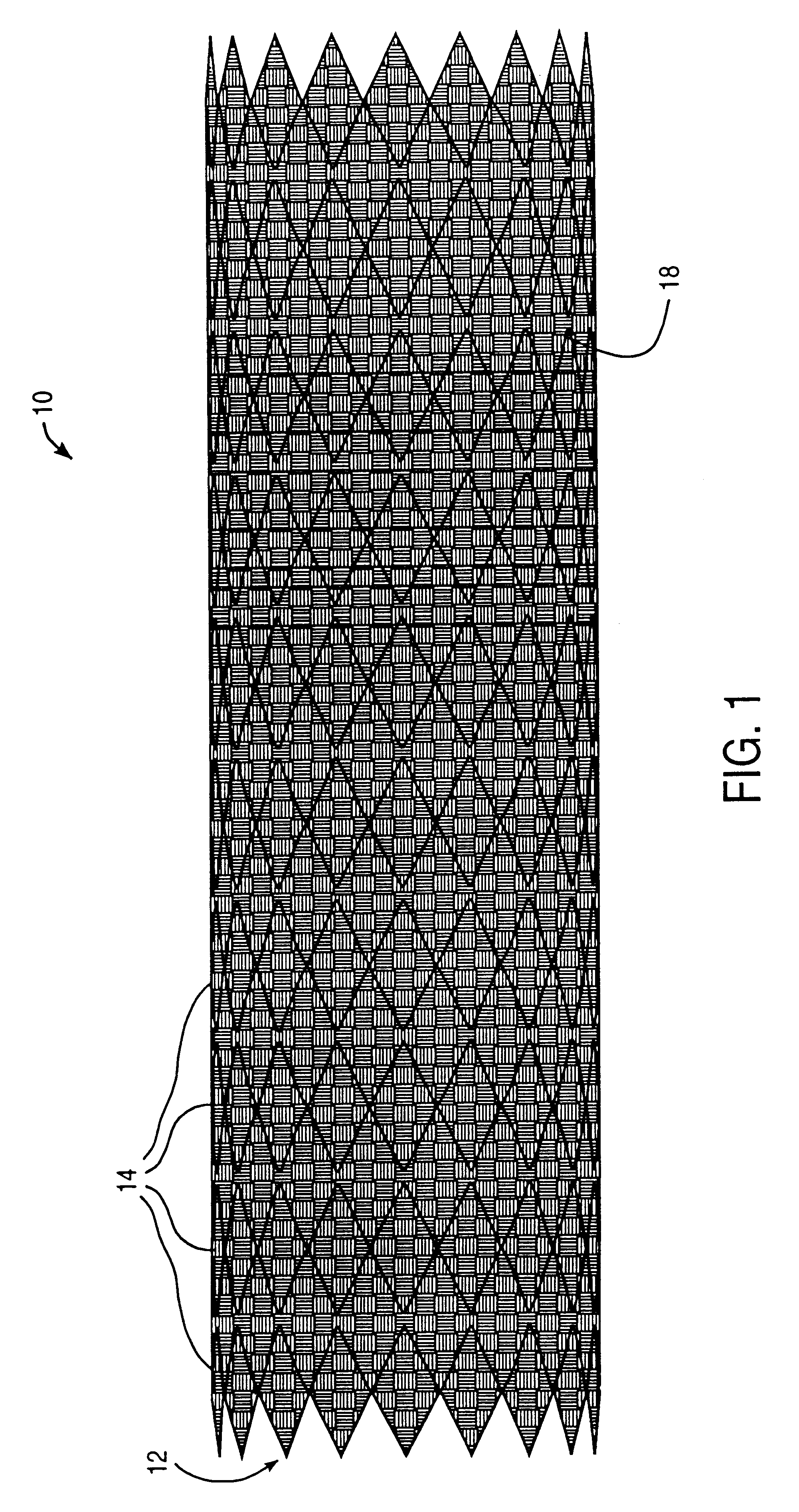
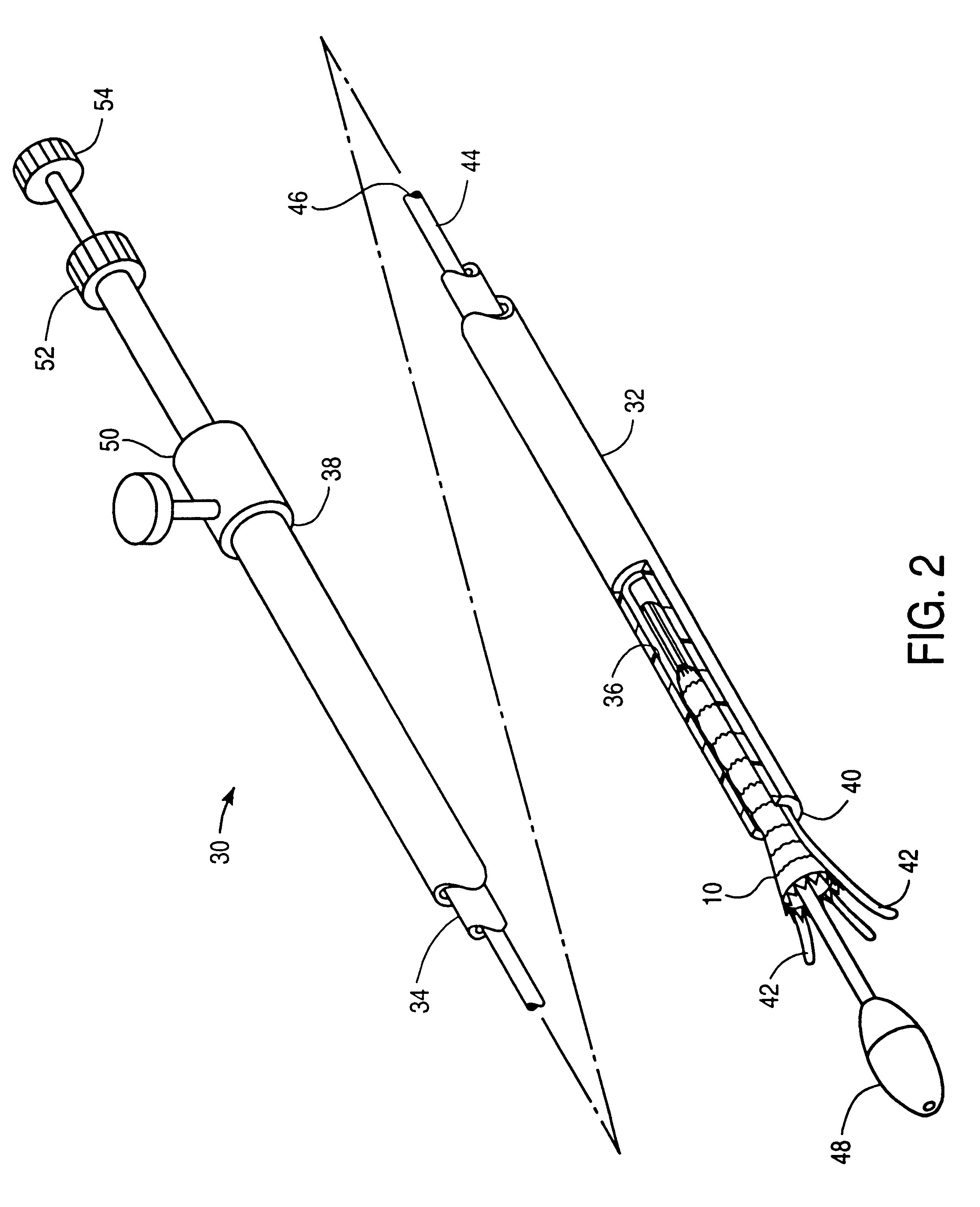
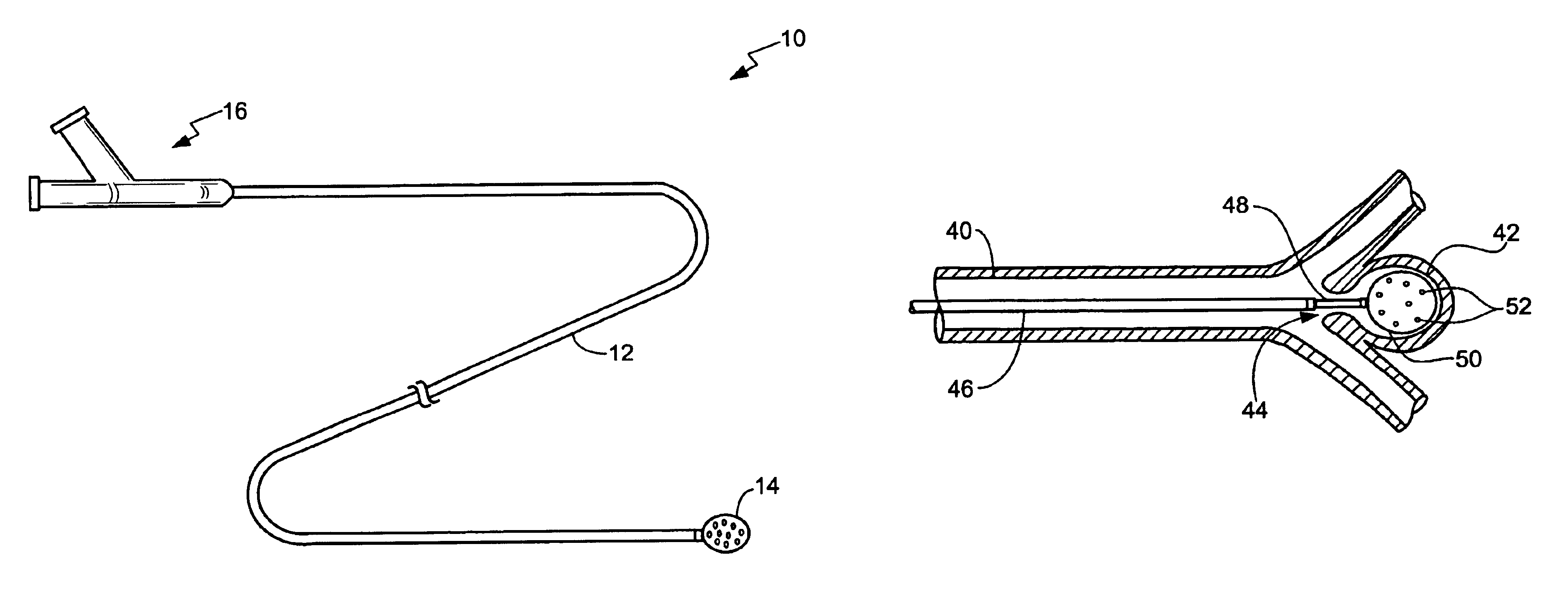
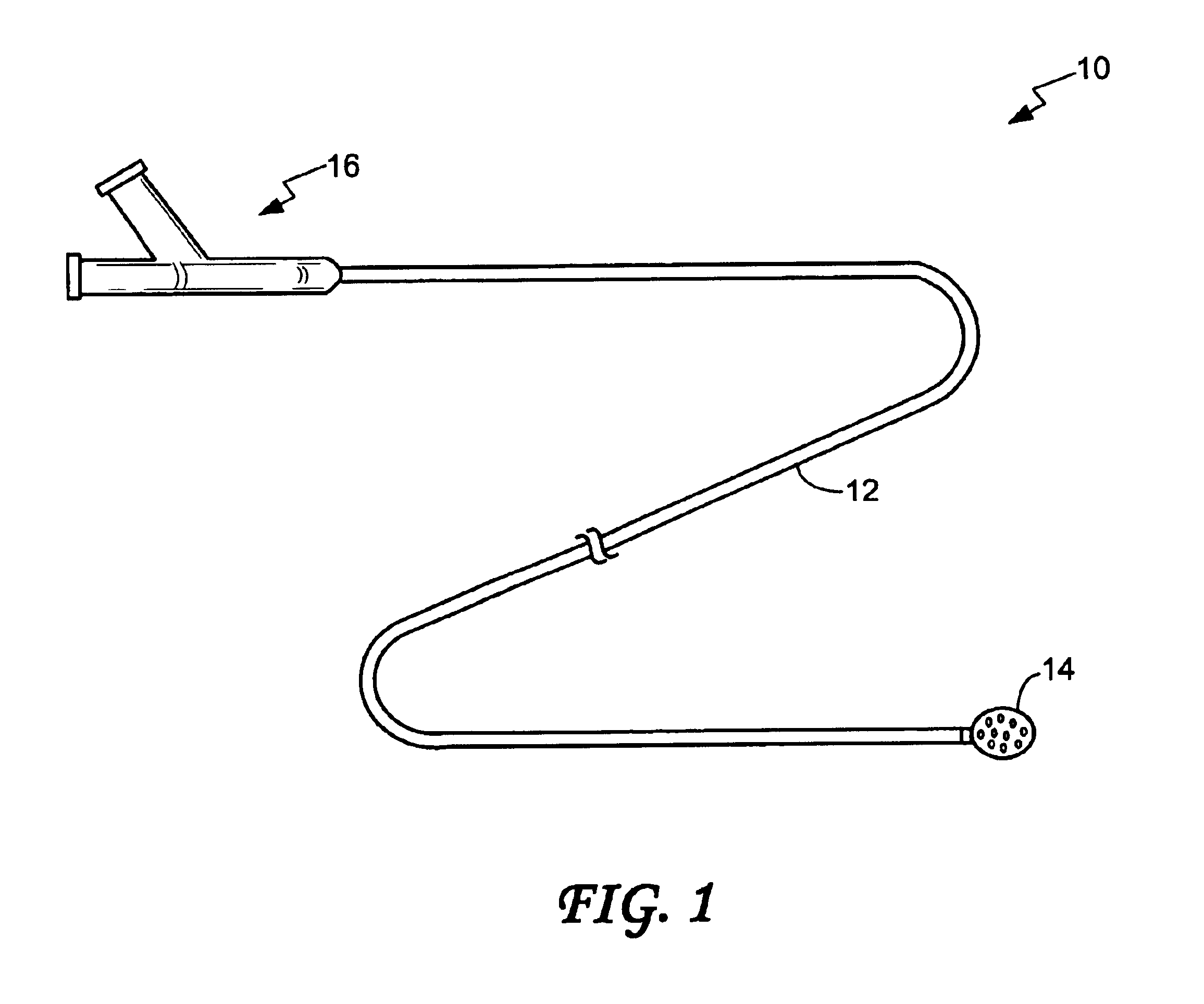
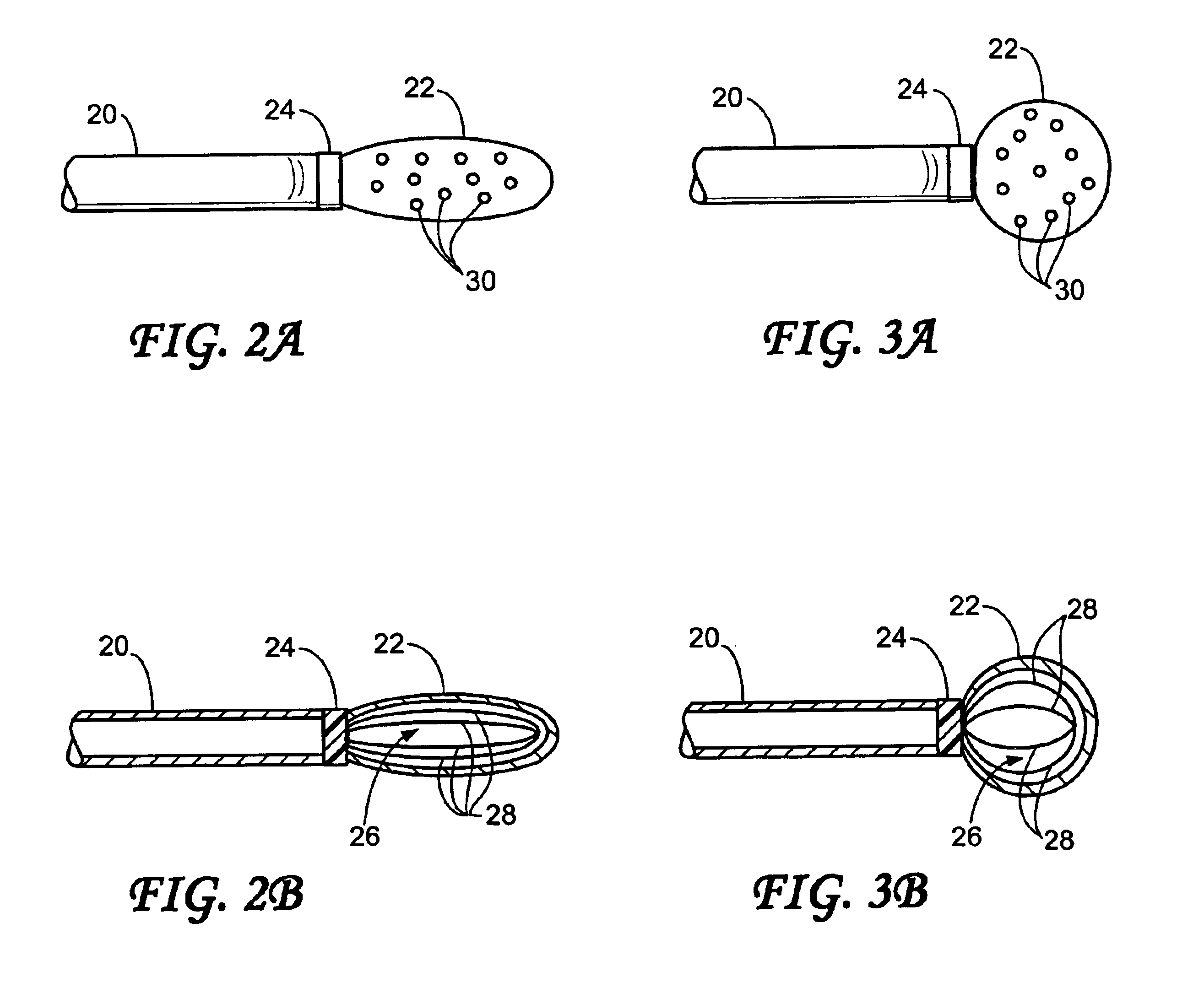
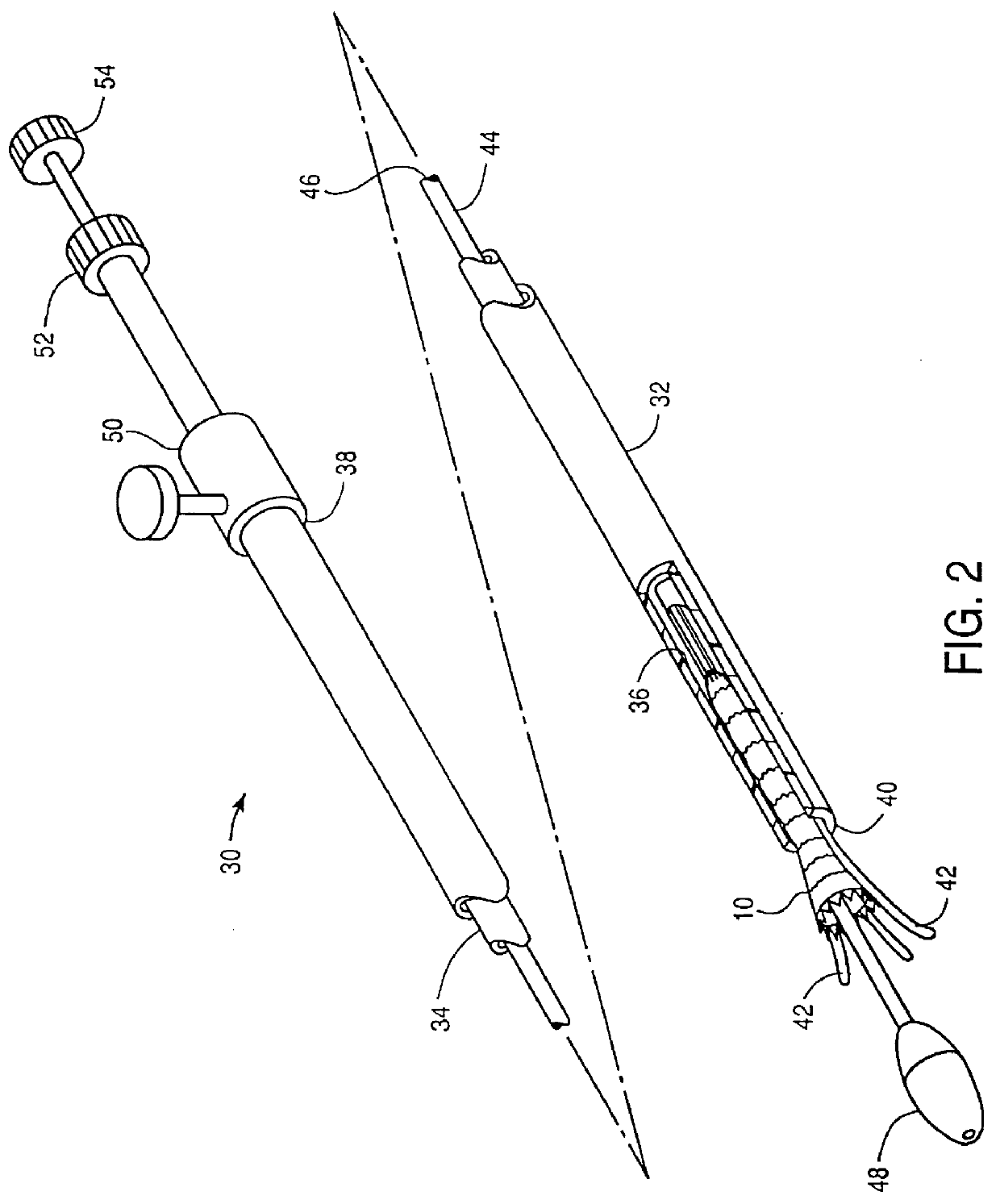
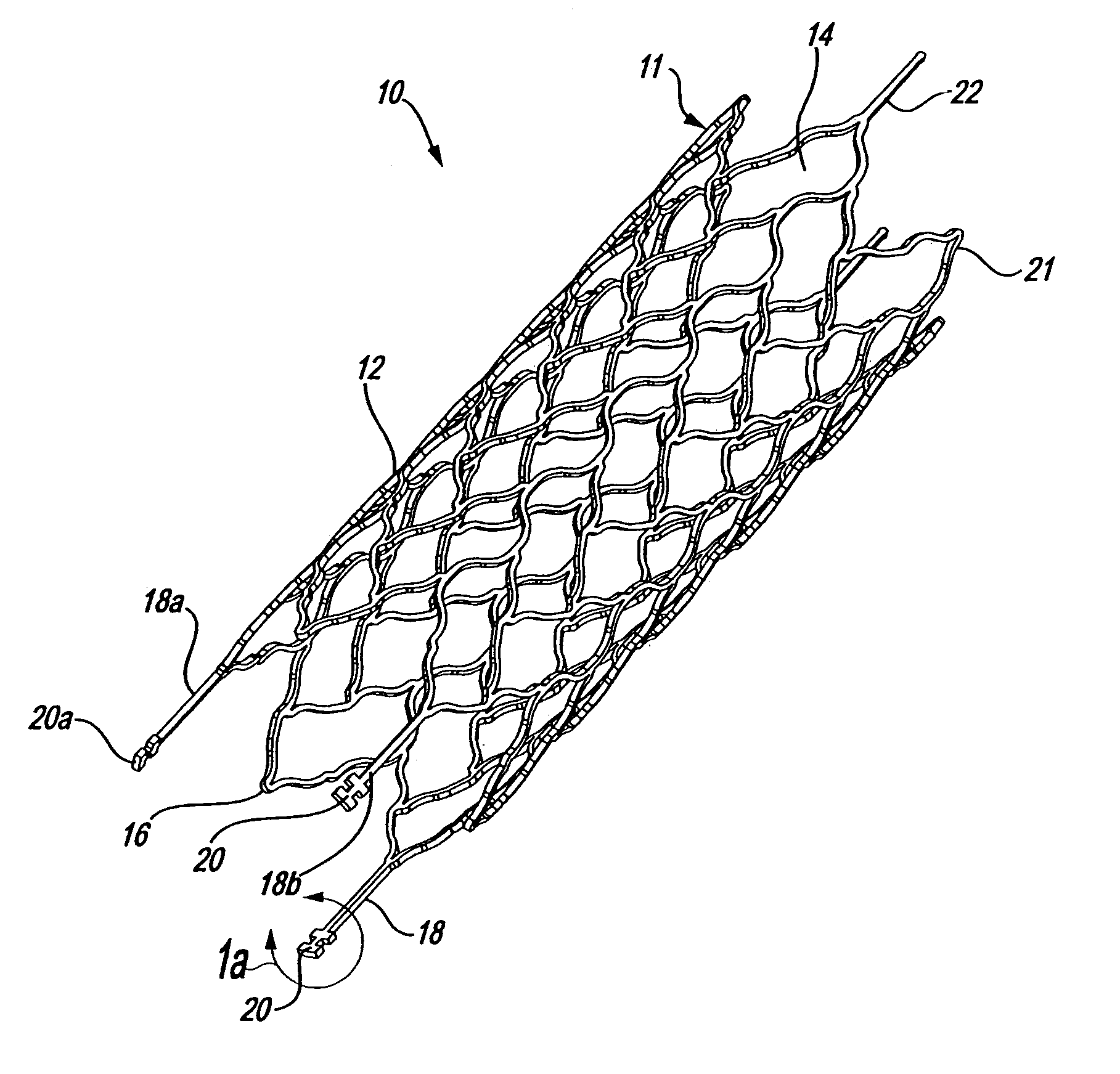
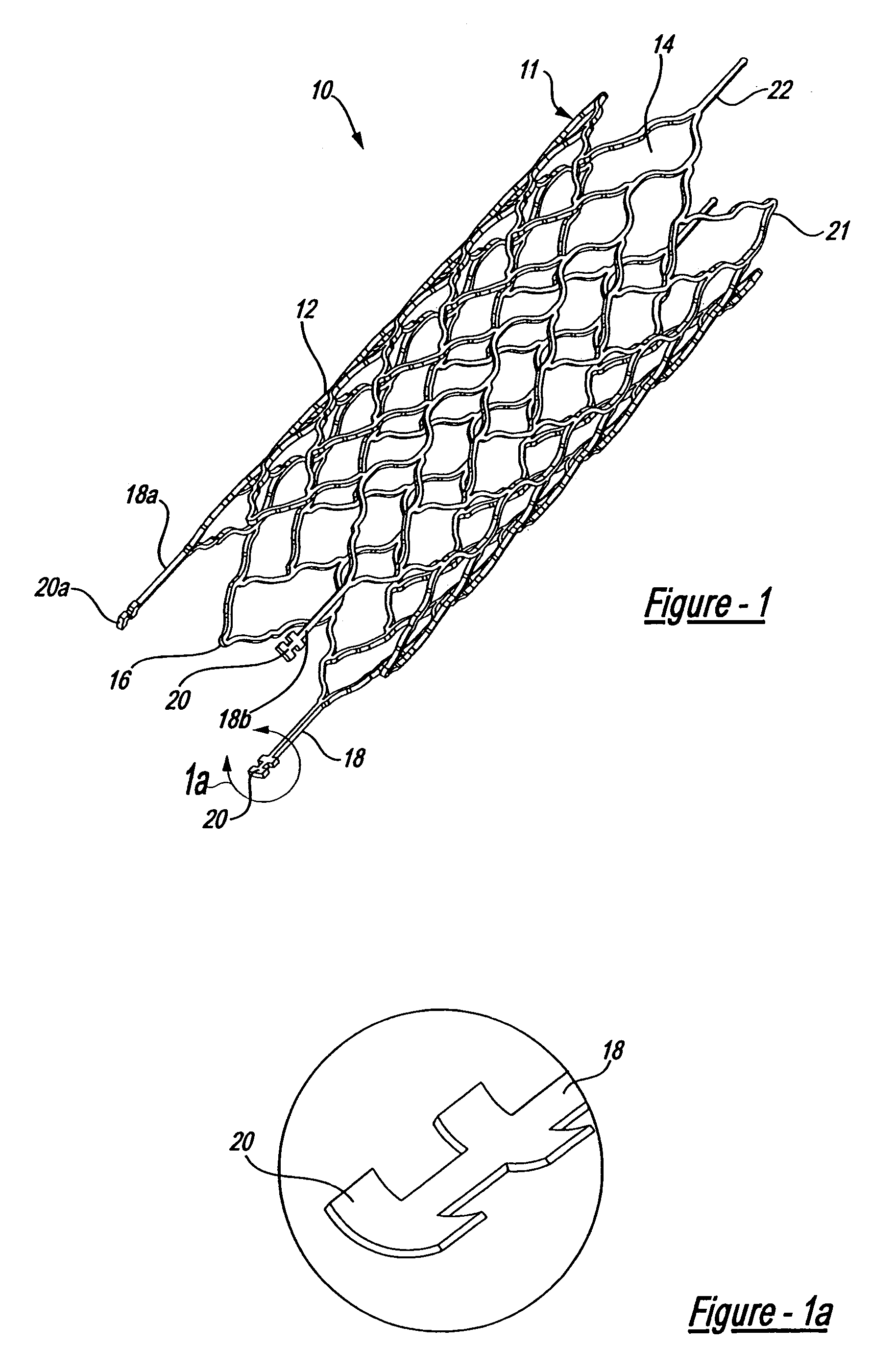
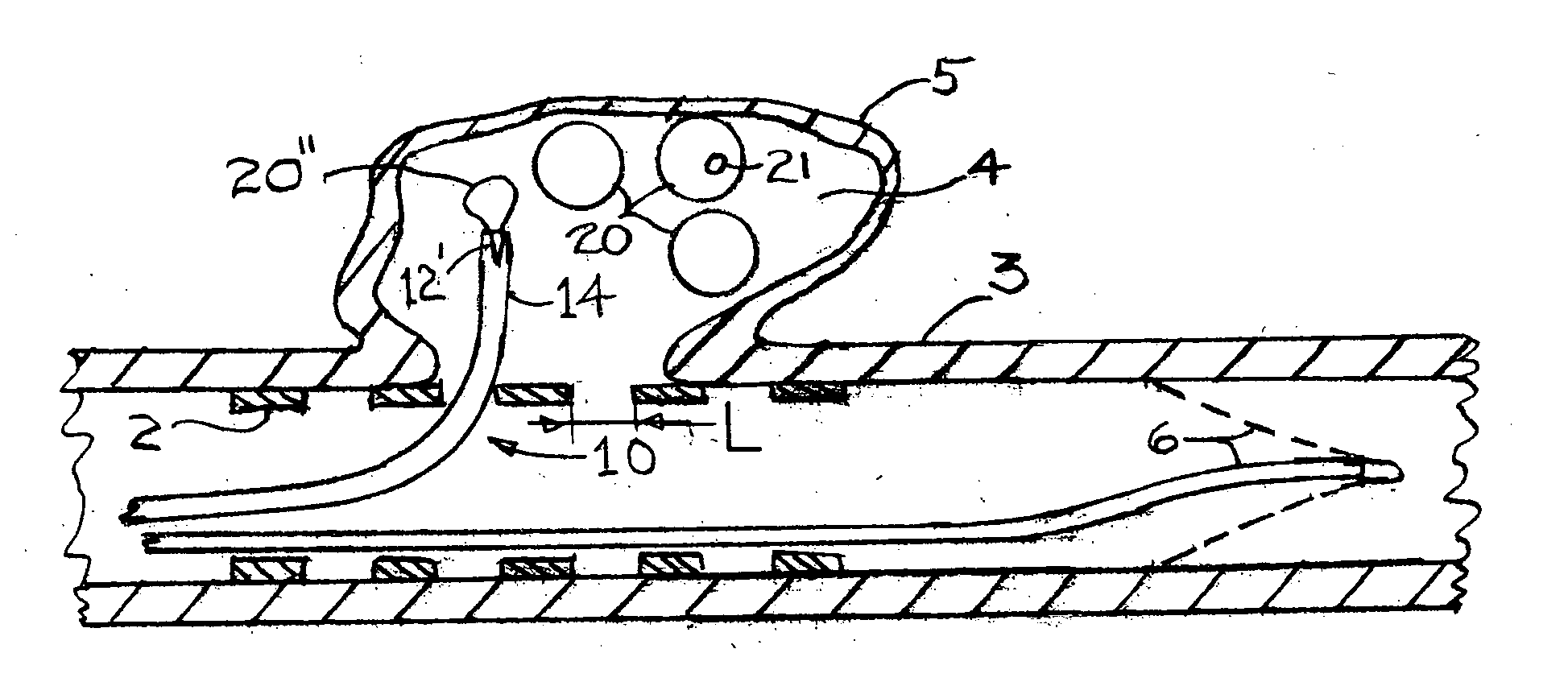
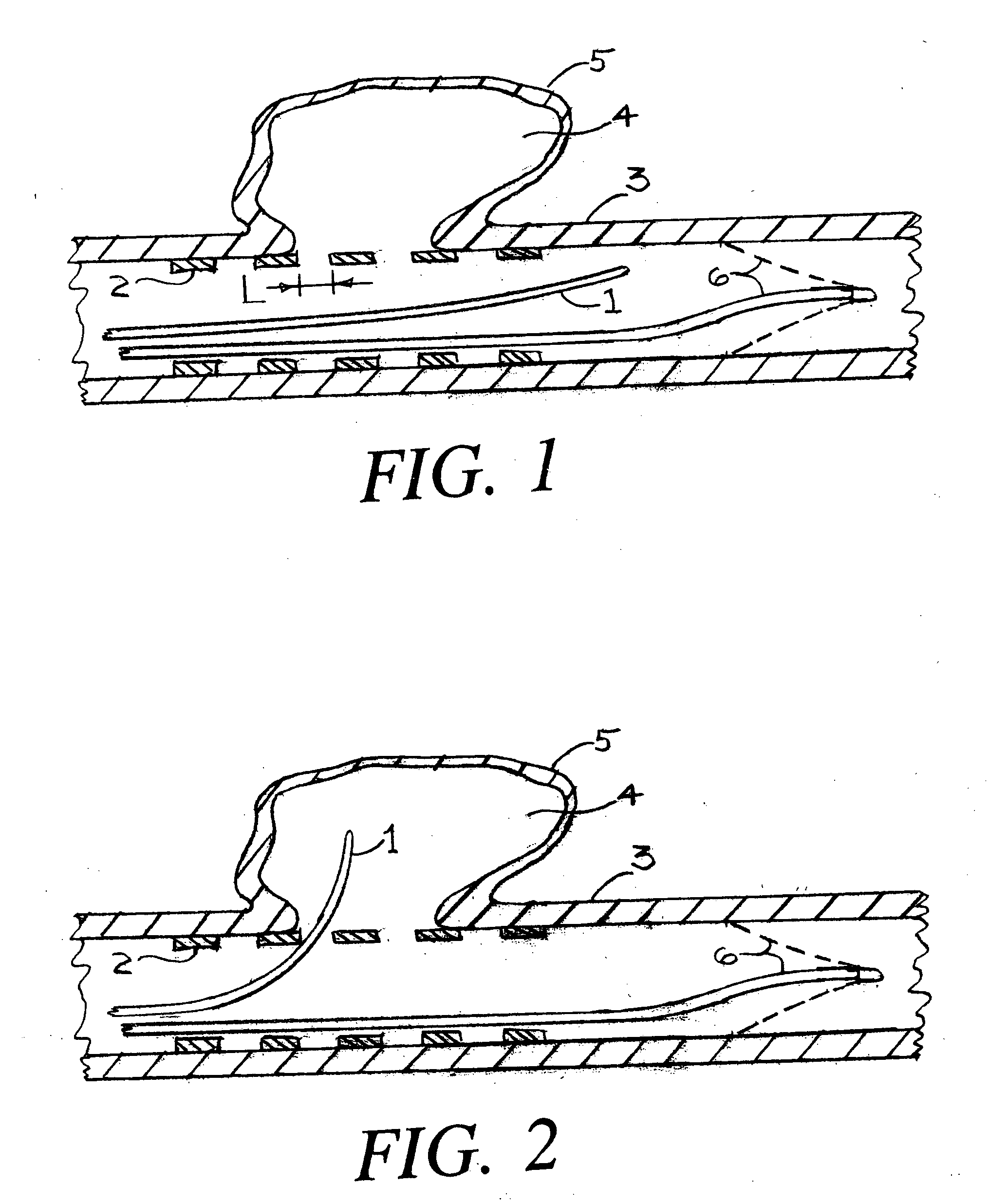
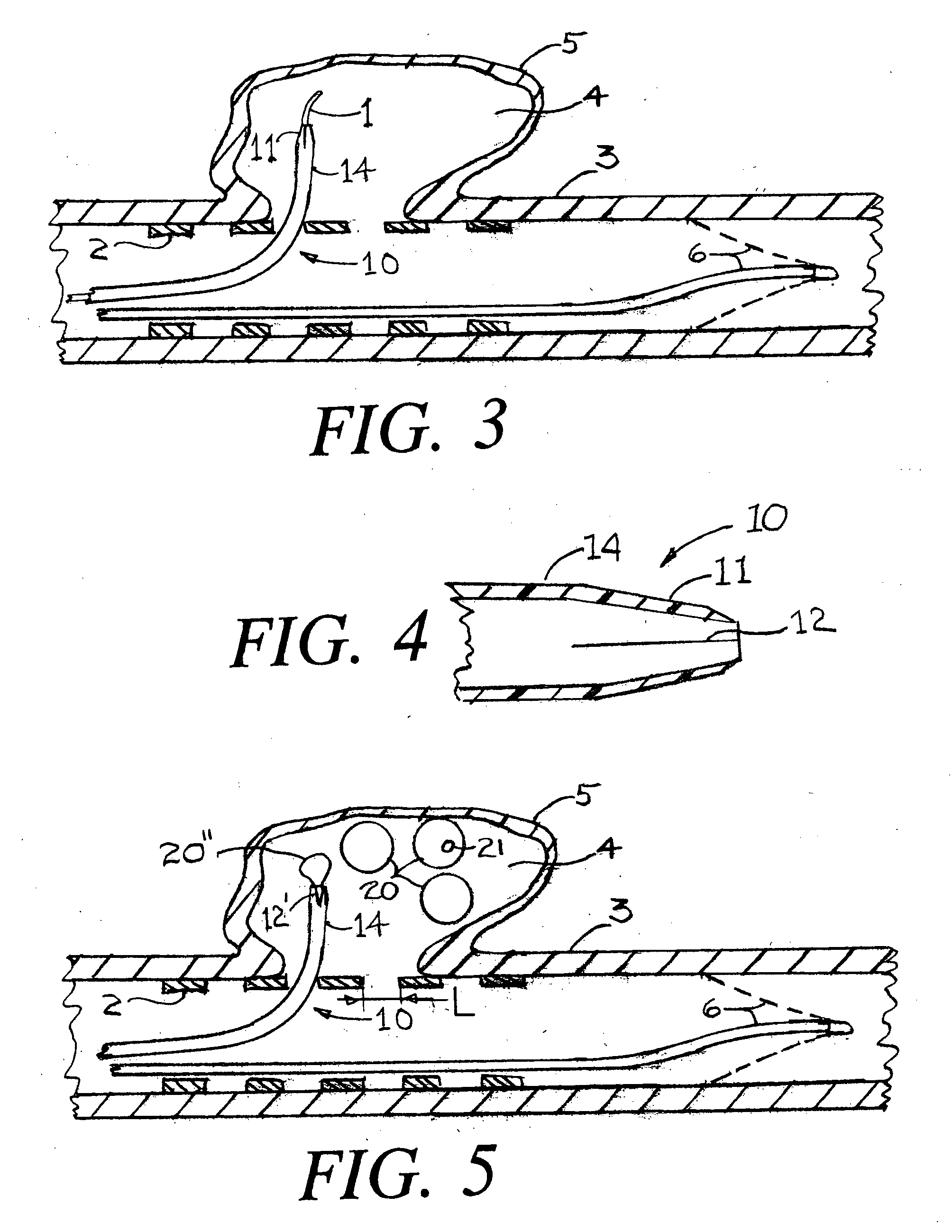
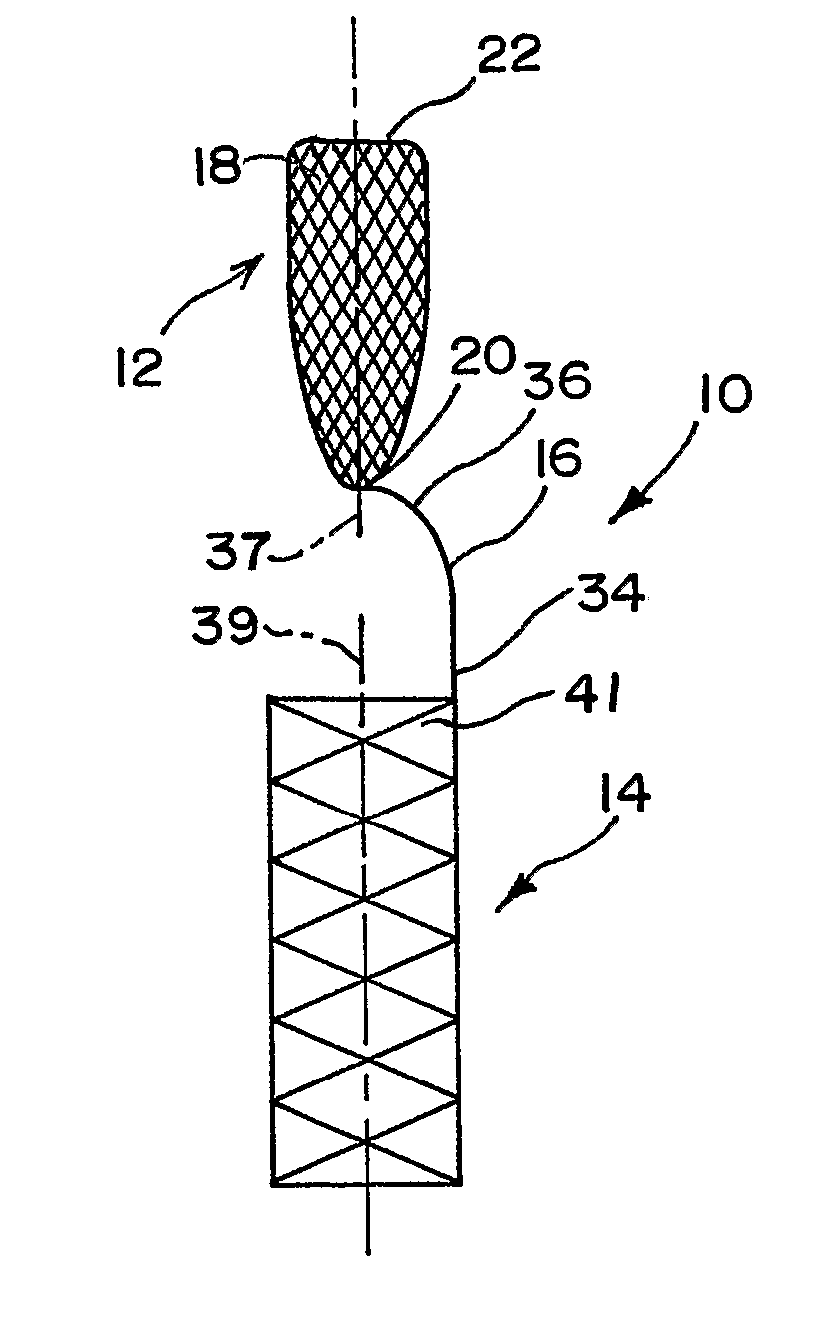
Endovascular aneurysm devices, systems, and methods
Devices, systems, and methods for implanting prostheses in the body lumens rely on tacking or anchoring the prostheses with separately introduced fasteners. After initial placement, a fastener applier system is introduced within the expanded prostheses to deploy a plurality of fasteners to at least one prosthesis end. The fasteners are usually helical fasteners which are releasably restrained on the fastener driver, and are delivered by rotation of the fastener driver. The fasteners may be applied singly, typically in circumferentially spaced-apart patterns about the interior of at least one end of the prosthesis. A lumen extension or lumens may be coupled to the prosthesis to extend the reach of the prosthesis within the implantation site. Fasteners may also be applied to the lumen extensions.
