Endovascular aneurysm devices, systems, and methods
a technology of endovascular aneurysm and endovascular aneurysm, applied in the field of endovascular aneurysm devices, systems, and methods, can solve the problems of aneurysm formation, aneurysm growth, and the formation of aneurysms, and achieve the effects of reducing the risk of aneurysm formation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
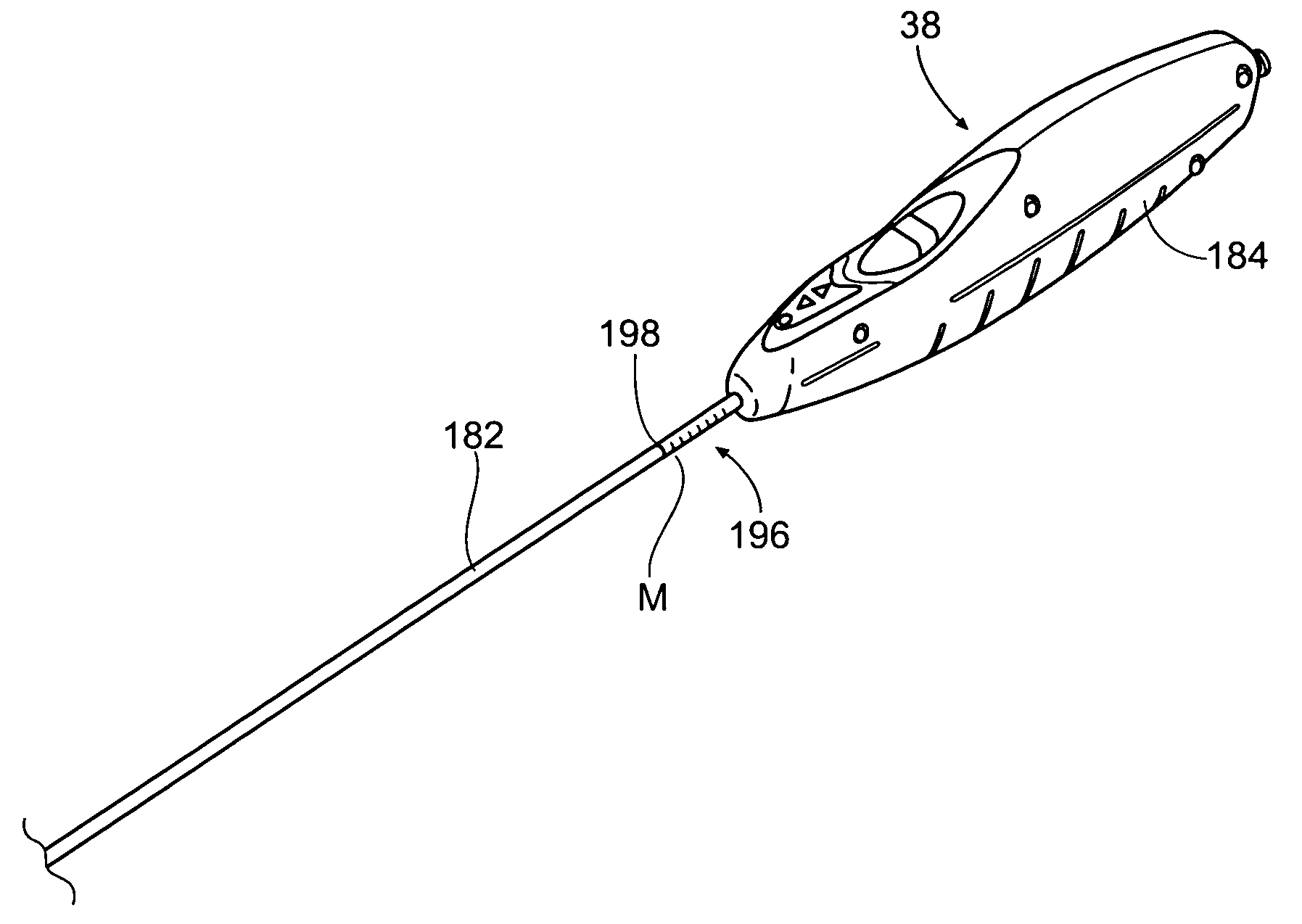