Owner:MEDTRONIC VASCULAR INC
Endovascular thin film devices and methods for treating and preventing stroke
InactiveUS6605111B2Treating and preventing ischemic and hemorrhagic strokeInhibit migrationStentsCatheterIn situ polymerizationProsthesis
Owner:NEW YORK UNIV
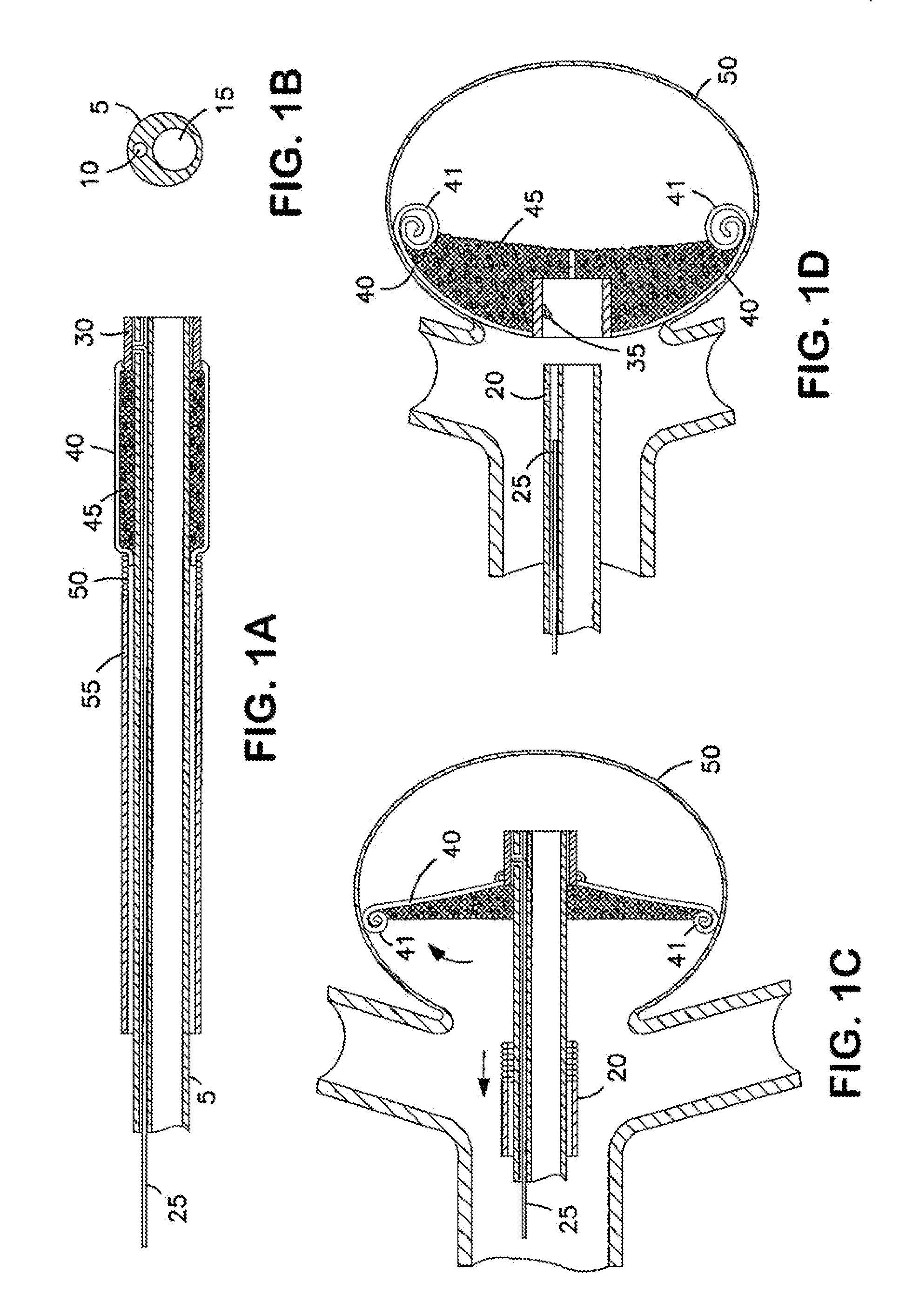
Bag for use in the intravascular treatment of saccular aneurysms
InactiveUS6346117B1Easy to controlHigh yield stressDilatorsOcculdersSaccular aneurysmsIliac Aneurysm
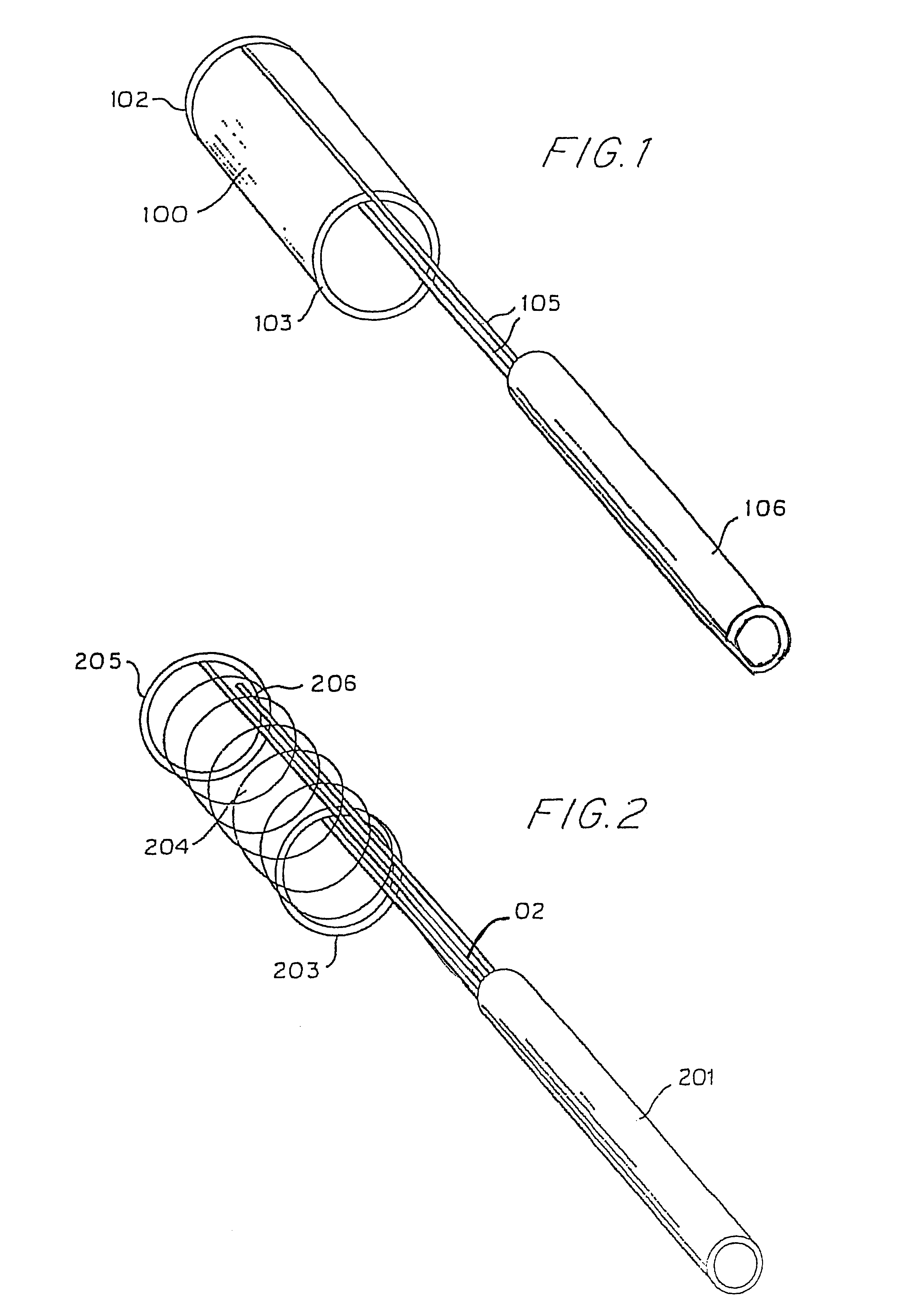
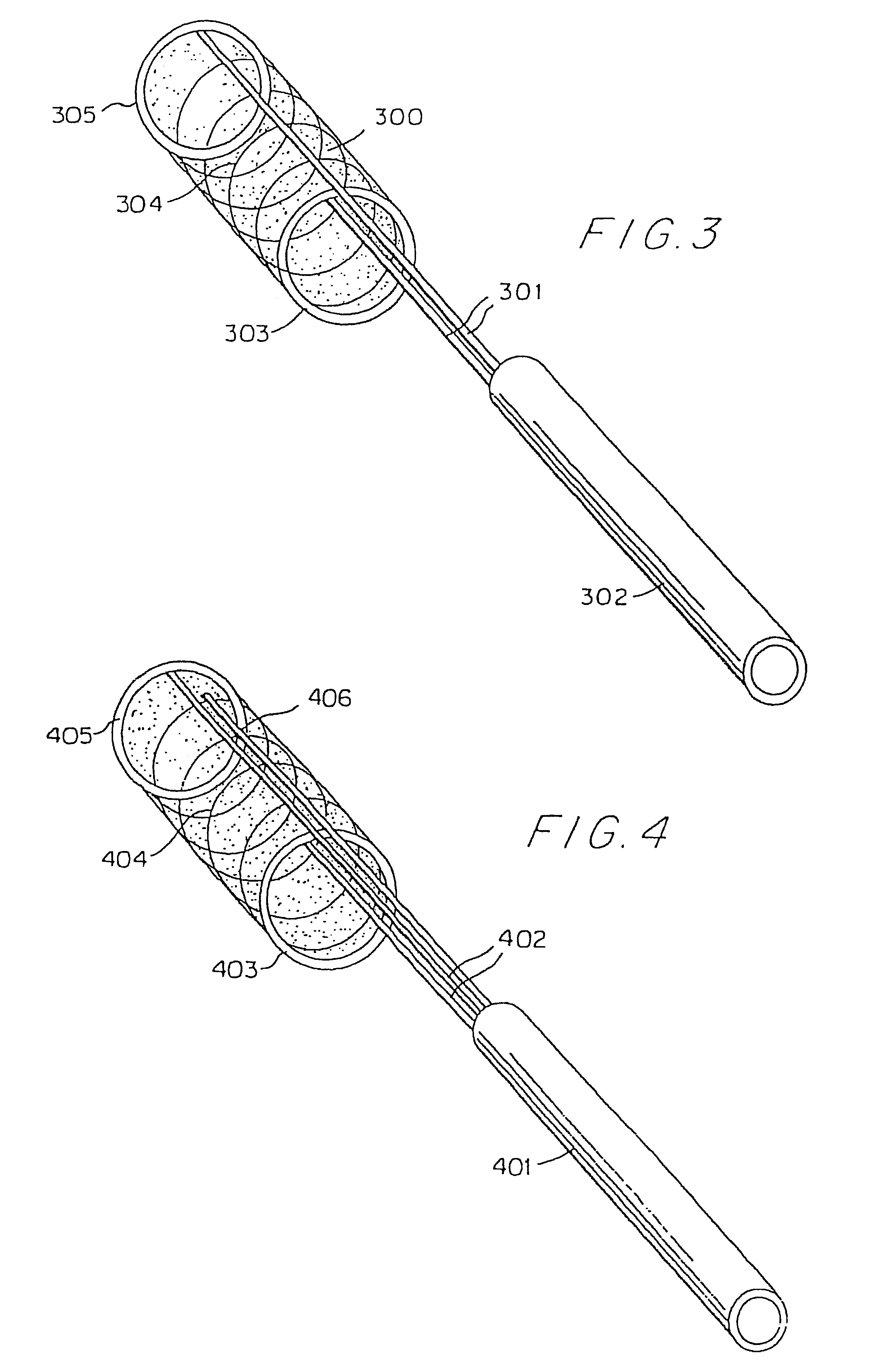
A bag for use in the intravascular treatment of saccular aneurysms and a method of forming the bag are disclosed. The bag is formed from a plurality of flexible, resilient filamentary members braided into a tubular sleeve and biased into a first shape having an expanded first diameter sized to substantially fill the aneurysm. The bag is resiliently deformable into a second shape having a diameter smaller than the first and sized to slidingly interfit within the lumen of a catheter. An opening is provided in the bag to receive a clotting medium, such as a platinum wire, on which blood clots can be induced to form by mechanical or electrolytic means. A closure is provided by biasing the filamentary members to form a constriction around the opening. In use the, bag is inserted into a saccular aneurysm via the catheter and expands to its first diameter upon release therefrom. Interstices between the interbraided filamentary members provide pores allowing blood from the aneurysm to enter the bag when the bag is positioned within the aneurysm. The clotting medium wire is packed into the bag, blood clots on the wire and occludes the aneurysm, sealing it off from the blood stream and preventing rupture. The wire is released from the catheter and is contained within the bag in the aneurysm.
Owner:PRODESCO
Modular intraluminal prosteheses construction and methods
InactiveUS6193745B1Unnecessary expansive forcePrevent radial movementStentsBlood vesselsDiseaseExtensibility
The present invention provides modular intraluminal tubular prostheses, particularly stents and stent-grafts, for the treatment of disease conditions, particularly aneurysms. Modular sections of the prostheses, or "prosthetic modules," may be selectively combined to form a composite prosthesis having characteristics which are tailored to the specific requirements of the patient. Each prosthetic module preferably includes one or more standard interface ends for engaging another module, the module / module interface typically comprising ends which overlap and / or lock within a predetermined axial range. Advantageously, the axial length, cross-section, perimeter, resilient expansive force, axial flexibility, liner permeability, liner extensibility, radial conformability, liner / tubal wall sealing and anchoring, and other prosthetic characteristics may be varied along the axis of the composite prosthesis, and also along the axis of each prosthetic module. The modules are preferably individually introduced into a lumen system of a patient body so that the composite prosthesis is assembled in situ. Ideally, selection of appropriate prosthetic modules and the flexibility of the interface overlap range provides a custom fit intraluminal prosthesis which provides a therapy tailored to the individual patient's needs.
Owner:MEDTRONIC AVE
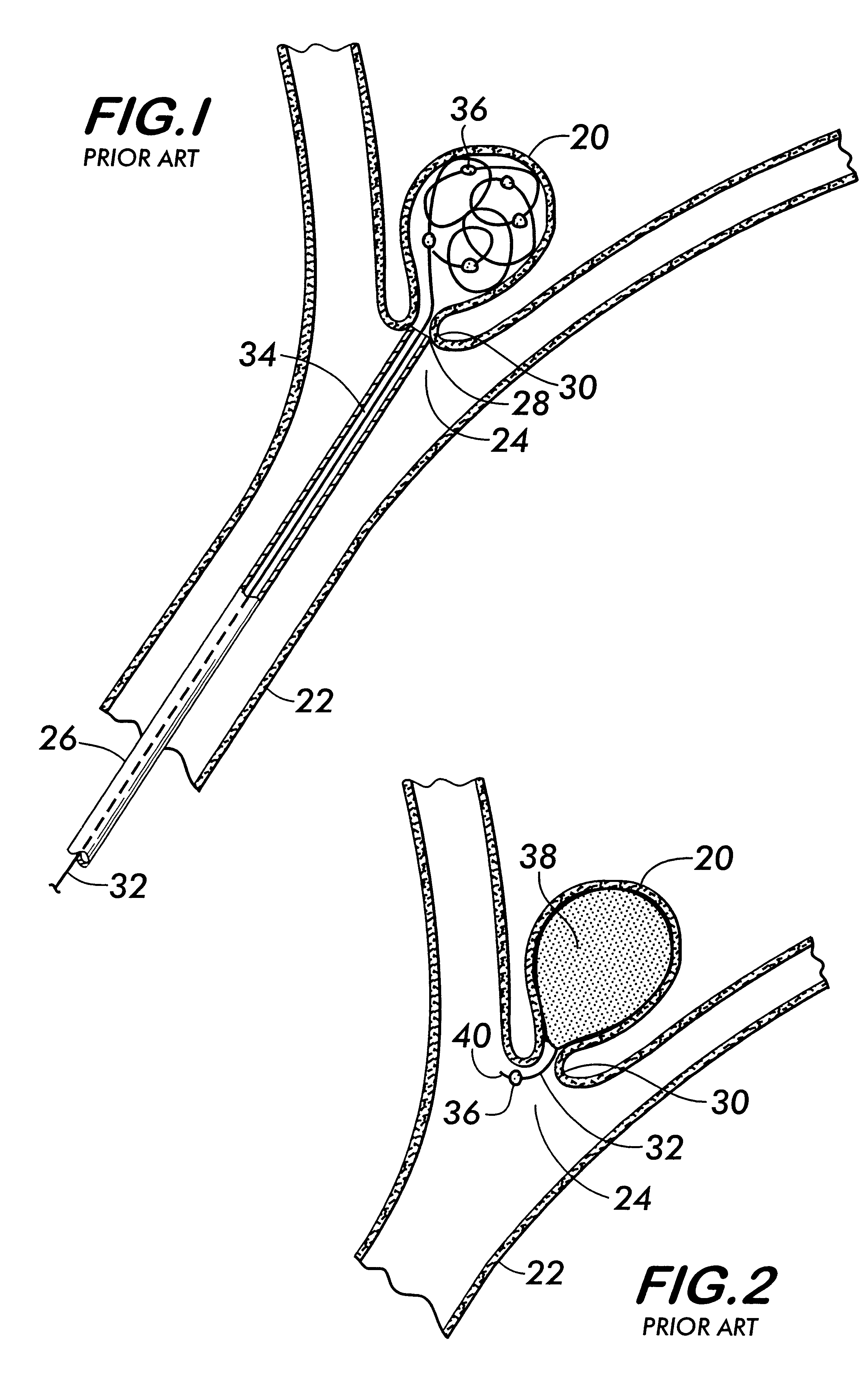
Endovascular aneurysm embolization device
The present invention relates to a medical device for placement at a predetermined location within a vessel of the human body, and more particularly, relates to a collapsible aneurysm embolization device that is delivered through the lumen of a catheter and exits the catheter at a predetermined position within the vessel to thereby embolize a blood vessel defect, such as an aneurysm.
Owner:CODMAN & SHURTLEFF INC
System for tissue cavity closure
InactiveUS20060004388A1Lower the volumeLess invasive treatmentSuture equipmentsSurgical staplesSurgical operationAnatomical structures
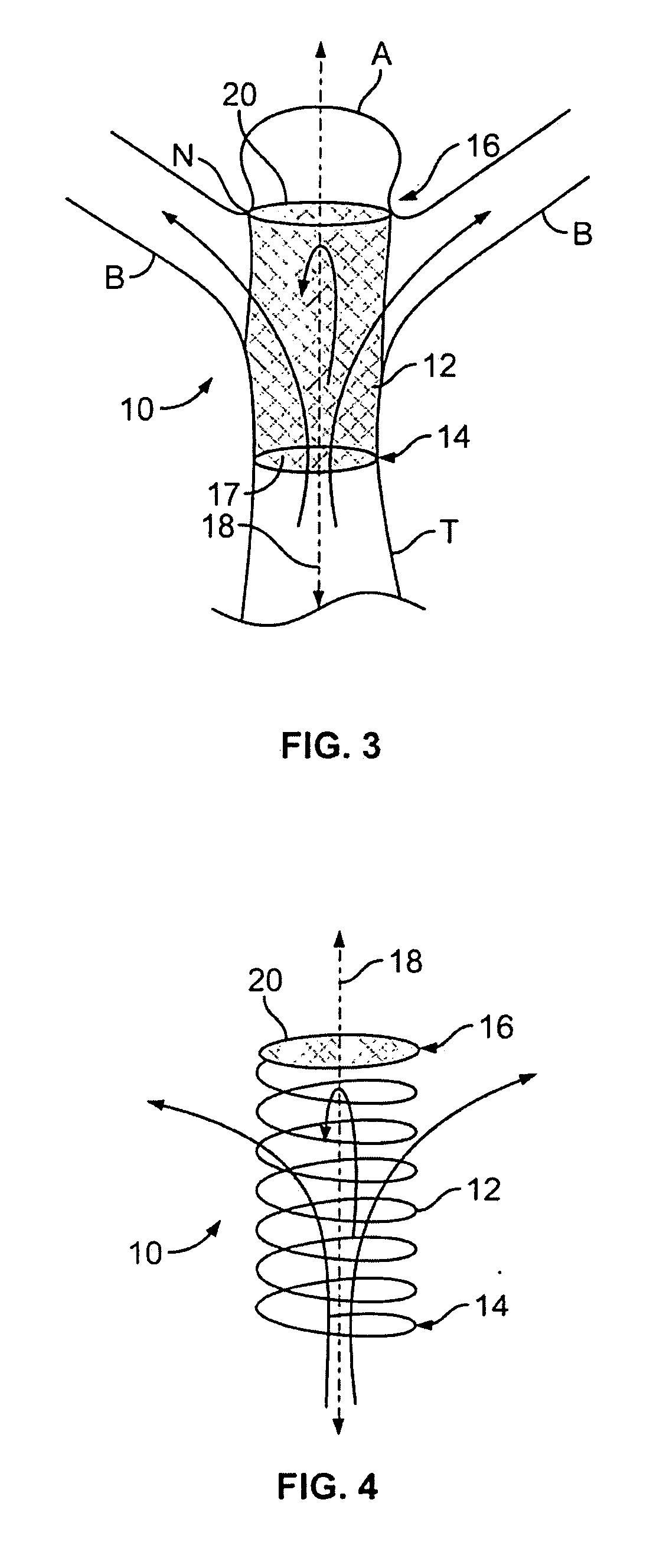
Surgical systems for less invasive access to and isolation of an atrial appendage are provided. A suturing grasper compresses soft tissue structures and deploys one or more sutures through complimentary pledget(s) carried by the grasper. The pledgets are reinforced, containing support members that define the profile of the stitch and distribute stresses applied by the stitch, once tightened, over a length of tissue. This hardware may be applicable to other surgical and catheter based applications as well including: compressing soft tissue structures lung resections and volume reductions; gastric procedures associated with reduction in volume, aneurysm repair, vessel ligation, or other procedure involving isolation of, resection of, and reduction of anatomic structures.
Owner:ATRICURE
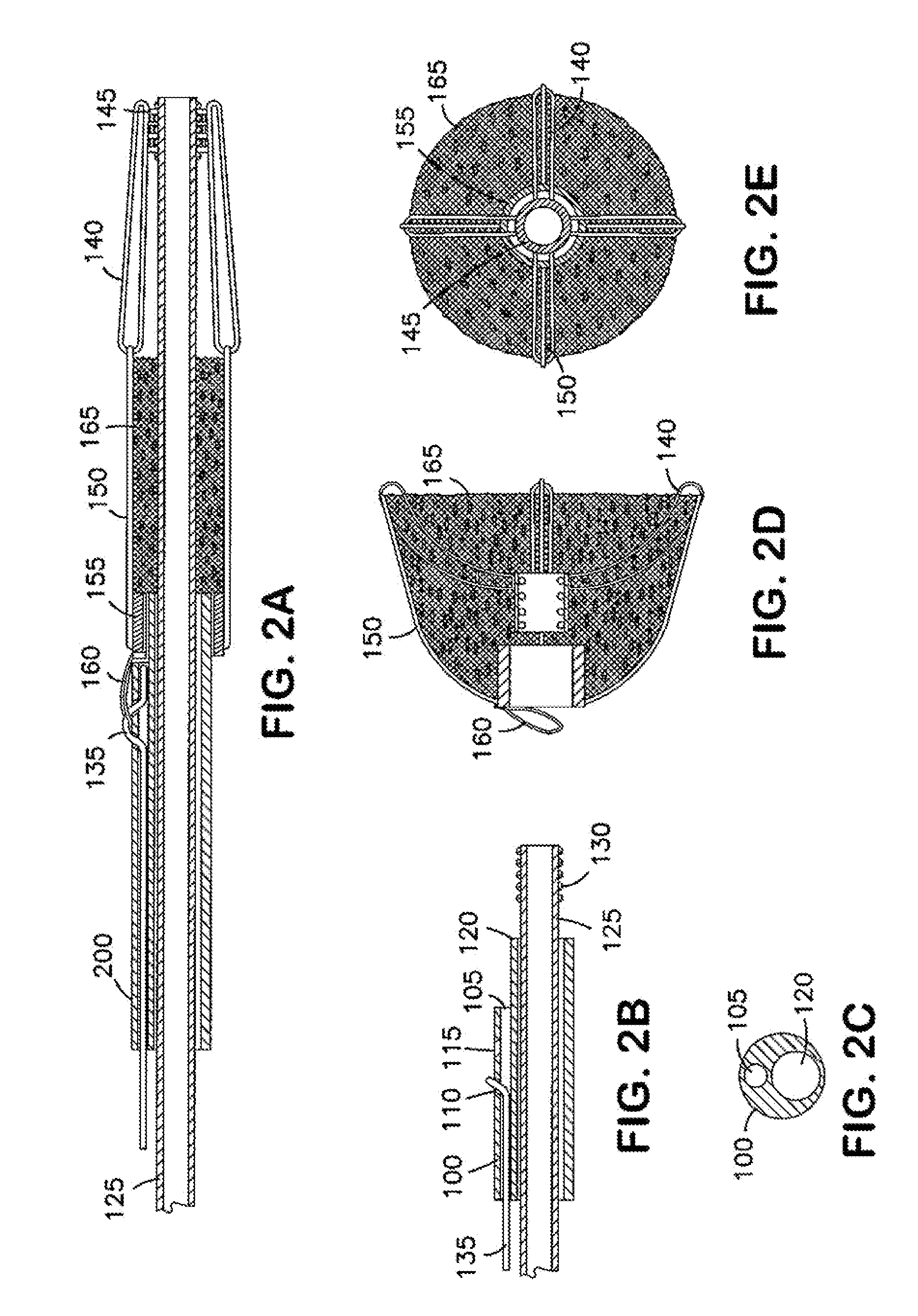
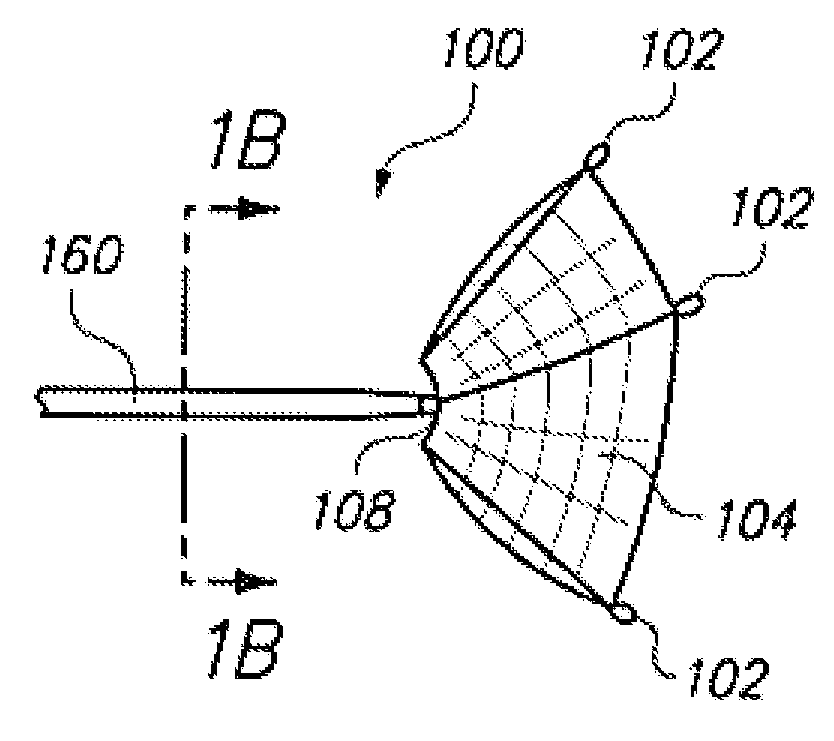
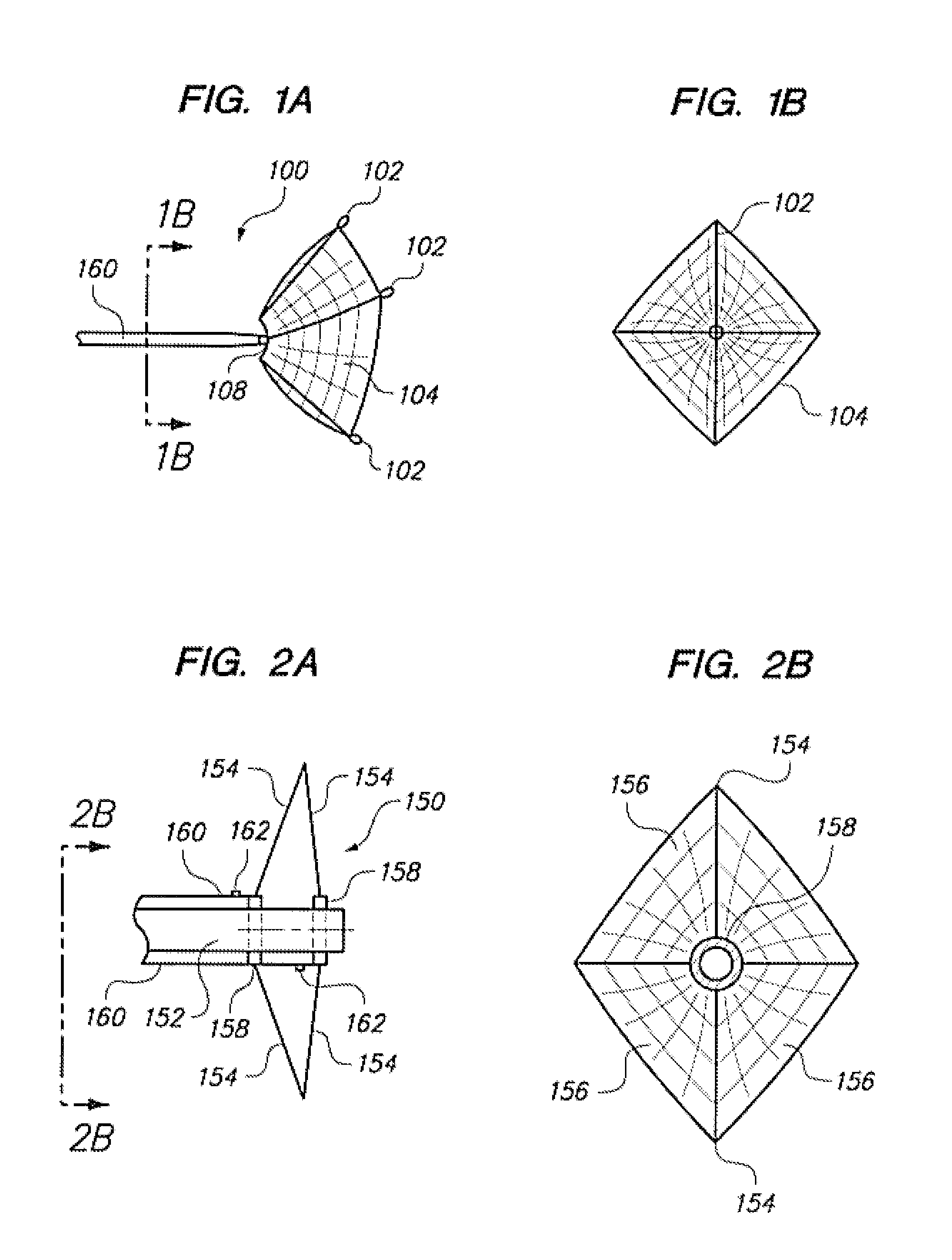
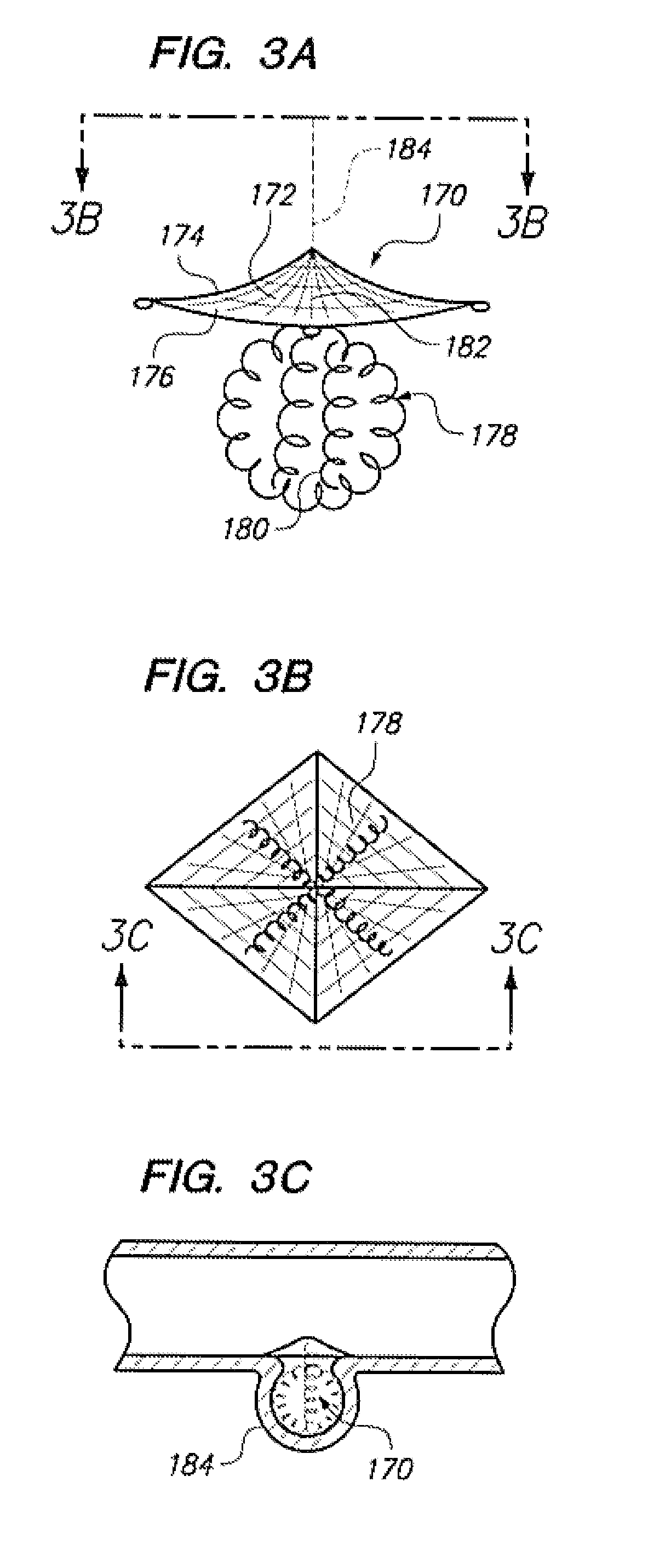
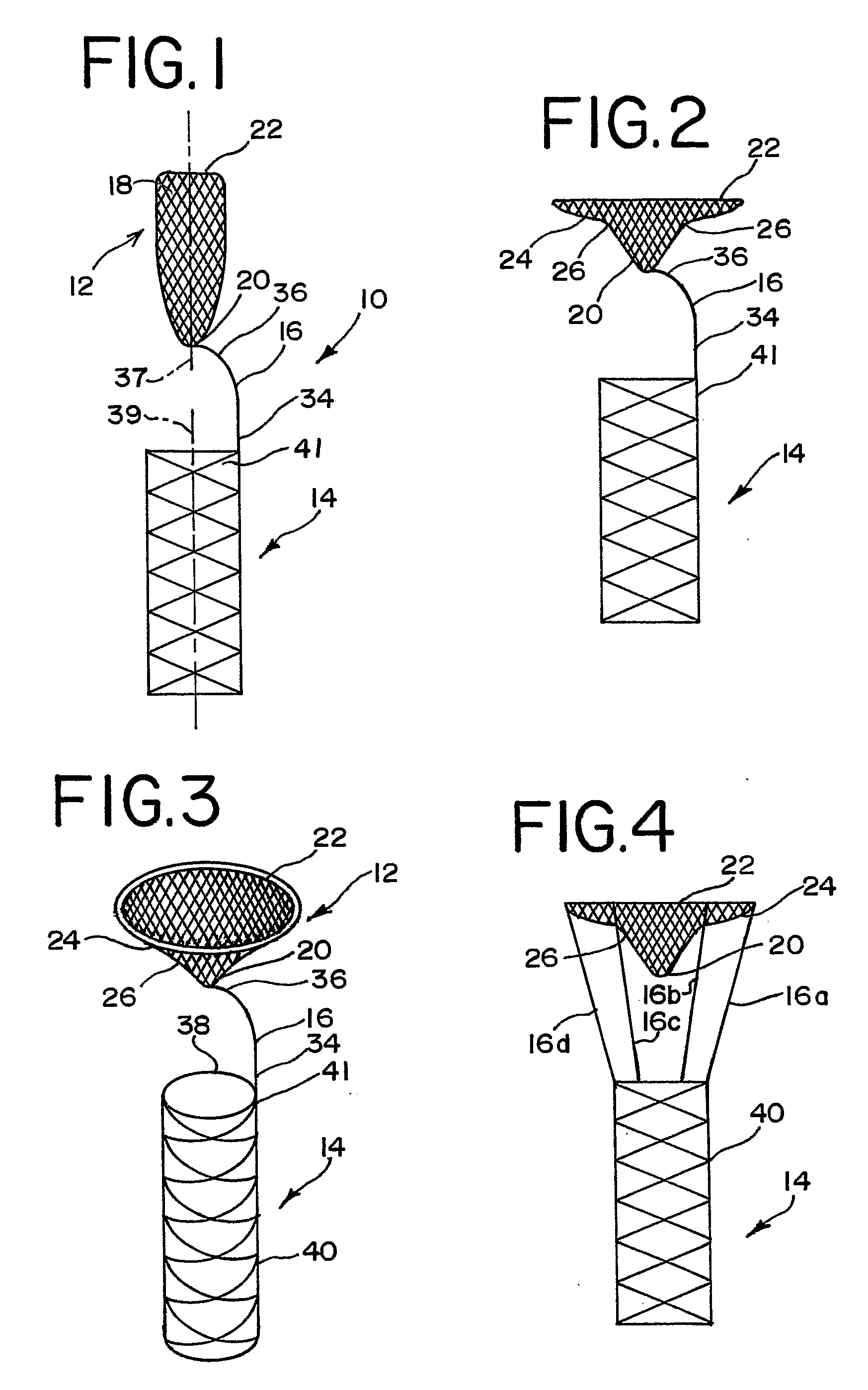
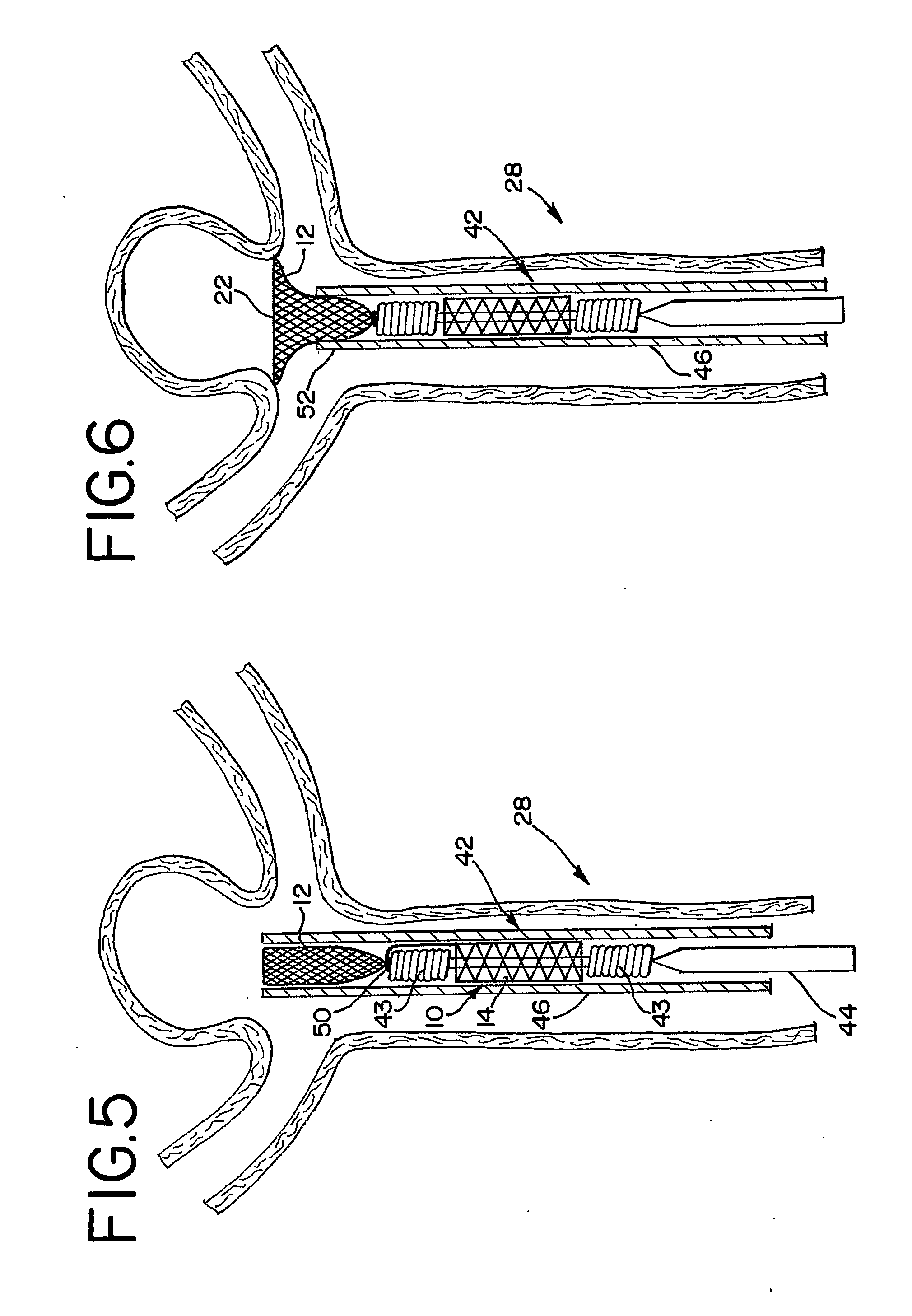
Aneurysm Occlusion Devices
InactiveUS20080281350A1Promote endothelial growthEliminate riskDilatorsCatheterMedicineImplanted device
An implantable occlusion device for bridging the neck of an aneurysm comprises a biocompatible matrix. The device is movable between a compressed position prior to implantation and a generally concave or cup-shaped position following implantation. The device may comprise a frame having a plurality of elements. The frame elements have a first pre-deployment position generally parallel to a major axis of the delivery lumen, and a second post-deployment position spread radially from the major axis of the delivery lumen. The biocompatible matrix and / or the frame elements may also form or be manipulated to form a generally concave or cupped shape. The matrix can be porous or semiporous, such as a foam or a reticulated matrix. The occlusion device can be folded, twisted and / or stretched to adopt a narrow profile for loading into a coaxial delivery device and expand in place as it adopts its original shape on release. The device may be released or manipulated to a desired shape to occlude an aneurysm. Methods of using the implantable device are also provided.
Owner:BIOMERIX CORP
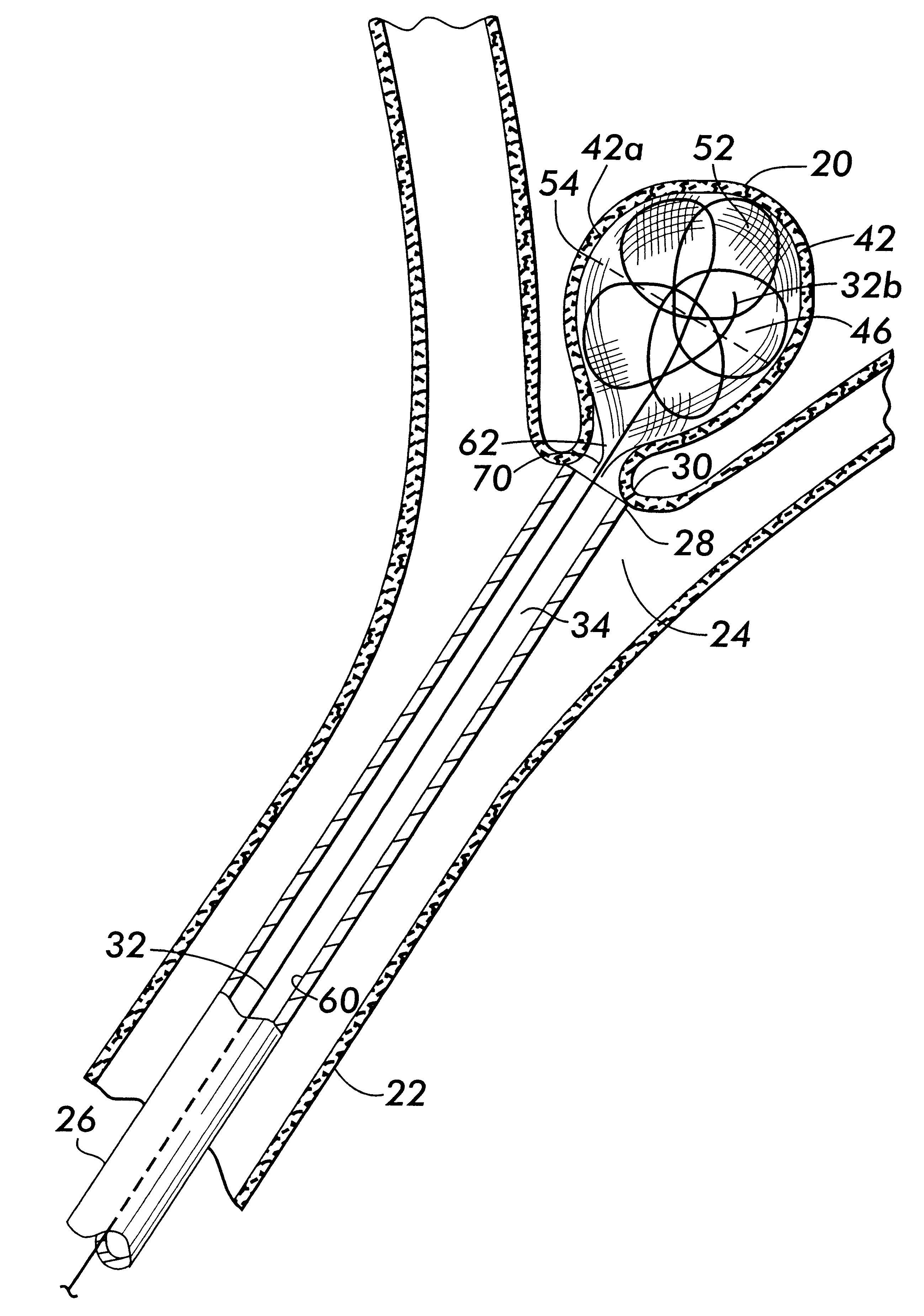
Cranial aneurysm treatment arrangement
InactiveUS20060064151A1Minimize migrationMaximize flowStentsOcculdersInsertion stentAneurysm treatment
A stent for application within an aneurysm the stent comprising an elongated tubular member having a proximal end and a distal end. The stent has a proximal portion expandable from a first diameter to a second diameter. The distal end of the stent may be expandable to a third diameter, which third diameter is larger than the second diameter. The stent may be left disposed across the neck of the aneurysm.
Owner:GUTERMAN LEE R +1
Embolic balloon
An embolic balloon assembly is disclosed herein. The assembly includes a detachable balloon system which expands while aspirating a quantity of surrounding blood to occlude a vessel or aneurysm. The balloon has a distensible membrane having a plurality of orifices throughout its surface. Within the distensible membrane is a plurality of expandable members made from a shape memory alloy, e.g., Ni—Ti alloy, which expand upon application of a stimulus. Alternatively, the expandable members or a single expandable wire may be inserted separately into the distensible membrane. Once the balloon begins to expand, internal pressure within a volume defined by the distensible membrane begins to drop, forcing the device to aspirate a quantity of surrounding blood inside the volume which then begins to coagulate by stasis or some stimulus. The balloon may be configured to automatically release into the aneurysm or vessel or it may be released by a detachable joint.
Owner:SAADAT VAHID
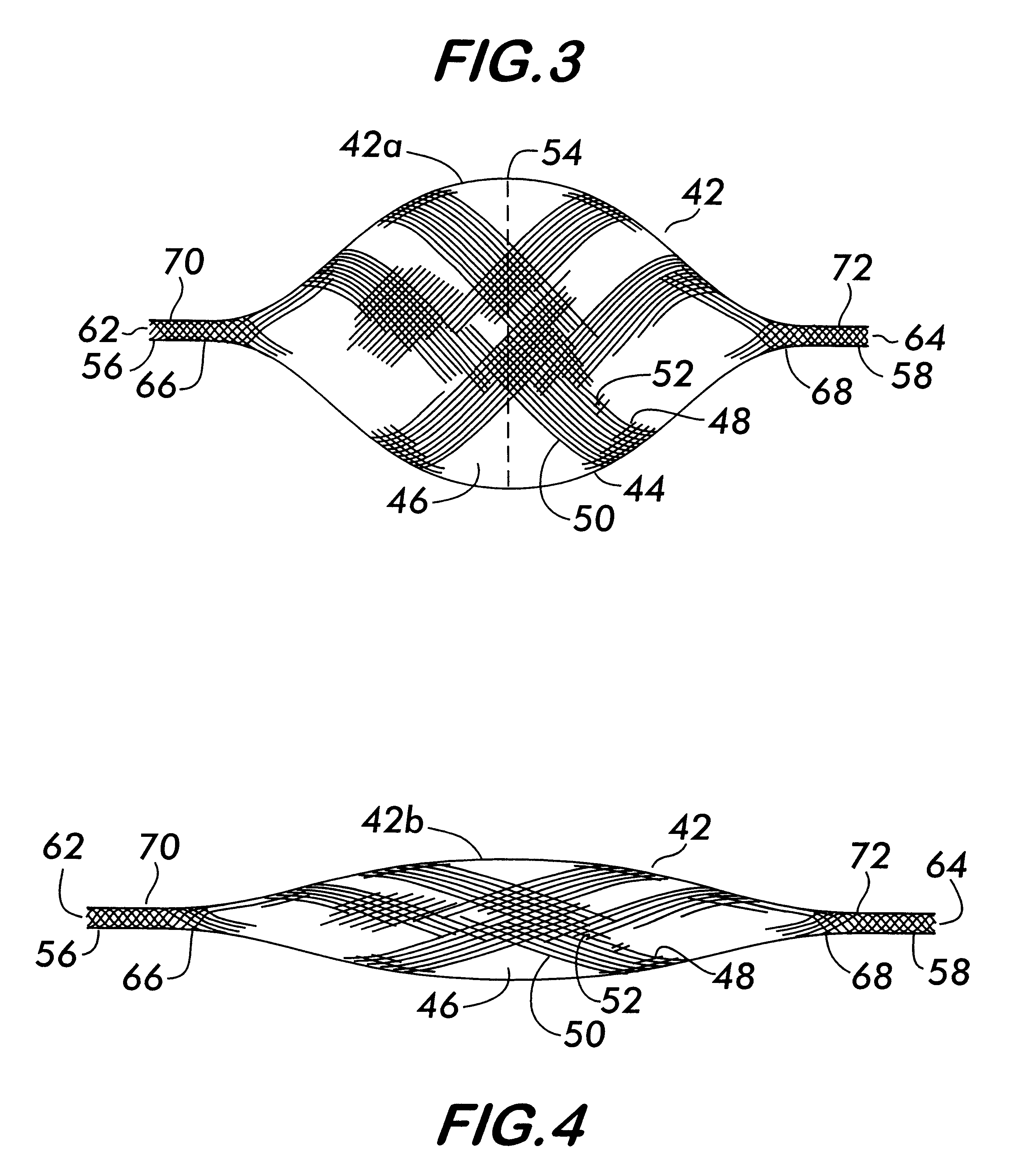
Stitched stent grafts and methods for their fabrication
InactiveUS6123722AUnnecessary expansive forcePrevent radial movementStentsBlood vesselsExtensibilityStent grafting
The present invention provides modular intraluminal tubular prostheses, particularly stents and stent-grafts, for the treatment of disease conditions, particularly aneurysms. Modular sections of the prostheses, or "prosthetic modules," may be selectively combined to form a composite prosthesis having characteristics which are tailored to the specific requirements of the patient. Each prosthetic module preferably includes one or more standard interface ends for engaging another module, the module / module interface typically comprising ends which overlap and / or lock within a predetermined axial range. Advantageously, the axial length, cross-section, perimeter, resilient expansive force, axial flexibility, liner permeability, liner extensibility, radial conformability, liner / tubal wall sealing and anchoring, and other prosthetic characteristics may be varied along the axis of the composite prosthesis, and also along the axis of each prosthetic module. The modules are preferably individually introduced into a lumen system of a patient body so that the composite prosthesis is assembled in situ. Ideally, selection of appropriate prosthetic modules and the flexibility of the interface overlap range provides a custom fit intraluminal prosthesis which provides a therapy tailored to the individual patient's needs.
Owner:MEDTRONIC AVE
Intravascular stent device
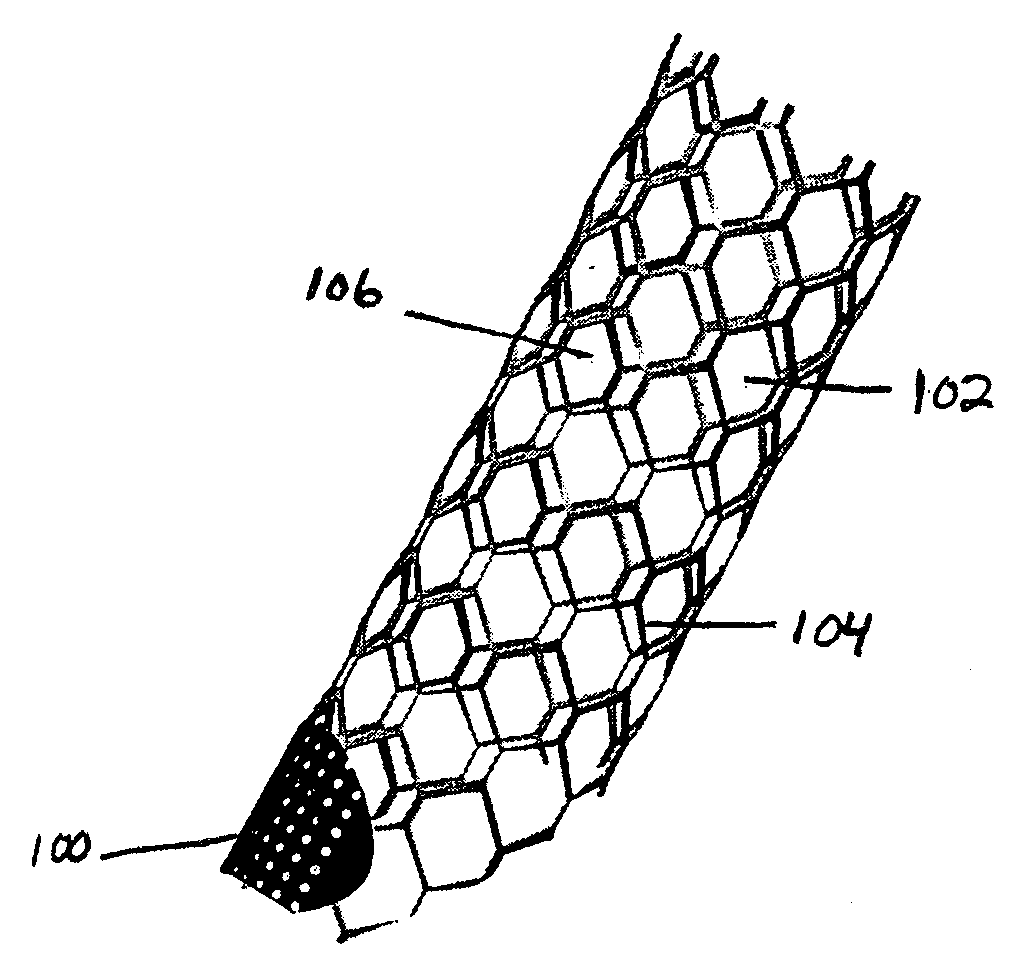
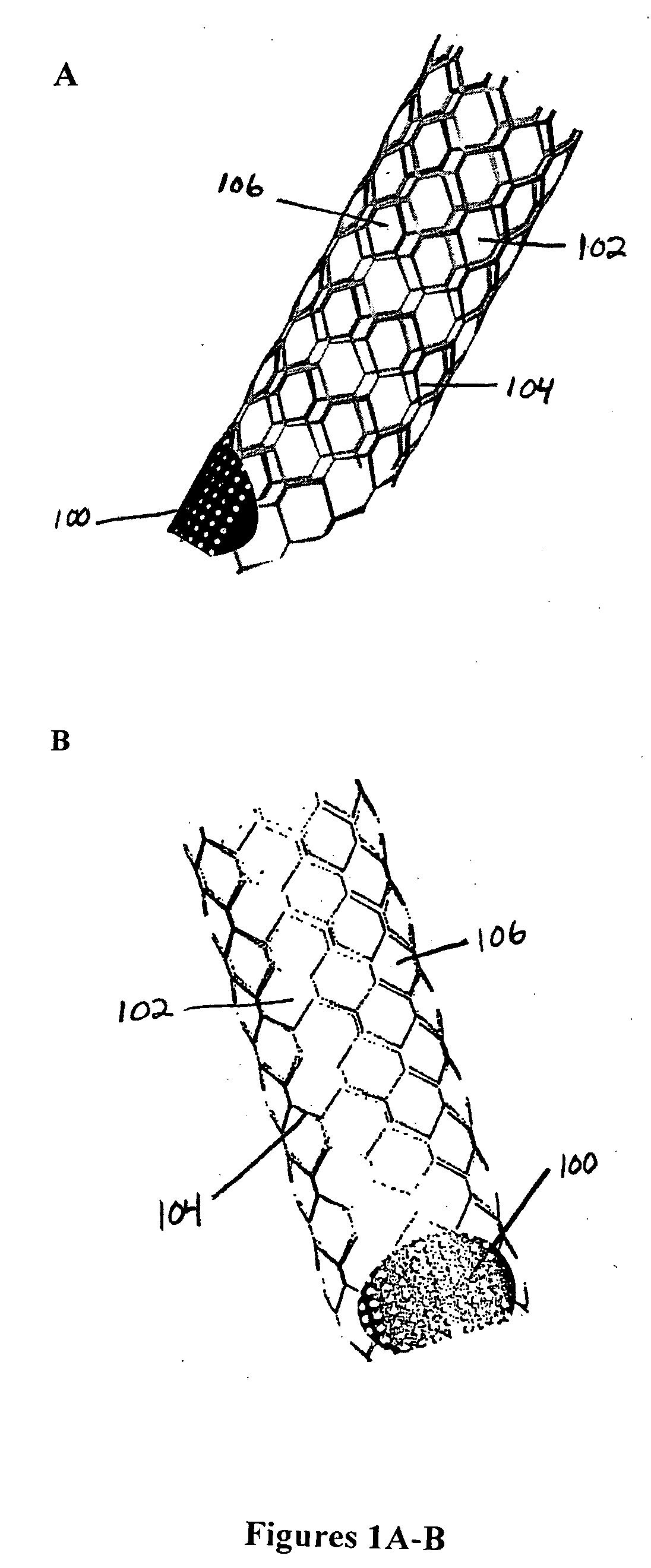
A very small diameter intravascular stent device which may be used to occlude or partially occlude an aneurysm in the human brain which is comprised of a thin-walled skeletal cylindrical tube formed of undulating or sinusoidal elements which, when compressed, nest tightly with each other.
Owner:CODMAN & SHURTLEFF INC
Bilateral extension prosthesis and method of delivery
The invention is a system, apparatus, and method for treating, and / or repairing an aneurysm, preferably an aortic aneurysm, and most preferably, an abdominal aortic aneurysm. The systems, devices, and methods of the present invention include a prosthesis assembly for establishing a fluid flow path between an upstream portion of an artery and at least one bifurcated downstream portion of the artery.
Owner:CARDINAL HEALTH SWITZERLAND 515 GMBH
Shape memory tubular stent
A stent radially expandable from a radially contracted introduction state into a radially expanded position state, in which the final shape of the stent can be controlled by varying the amount and places energy is delivered onto the interior surfaces of the tubes from which the stent is fabricated, and means to seal the opening into the aneurysm, thereby causing the blood therein to clot and preventing the aneurysm from growing or rupturing.
Owner:UNSWORTH JOHN DUNCAN +1
Intravascular deliverable stent for reinforcement of vascular abnormalities
InactiveUS20070168019A1Avoid interactionReduce overall outer diameterStentsCatheterVascular Skin TumorSaphenous veins
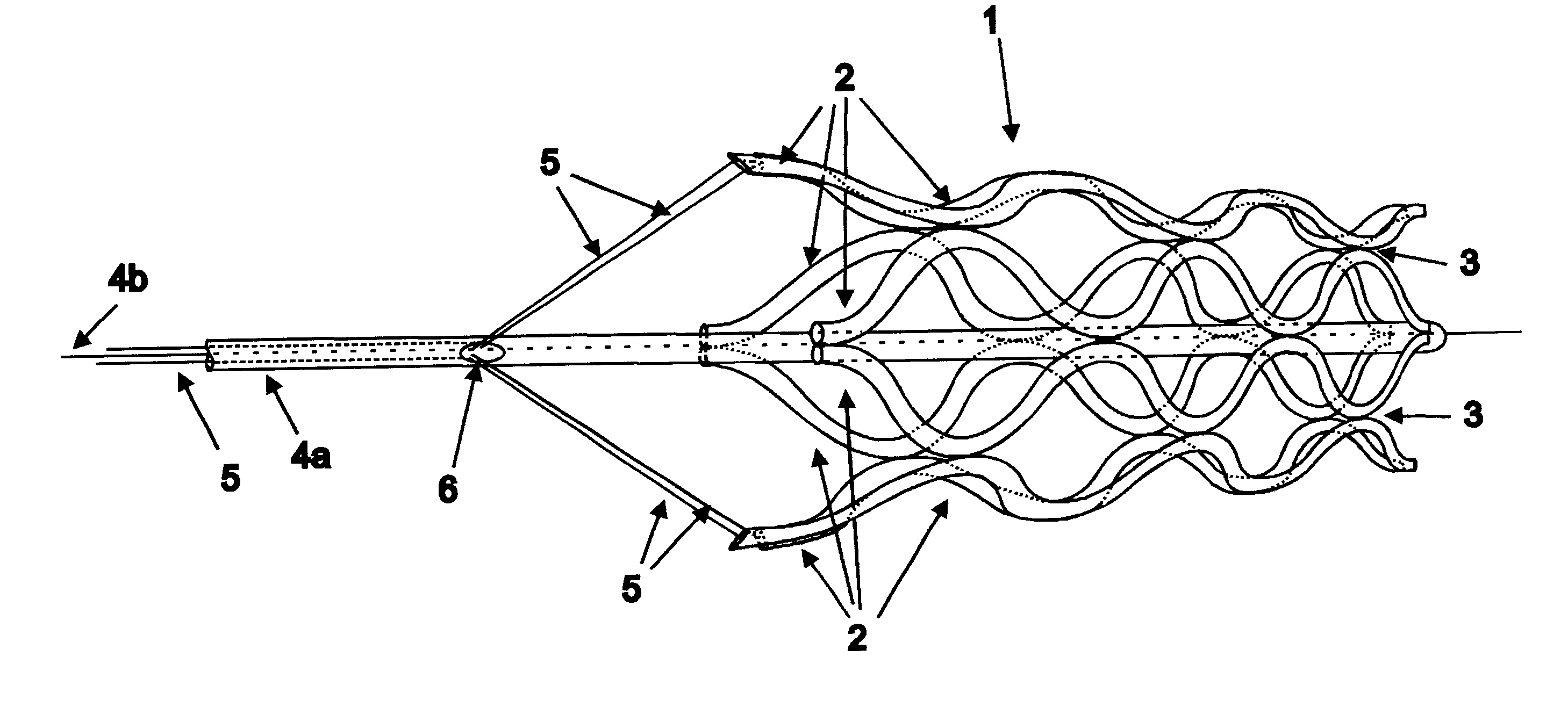
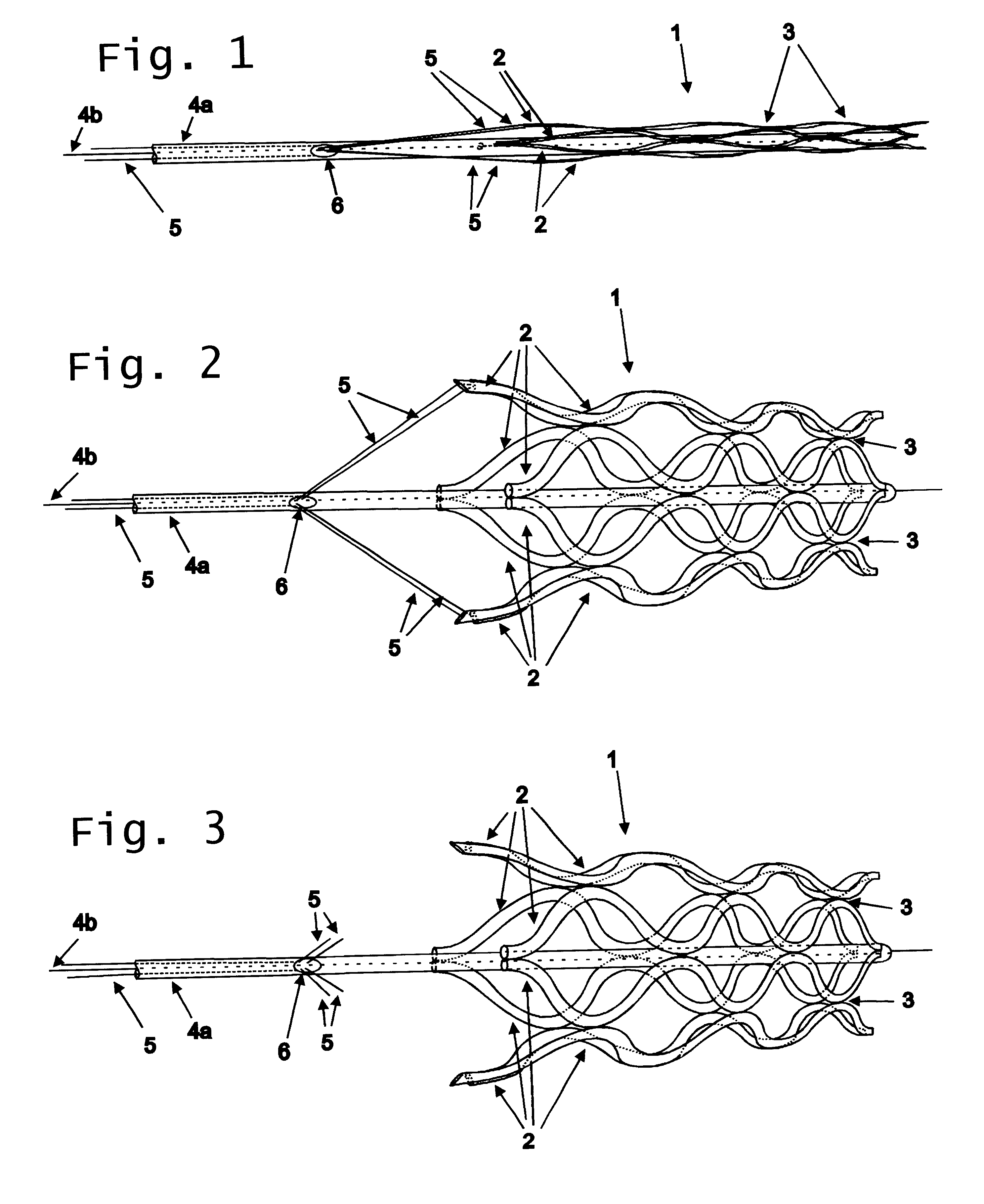
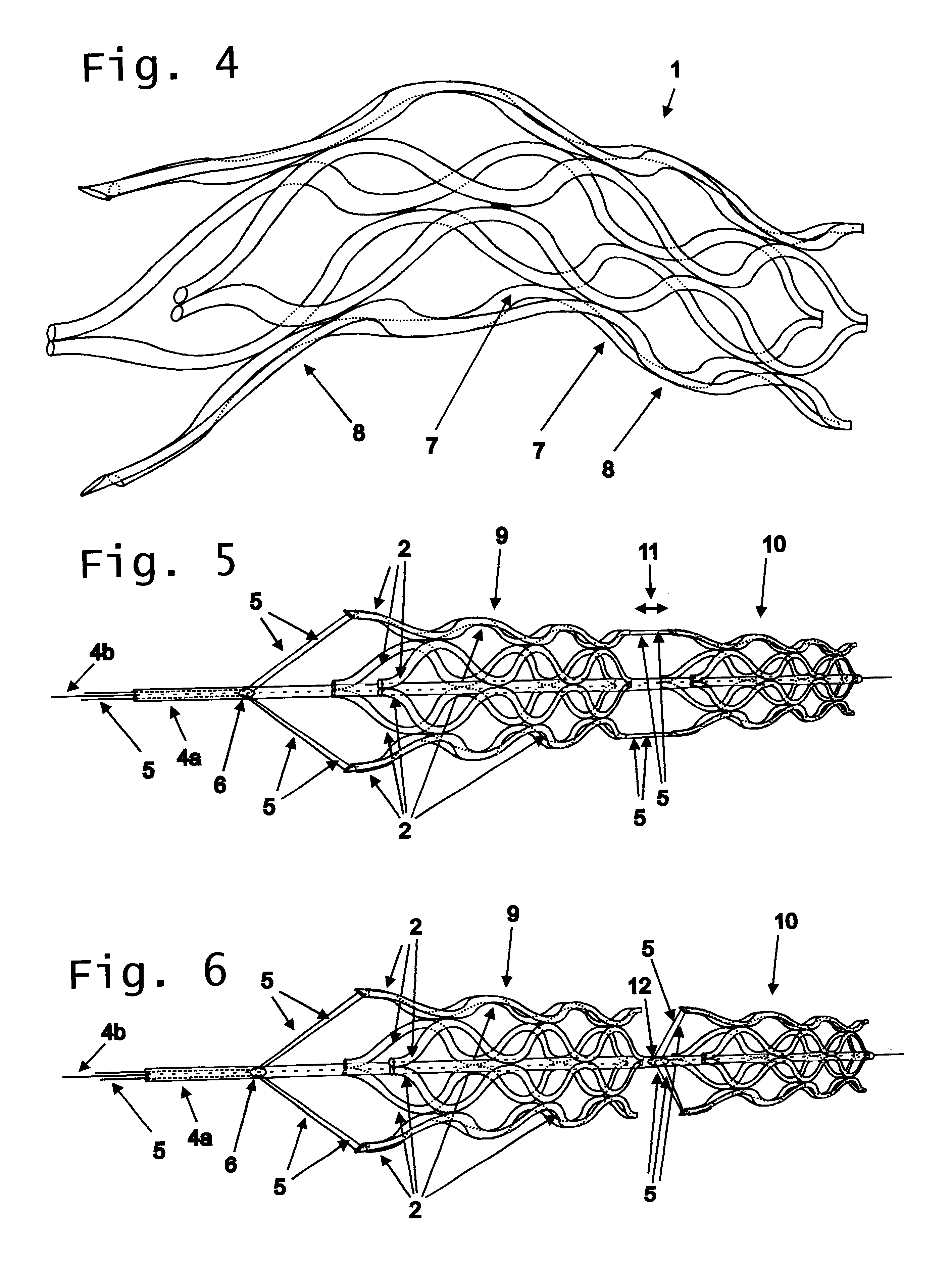
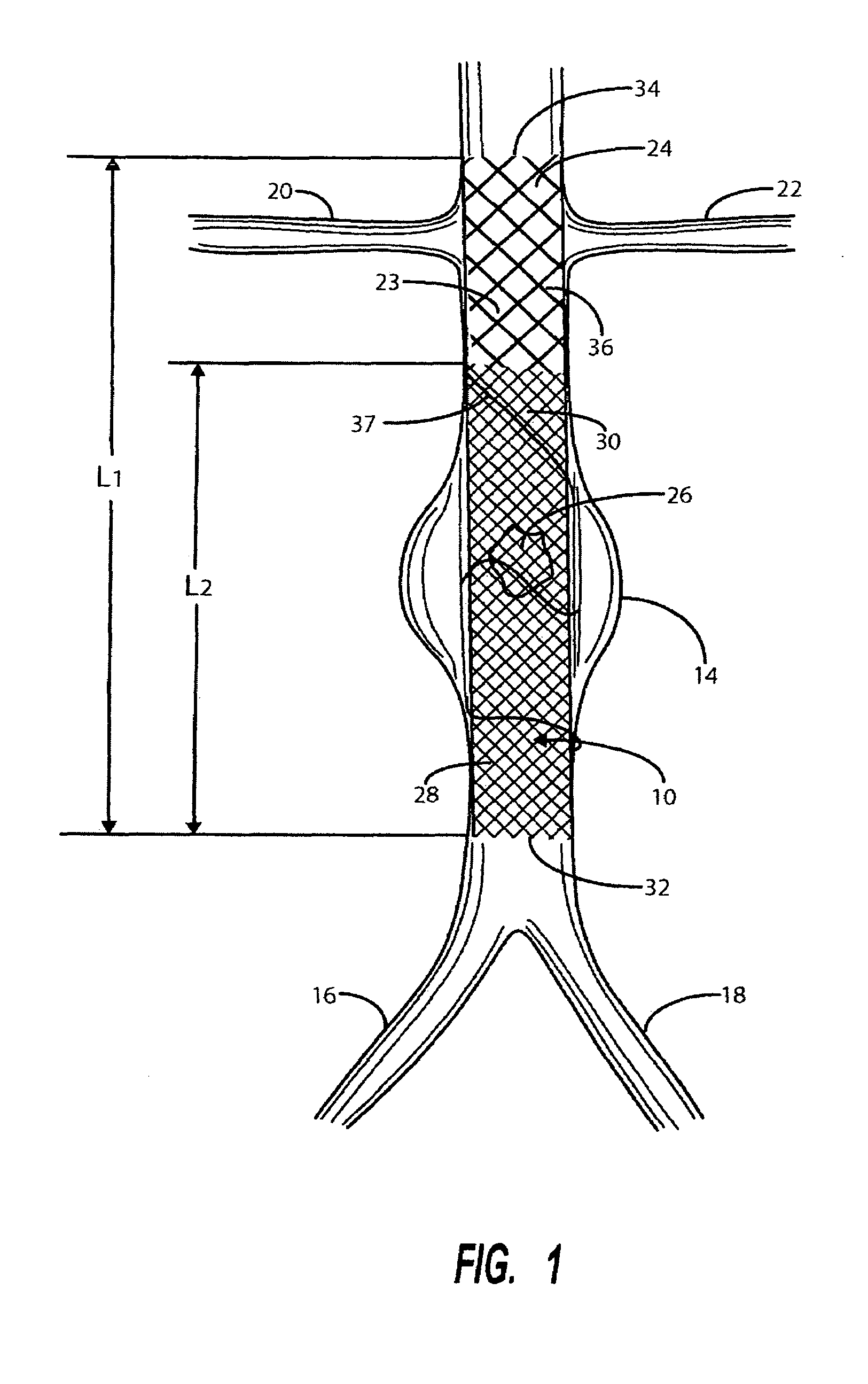
A catheter deliverable stent / graft especially designed to be used in a minimally invasive surgical procedure for treating a variety of vascular conditions such as aneurysms, stenotic lesions and saphenous vein grafts, comprises an innermost tubular structure and at least one further tubular member in coaxial arrangement. In one embodiment, the innermost tubular structure is of a length (L1) and is formed by braiding a relatively few strands of highly elastic metallic alloy. The pick and pitch of the braid are such as to provide relative large fenestrations in the tubular wall that permit blood flow through the wall and provide the primary radial support structure. A portion of the innermost tubular structure of a length L1 is surrounded by a further braided tubular structure having relatively many strands that substantially inhibit blood flow through the fenestrations of the innermost tubular structure. The composite structure can be stretched to reduce the outer diameter of the stent / graft, allowing it to be drawn into a lumen of a delivery catheter. The catheter can then be advanced through the vascular system to the site of treatment and then released, allowing it to self-expand against the vessel wall. Various optional embodiments are disclosed that allow one skilled in the art to tailor the design to the specific application.
Owner:ST JUDE MEDICAL CARDILOGY DIV INC
Intravascular devices and fibrosis-inducing agents
InactiveUS20050149173A1Reducing perigraft leakageFacilitate “anchoring”StentsPeptide/protein ingredientsFibrosisCoil embolization
Intravascular devices (e.g., stents, stent grafts, covered stents, aneurysm coils, embolic agents and drug delivery catheters and balloons) are used in combination with fibrosing agents in order to induce fibrosis that may otherwise not occur when the implant is placed within an animal or to promote fibrosis betweent the devices and the host tissues. Compositions and methods are described for use in the treatment of aneurysms and unstable arterial (vulnerable) plaque.
Owner:ANGIOTECH INT AG (CH)
Endovascular thin film devices and methods for treating and preventing stroke
InactiveUS20030060782A1Safely and permanently excludingPreventing initial or recurrent aneurysmal subarachnoid hemorrhageStentsCatheterIn situ polymerizationProsthesis
Devices for excluding aneurysms and treating atherosclerotic disease, for intra-aneurysmal occlusion; and devices for preventing distal emboli. The devices are generally pliable and collapsible thin film devices which can be delivered via a microcatheter into the desired location where they are deployed and undergo either a shape memory phase transformation or in situ polymerization to assume the stable configuration of a permanent endoluminal prosthesis. Prior to being caused to assume their final shape, the devices remain soft, collapsible and pliable to ensure atraumatic delivery through the vascular system. Upon reaching the endoluminal defect in the vessel, the device is extruded from the microcatheter. Devices are also provided for retrieving clots.
Owner:NEW YORK UNIV
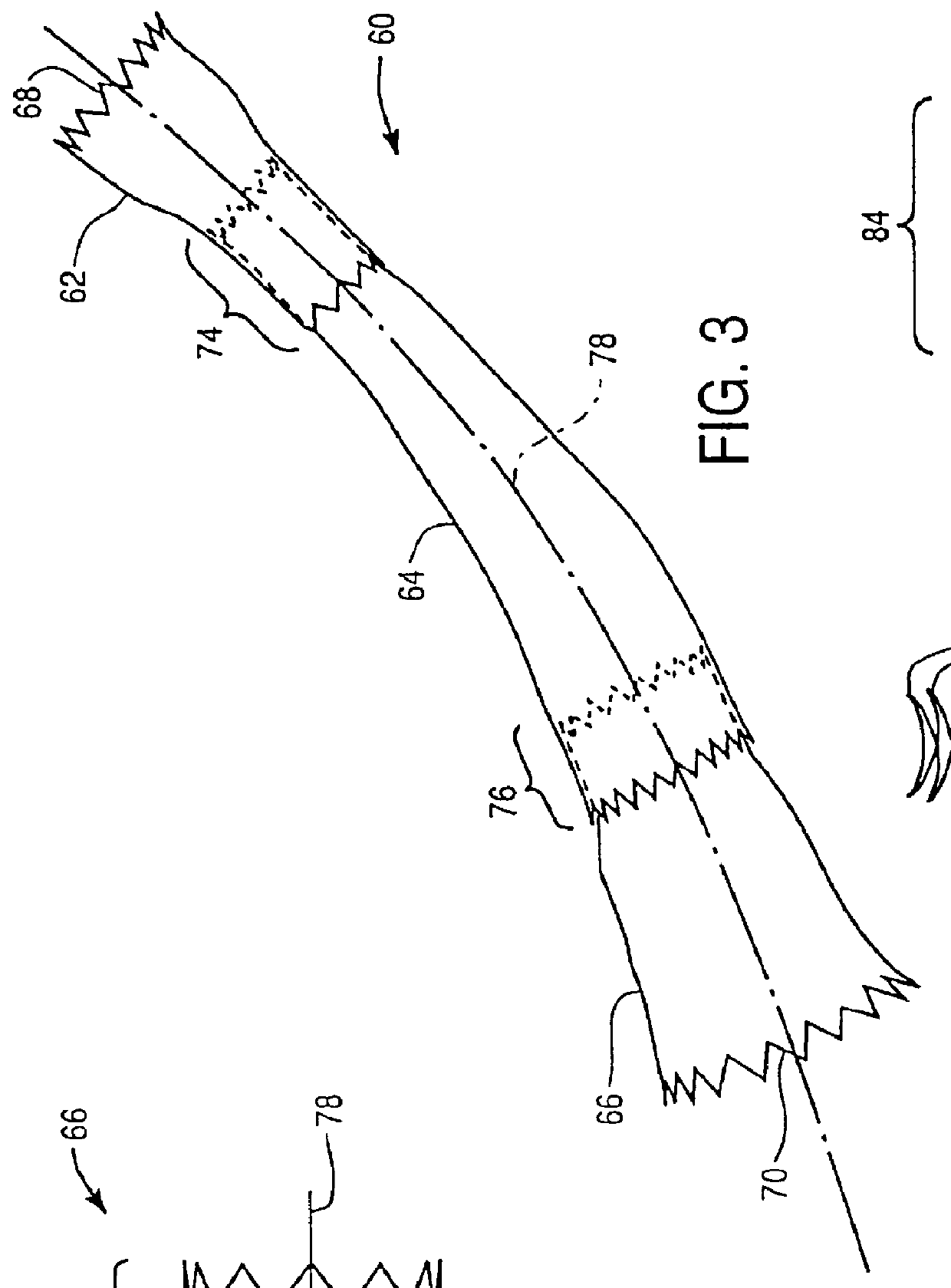
Stent vascular intervention device and methods for treating aneurysms
InactiveUS20070021816A1Shorten the construction periodGreat effect on aneurysmal blood flowStentsBlood vesselsPorosityInsertion stent
The present invention relates to a stent including a variable porosity, tubular structure having pores defined by structural surfaces. The tubular structure has a low porosity region in proximity to or at either end of the tubular structure, where the low porosity region is less porous than other regions located on the tubular structure and fully or partially obstructs passage of fluid. Any arcuate path that starts at one point within the low porosity region and goes around the perimeter of the tubular structure to stop at the same point within the low porosity region must have at least a portion that is outside of the low porosity region. Also disclosed is a method of modifying blood flow within and near an opening of an aneurysm in a blood vessel by deploying one or more stents of the present invention near an opening of the aneurysm in a blood vessel.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK
Removable occlusion system for aneurysm neck
A system for treating an aneurysm in a vessel includes a delivery device having a delivery portion suitable for delivery of embolic material. The delivery device is placed in a neck of the aneurysm and an expandable member is placed proximate the neck. The expandable member is expanded to overlie substantially the entire neck. Embolic material is delivered to the aneurysm with a delivery device. The expandable member is held over the neck to inhibit movement of the embolic material out of the aneurysm. Blood is allowed to flow out of the aneurysm, past the neck of the aneurysm, and through the vessel while the expandable member is held over the neck of the aneurysm.
Owner:STRYKER CORP +1
Isolation devices for the treatment of aneurysms
Device, systems and methods are provided to isolate aneurysms, particularly at bifurcations, while maintaining adequate blood flow through nearby vessels. These devices are deliverable to a desired target area and maintain position in a desired orientation so as to occlude flow in some aspect while allowing flow in others. In addition, devices, systems and methods are provided to occlude blood vessels, such as endoleaks, to improve the isolation of aneurysms.
Owner:TSUNAMI INNOVATIONS
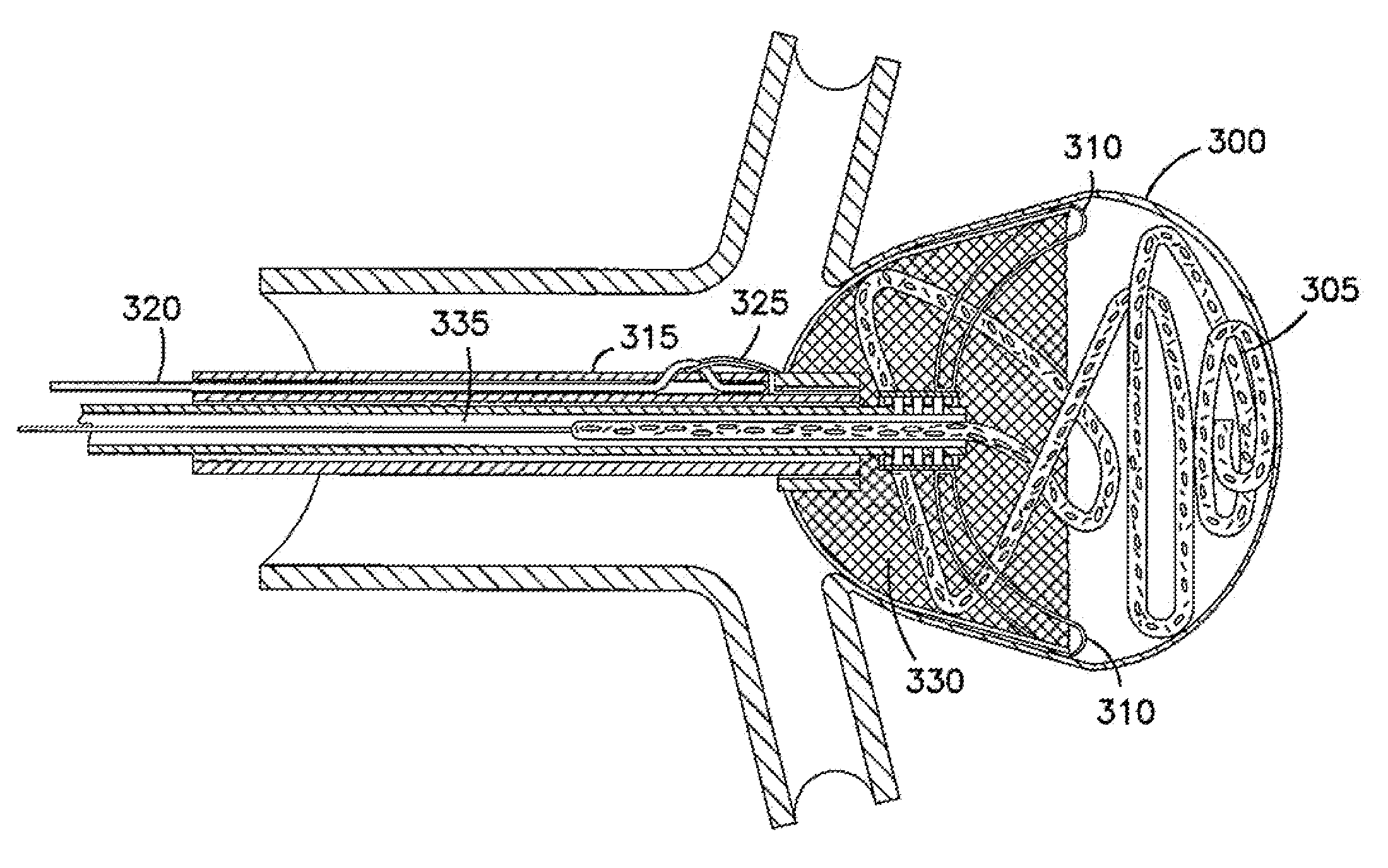
Intrasaccular embolic device
An intrasaccular device particularly adapted for treating body lumens. The intrasaccular device includes structure that provides a strong framework as well as improved covering across an opening to an aneurysm sac. The intrasaccular device is intended to retain foreign bodies within the aneurysm sac and includes members for accomplishing this objective. The intrasaccular device is also provided with structure that facilitates the alignment of a plurality of devices deployed within the sac.
Owner:ABBOTT CARDIOVASCULAR
Intra-aneurysm devices
Devices for occluding an aneurysm are provided. In particular, the device include an upper member that sits against the dome of the aneurysm, a lower member that sits in the neck of the aneurysm, and a means of adjusting the overall dimensions of the device. Also provided are methods of making and using these devices.
Owner:STRYKER EURO OPERATIONS HLDG LLC +1
Device forming an endoluminal intracorporeal endoprosthesis, in particular for the abdominal aorta
The invention relates to a device forming an endoluminal endoprosthesis. The device comprises at least a first segment, and upstream from said segment, at a predetermined distance therefrom, it comprises a "fixing" upstream segment made of a biocompatible material so as to be deployable from a closed or non-deployed position for insertion purposes to a deployed working position, the deployed working position being designed to be located in a healthy zone of blood vessel and being separated from the first segment by a predetermined distance defined by links of predetermined length. The invention makes it easy to recatheterize a blood vessel such as the abdominal aorta suffering from an aneurysm.
Owner:LEGONA ANSTALT
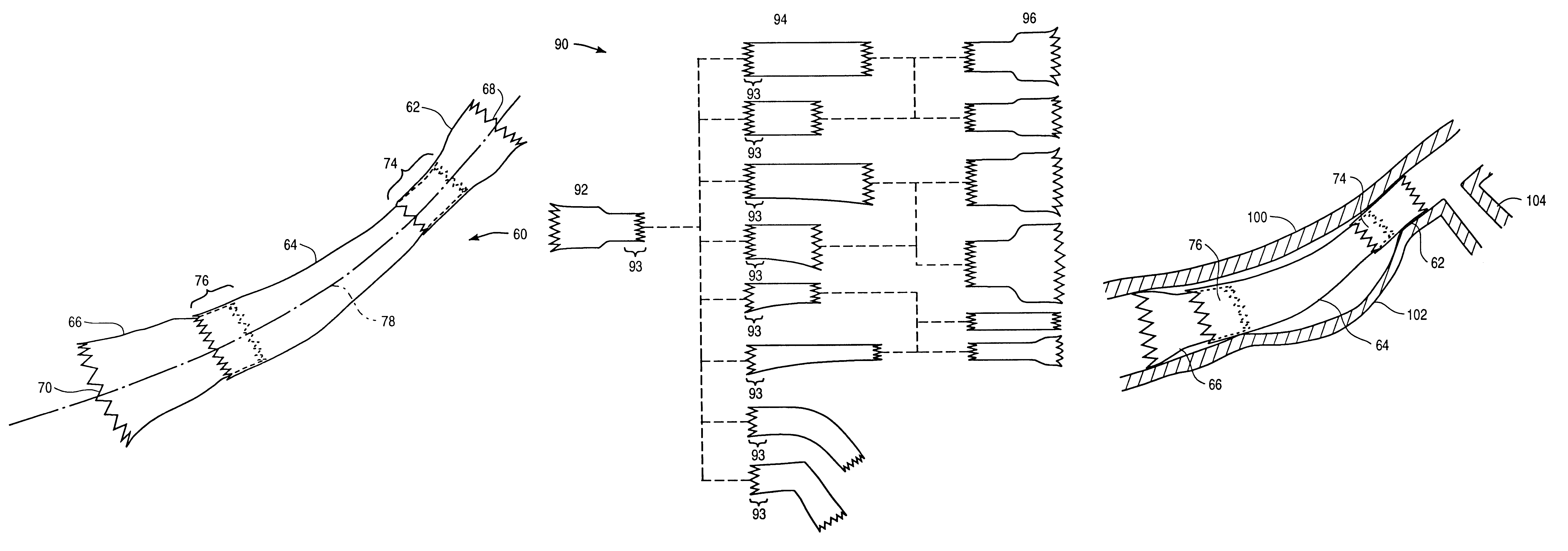
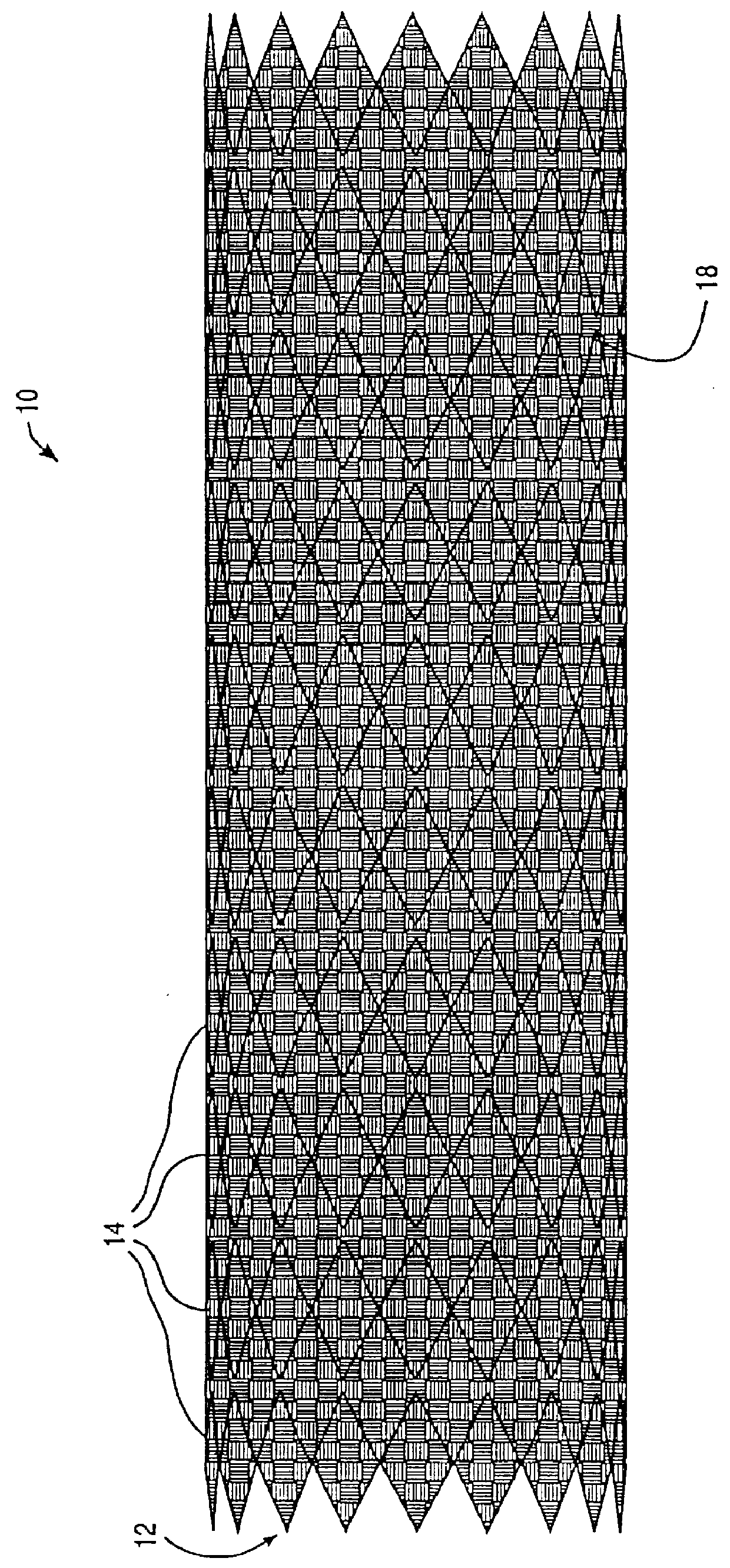
Modular stent graft assembly and use thereof
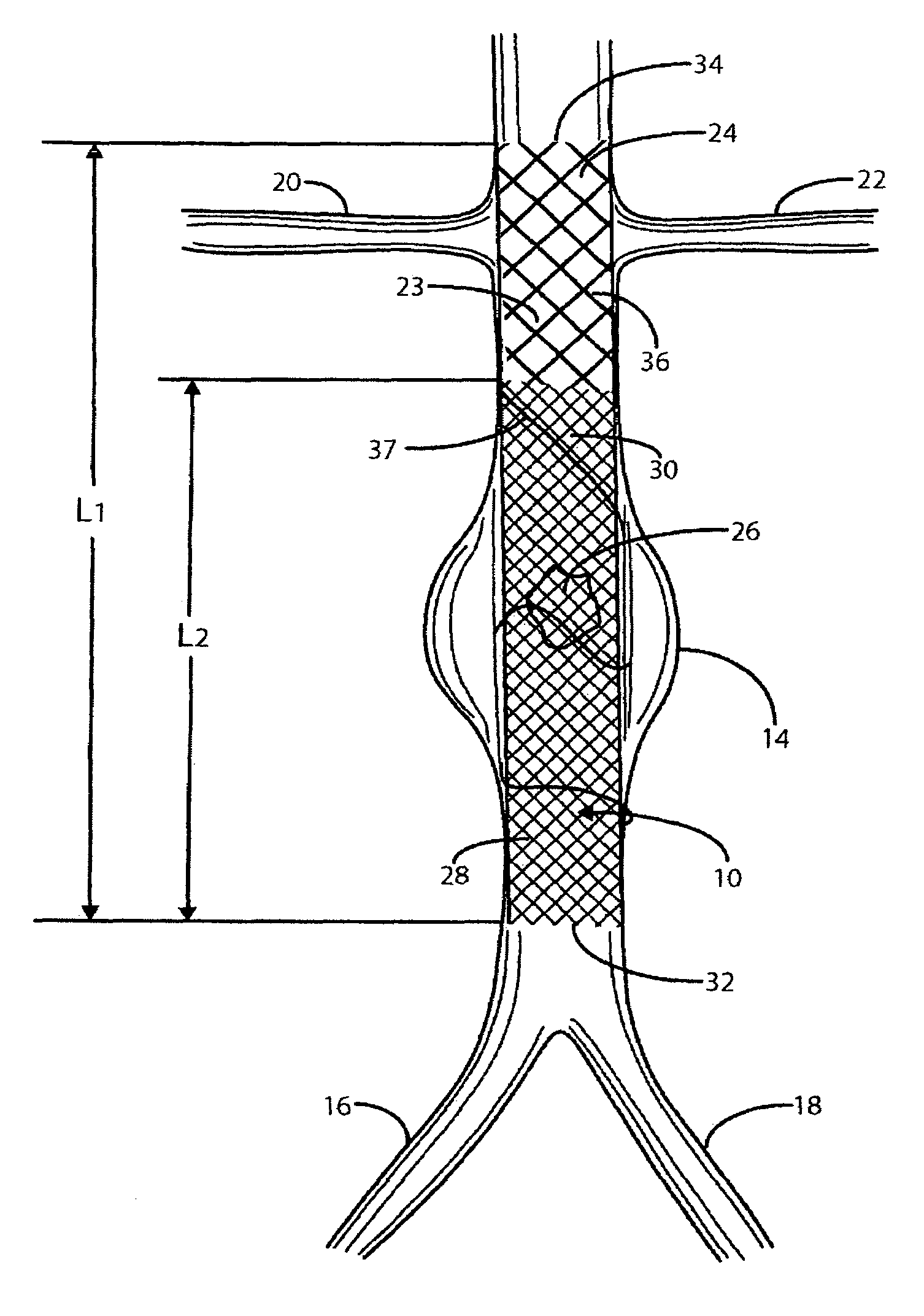
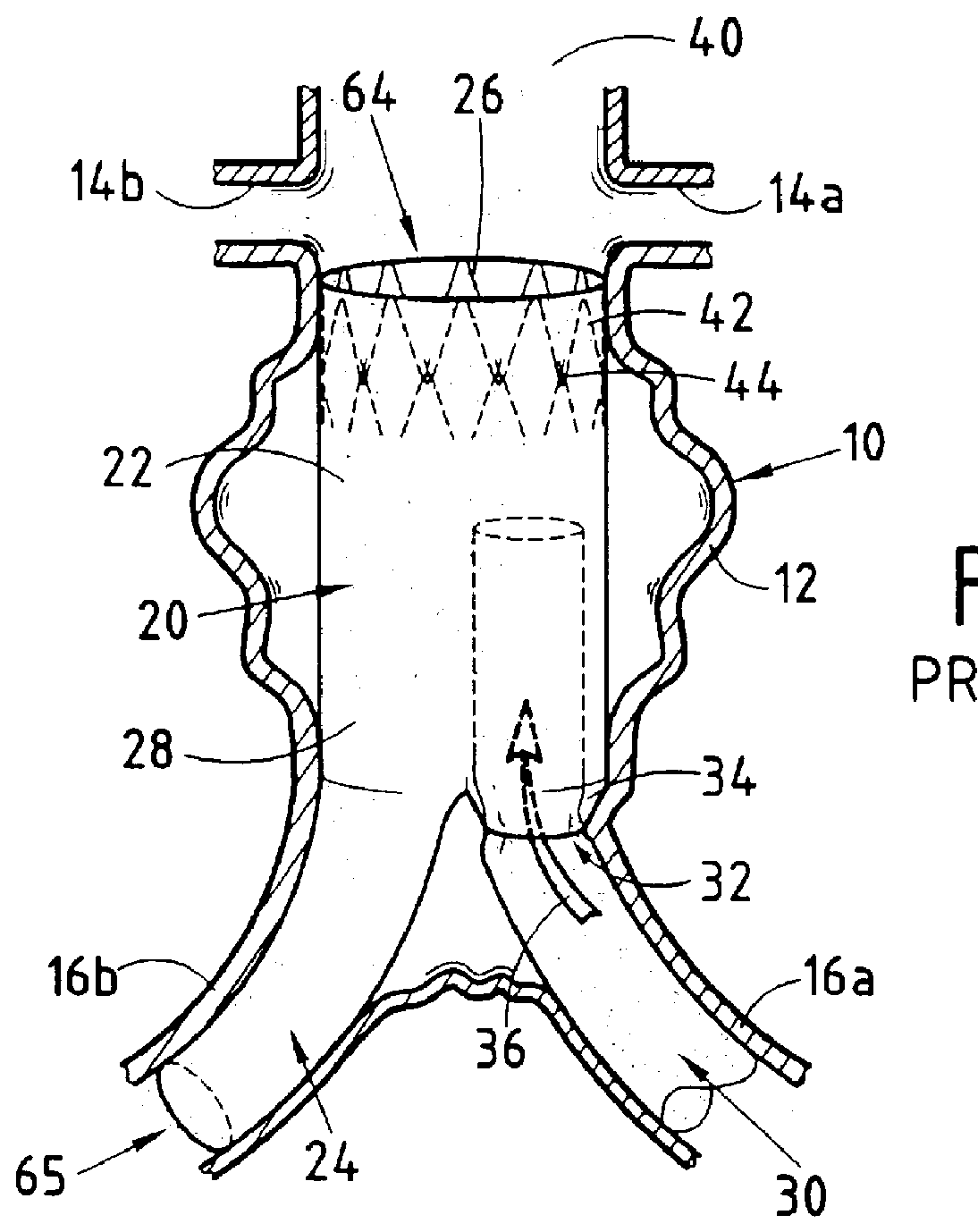
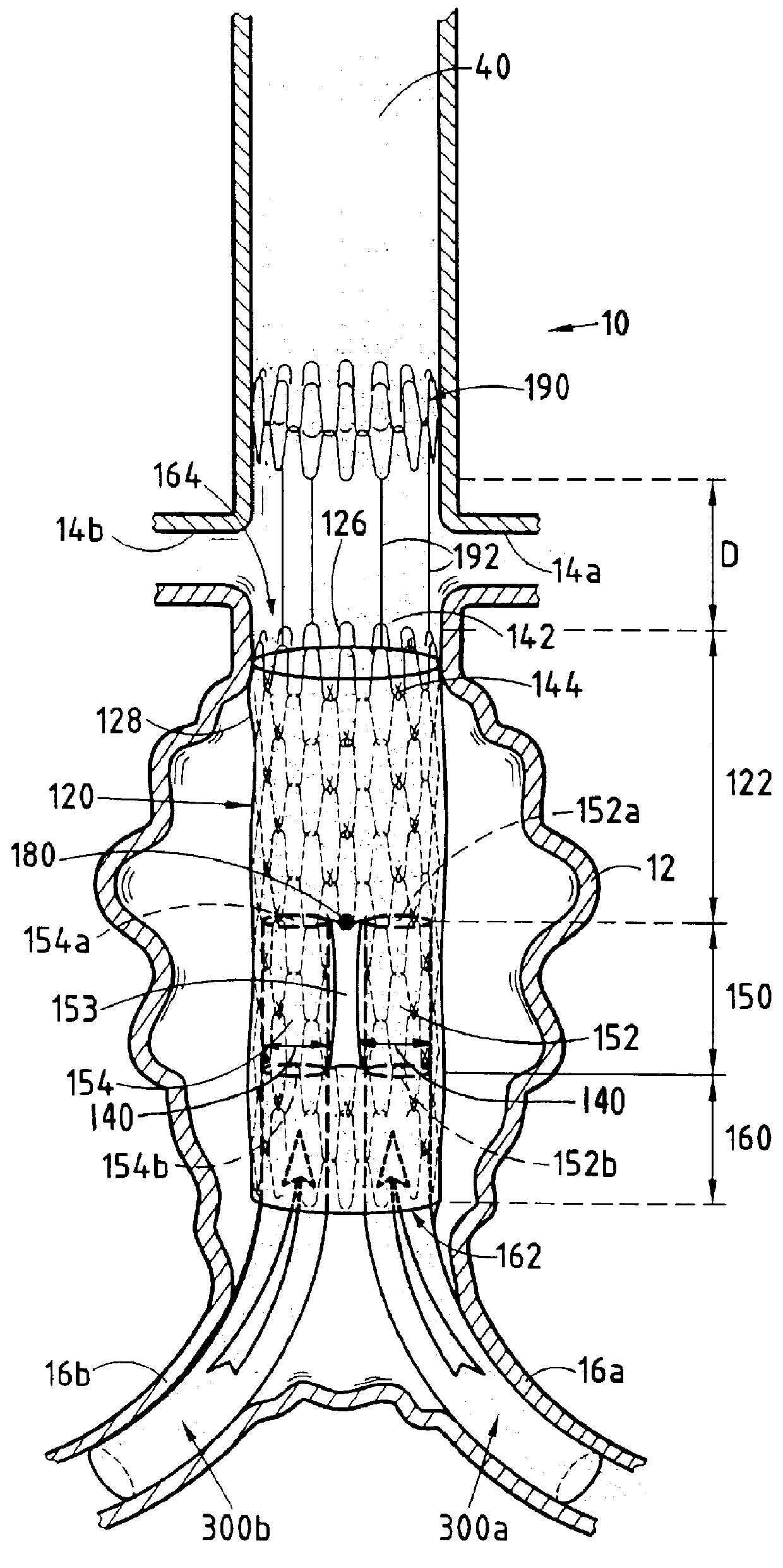
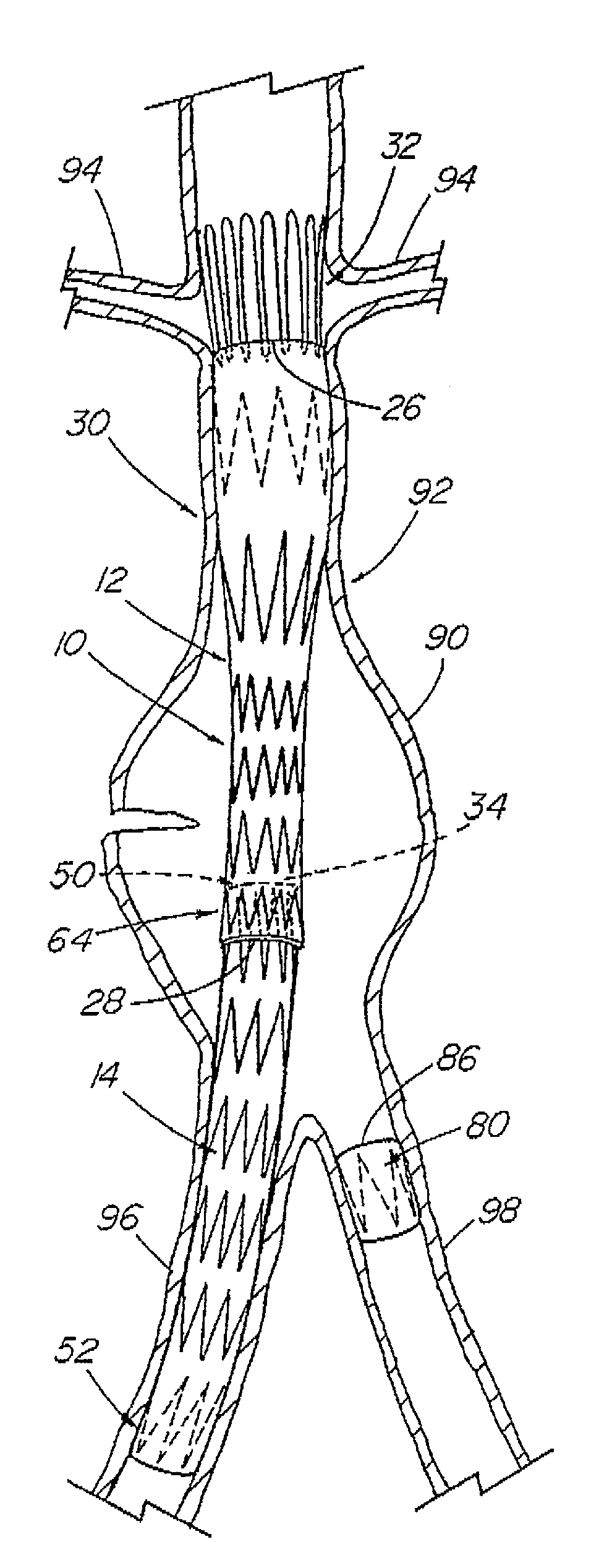
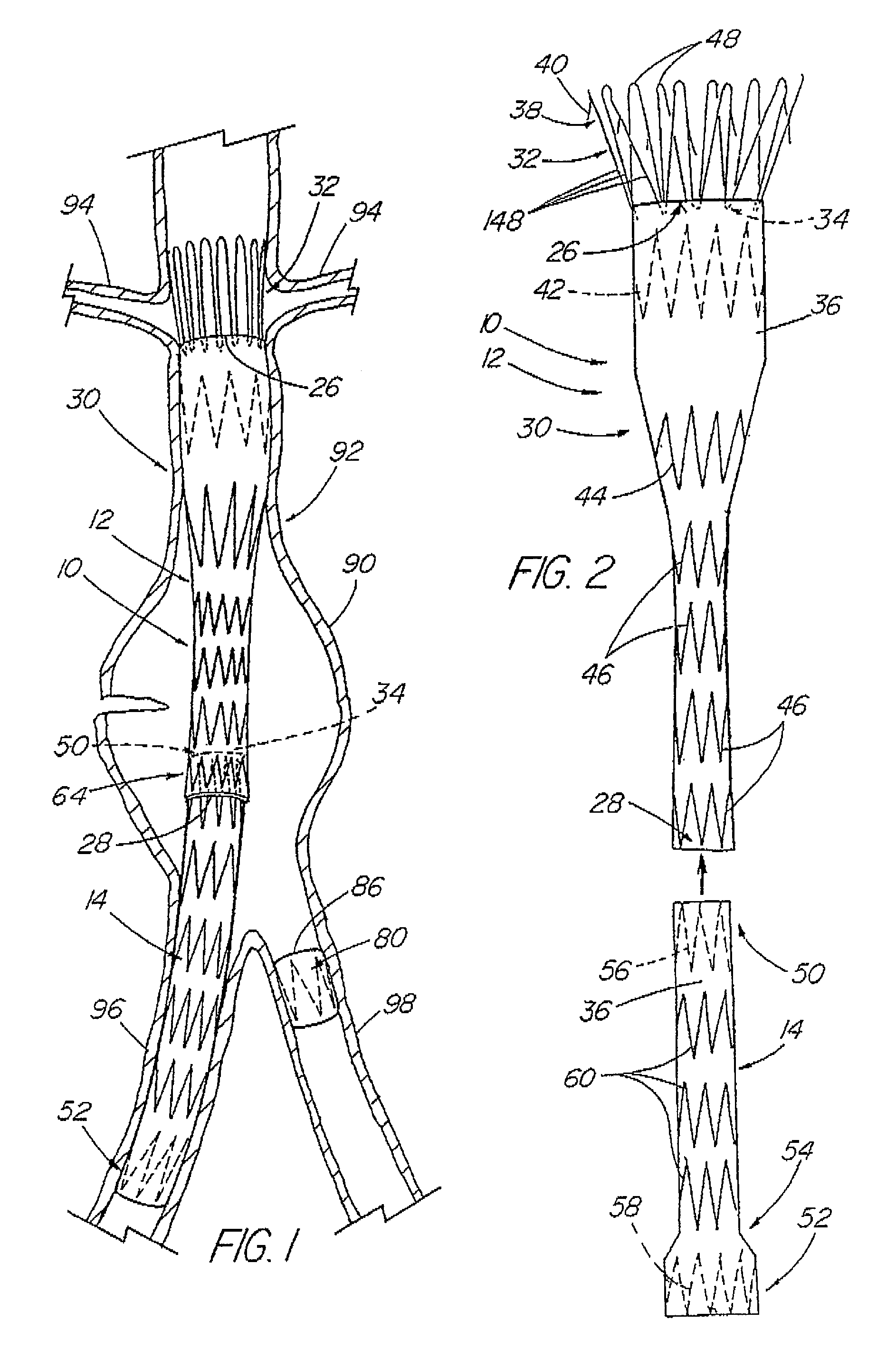
A modular stent graft assembly (10) for repairing a ruptured abdominal aorta aneurysm (90) and having an aortic section tubular graft (12) and an iliac section tubular graft (14). The aortic section graft has a proximal attachment stent (32) thereon for suprarenal attachment of the assembly (10) to the aorta. A proximal end portion (50) of the iliac section graft (14) underlies the distal end portion (28) of the aortic graft (12) and presses outwardly thereagainst forming a a friction fit, at a telescoping region (64). The assembly (10) can be selected from an inventory (300) containing a set of delivery systems (100) of four size aortic section grafts (12) and a set of delivery systems (200) of four size iliac section grafts (14), that together accommodate a large majority of aneurysm sizes, and delivery systems (250) containing four standard sizes of occluders (80).
Owner:COOK MEDICAL TECH LLC
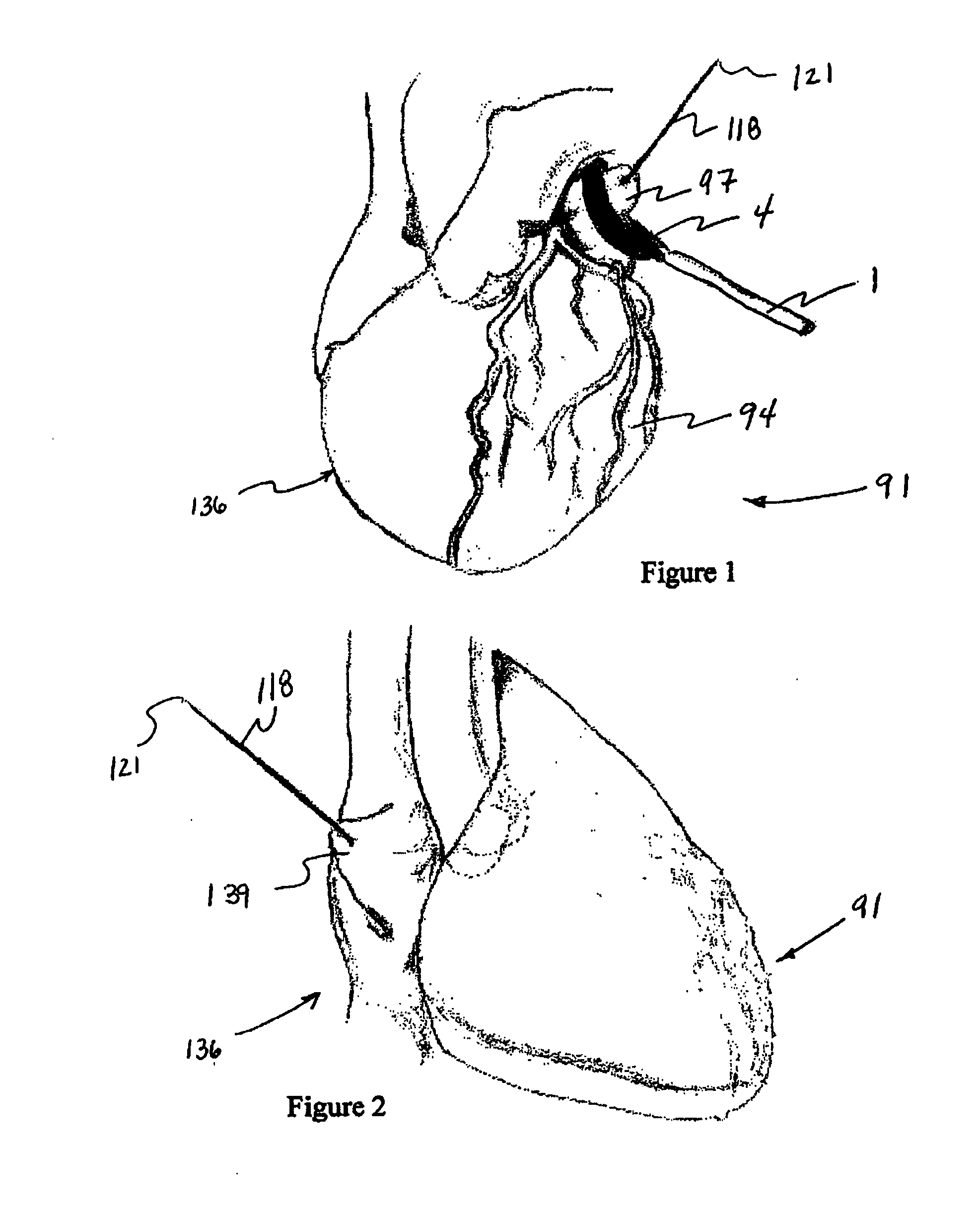
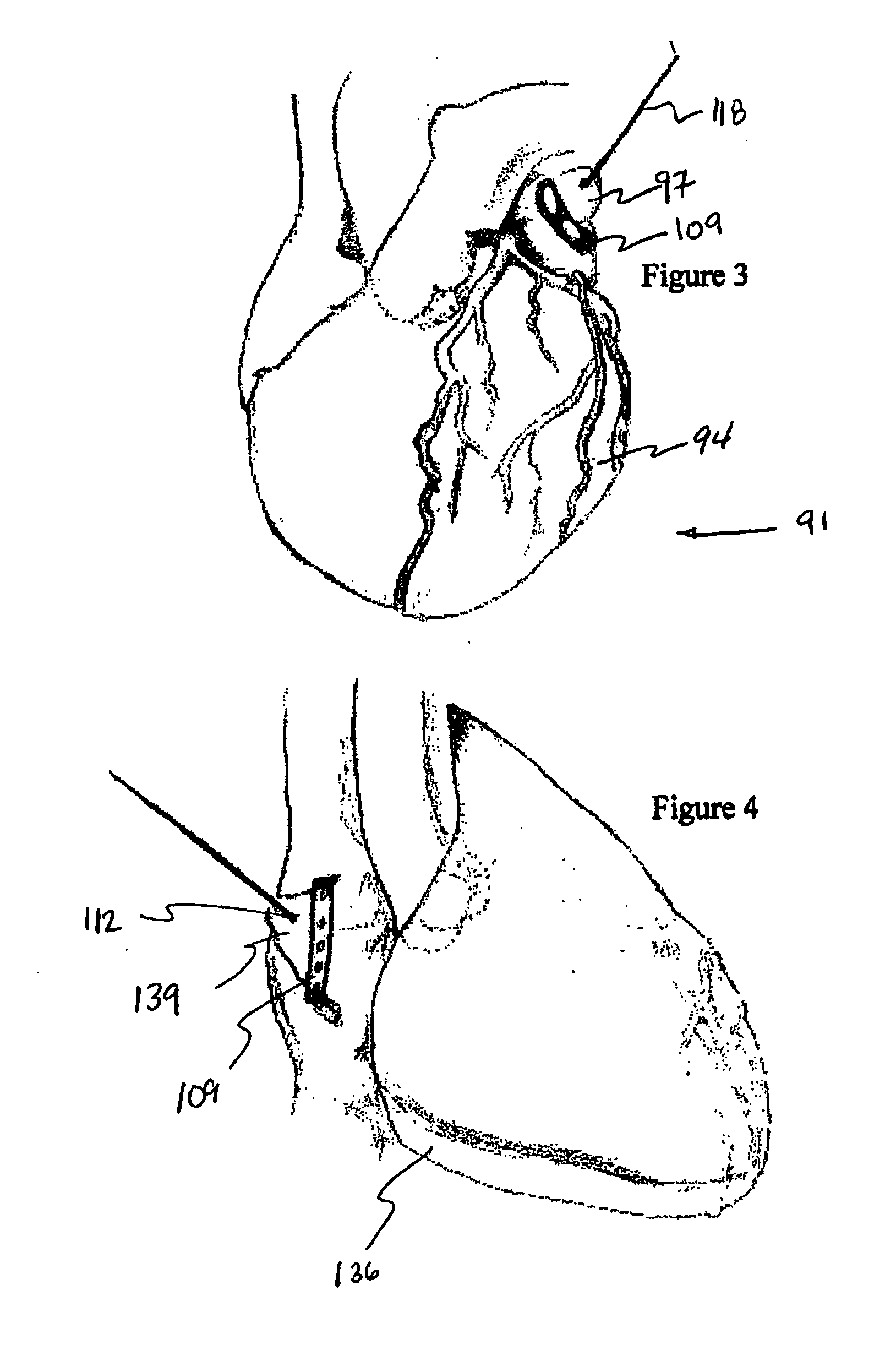
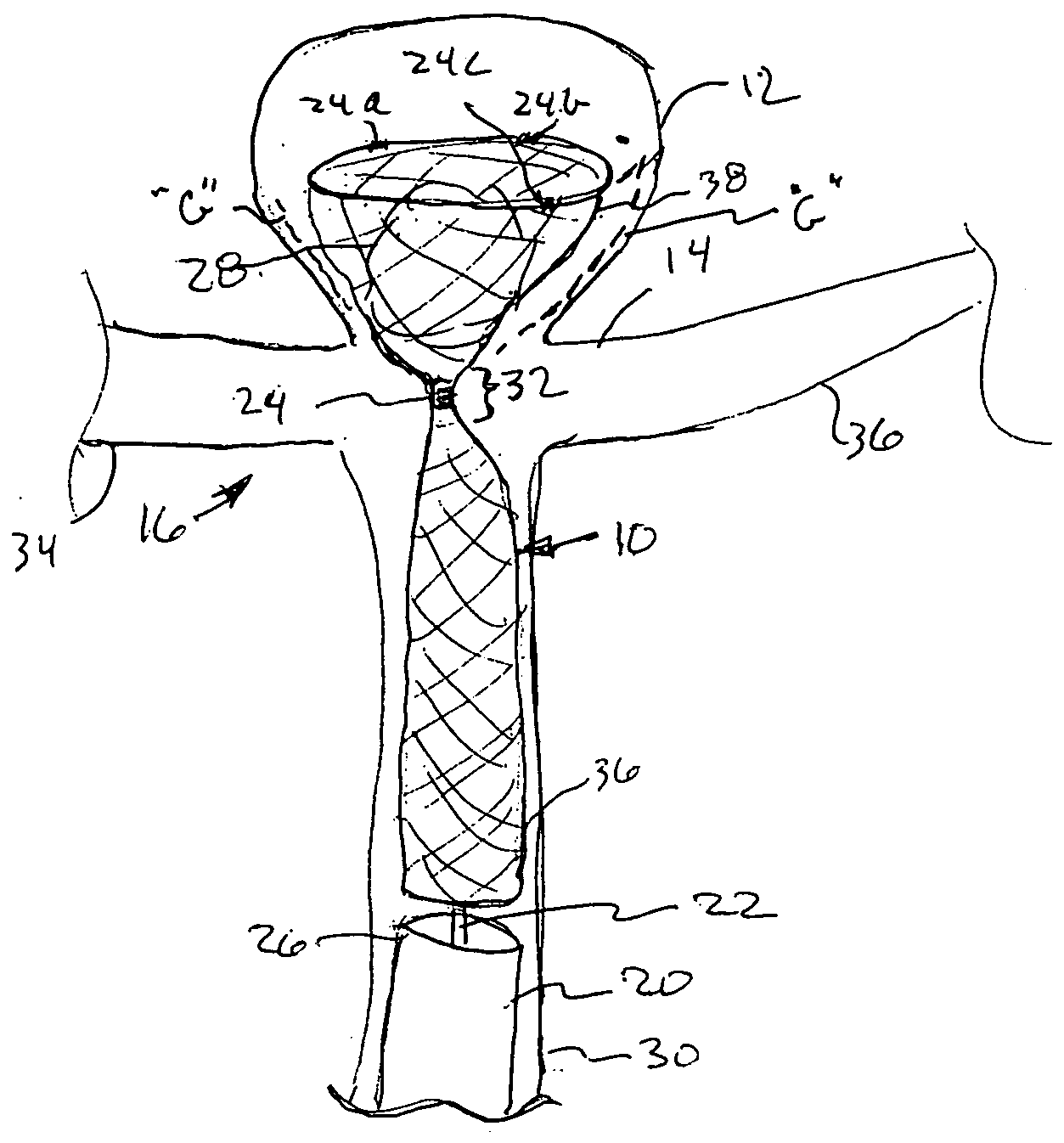
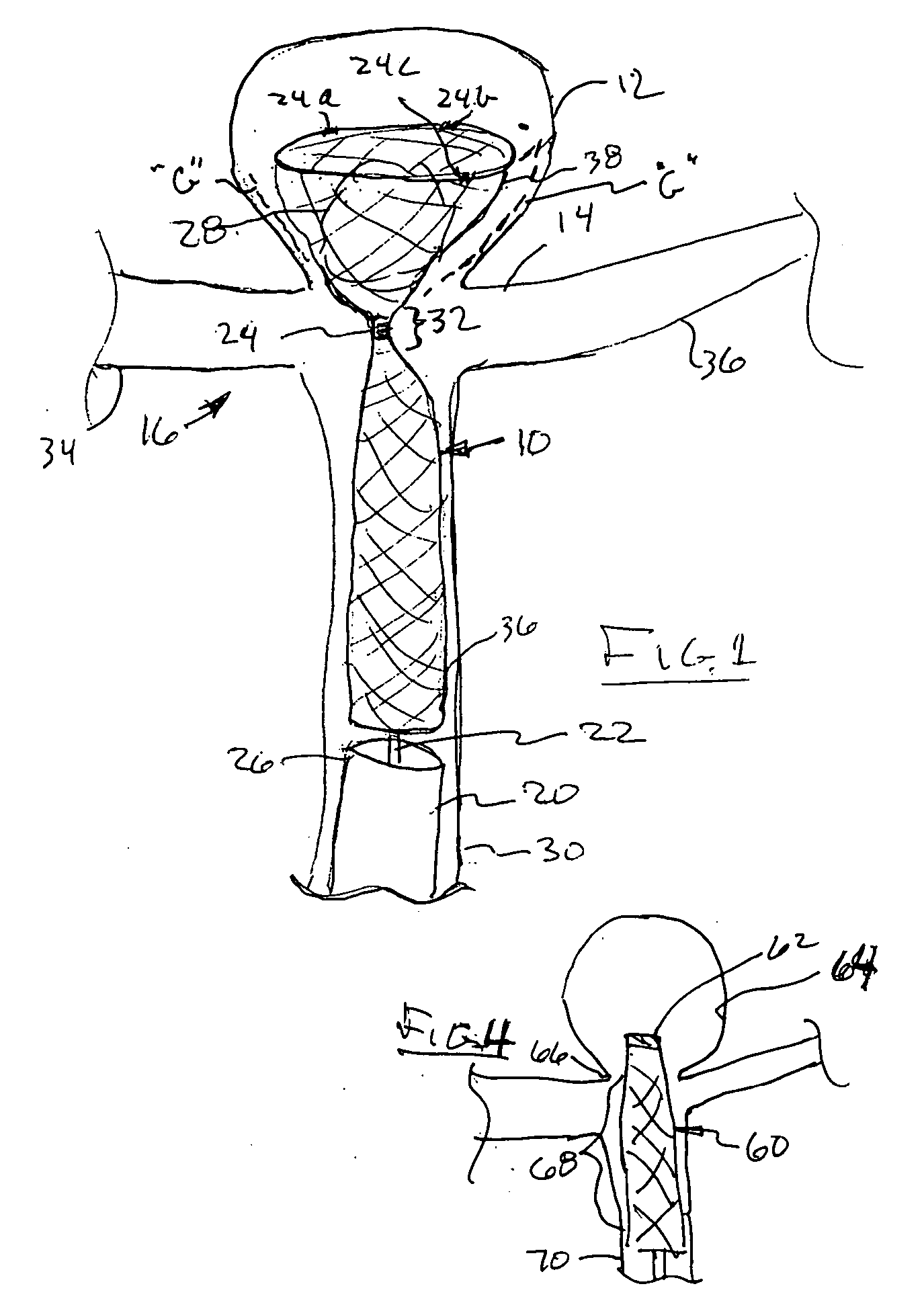
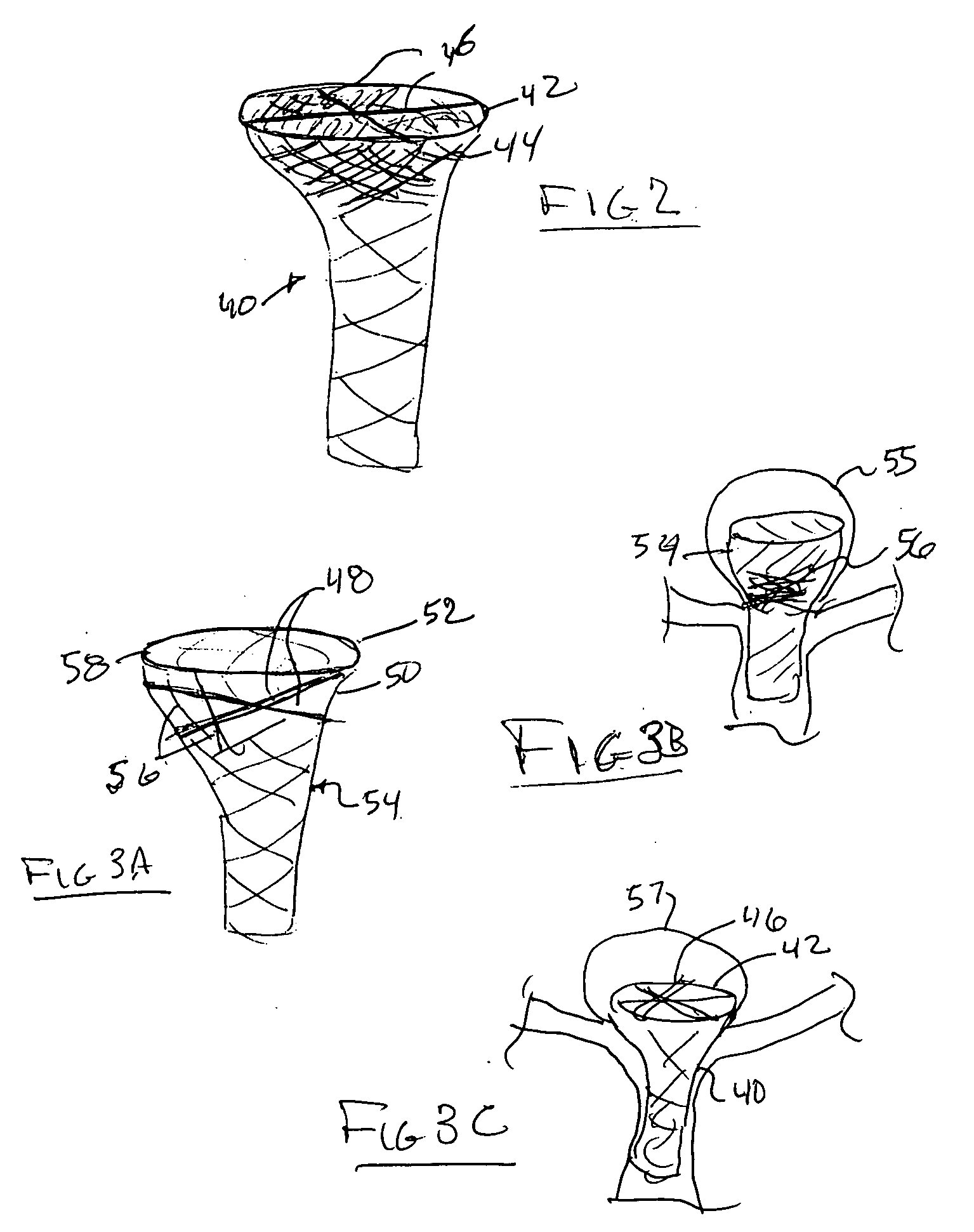
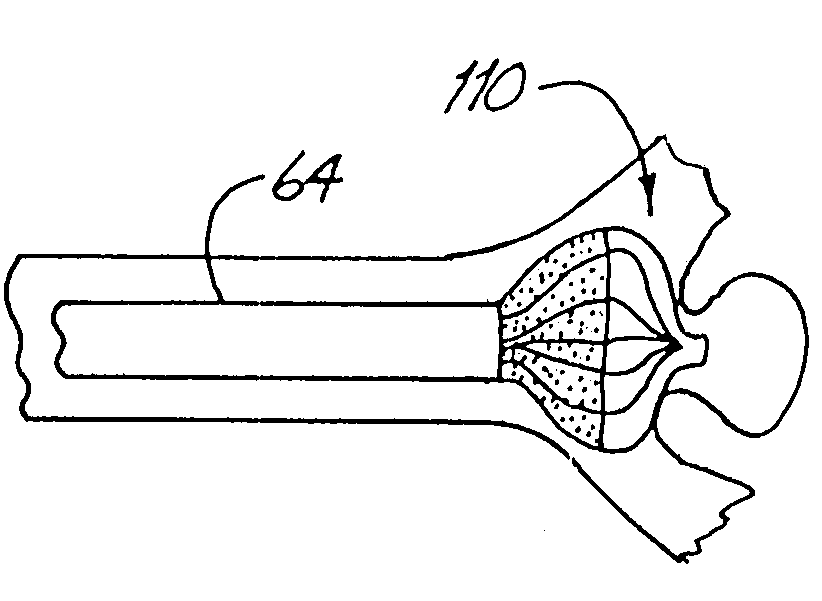
Detachable aneurysm neck closure patch
This is a device for bridging the neck of either an aneurysm in the vasculature and stabilizing the presence of vaso-occlusive devices (such as helically wound coils) in that aneurysm. The closure patch may be delivered either from the exterior of the distal end of a catheter or delivered from the catheter lumen. It is preferably implanted by the severance of an included electrolytically severable joint. The retainer assembly itself typically has a number of radially projecting elements which are, in turn, attached to a scrim-like fabric, preferably collagen-coated Dacron, extending among the various radially projecting elements. The closure patch is intended to be resident within the aneurysm after it is deployed. After deployment, the aneurysm may be at least partially filled with vaso-occlusive devices such as helically wound coils.
Owner:STRYKER EURO OPERATIONS HLDG LLC +1
Expandable support device and methods of use
InactiveUS20080071356A1Uniform deploymentIncrease maximum radial and shear forceStentsSpinal implantsIntervertebral discBiomedical engineering
An expandable support device and methods of using the same are disclosed herein. The expandable support device can expand radially when compressed longitudinally. The expandable support device can be deployed in a bone, such as a vertebra, for example to repair a compression fraction. The expandable support device can be deployed into or in place of all or part of an intervertebral disc. The expandable support device can be deployed in a vessel, in an aneurysm, across a valve, or combinations thereof. The expandable support device can be deployed permanently and / or used as a removable tool to expand or clear a lumen and / or repair valve leaflets.
Owner:STOUT MEDICAL GROUP
Self-expandable aneurysm filling device, system and method of placement
The self-expandable aneurysm filling device, system and method provide for placement of the stent into an aneurysm to at least partially fill and stabilize the aneurysm. The self-expandable aneurysm filling device has a compressed undeployed configuration and an expanded three-dimensional deployed configuration, and a severable deployment junction releasably connects the self-expandable aneurysm filling device to a pusher wire. The severable deployment junction can be mechanically, electrolytically, or thermally severed to separate the self-expandable aneurysm filling device from the pusher wire.
Owner:PENUMBRA
Stent graft with branch leg
The present invention is directed to a system, apparatus, and method for treating, repairing, and / or replacing an aneurysm, preferably an aortic aneurysm, and most preferably, an abdominal aortic aneurysm. The systems, devices, and methods of the present invention include a first prosthesis or stent gasket, and at least one second prosthesis for bypassing the aneurysm. In preferred embodiments, the second prosthesis includes a branch leg that may be disposed and anchored in an either a cross artery or a downstream artery to facilitate fluid flow. In other preferred embodiments, at least one third prosthesis is provided for establishing a fluid flow channel through a second diseased artery to bypass an aneurysm disposed therein. In accordance with the invention, the first artery may be the abdominal aorta and the second artery may be an iliac artery.
Owner:CARDINAL HEALTH SWITZERLAND 515 GMBH
Implantable aneurysm closure systems and methods
An implantable closure structure is delivered using minimally invasive techniques and inhibits the migration of liquid and particulate matter from inside a physiological cavity or opening, such as an aneurysm, as well as inhibiting the flow of liquid and particulate matter, such as from an associated blood vessel, into a physiological cavity or opening. The device has a flexible patch supported by a framework structure that covers the neck or opening of a cavity such as an aneurysm and may have anchoring structures for supporting the flexible patch in place across the opening.
Owner:PULSAR VASCULAR
Means and method for the treatment of cerebral aneurysms
Disclosed is a system for the treatment of cerebral aneurysms using a stent and a aneurysm pocket fill structure delivery system. One embodiment of the present invention uses a highly radiopaque, drug eluting stent that is deployed with its sidewall over the ostium of the aneurysm pocket. A fill structure delivery catheter is then advanced through the patient's vascular system until the catheter's distal end is situated within the aneurysm pocket. Compressed aneurysm pocket filling structures are then pushed through the fill structure delivery catheter. As the aneurysm pocket filling structures emerge from an opening in the catheter's distal end, they promptly expand so that their minimum dimension is sufficiently large so that they cannot pass through the spaces between the struts of the stent that cover the ostium of the aneurysm pocket.
Owner:FISCHELL ROBERT E +1
Thin Film Metallic Devices for Plugging Aneurysms or Vessels
InactiveUS20070270902A1Reduce riskFacilitates endoluminal deploymentStentsDilatorsBlood vesselEmbolization
Thin film metallic devices implantable within a human subject for occlusion of an aneurysm or blood vessel are provided. The devices include an embolization element that is moveable between a collapsed configuration for delivery to a deployed configuration within the body. The embolization device plugs the aneurysm or blood vessel preventing blood from flowing into or out of the aneurysm or other defective or diseased location of the blood vessel. The embolization element may be either self-supporting or supported by a strut structure. The occlusion device also includes an anchor element for anchoring the occlusion device and aiding in maintaining the embolization element in place. The anchor element is connected to the embolization device via a connector element.
Owner:DEPUY SYNTHES PROD INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com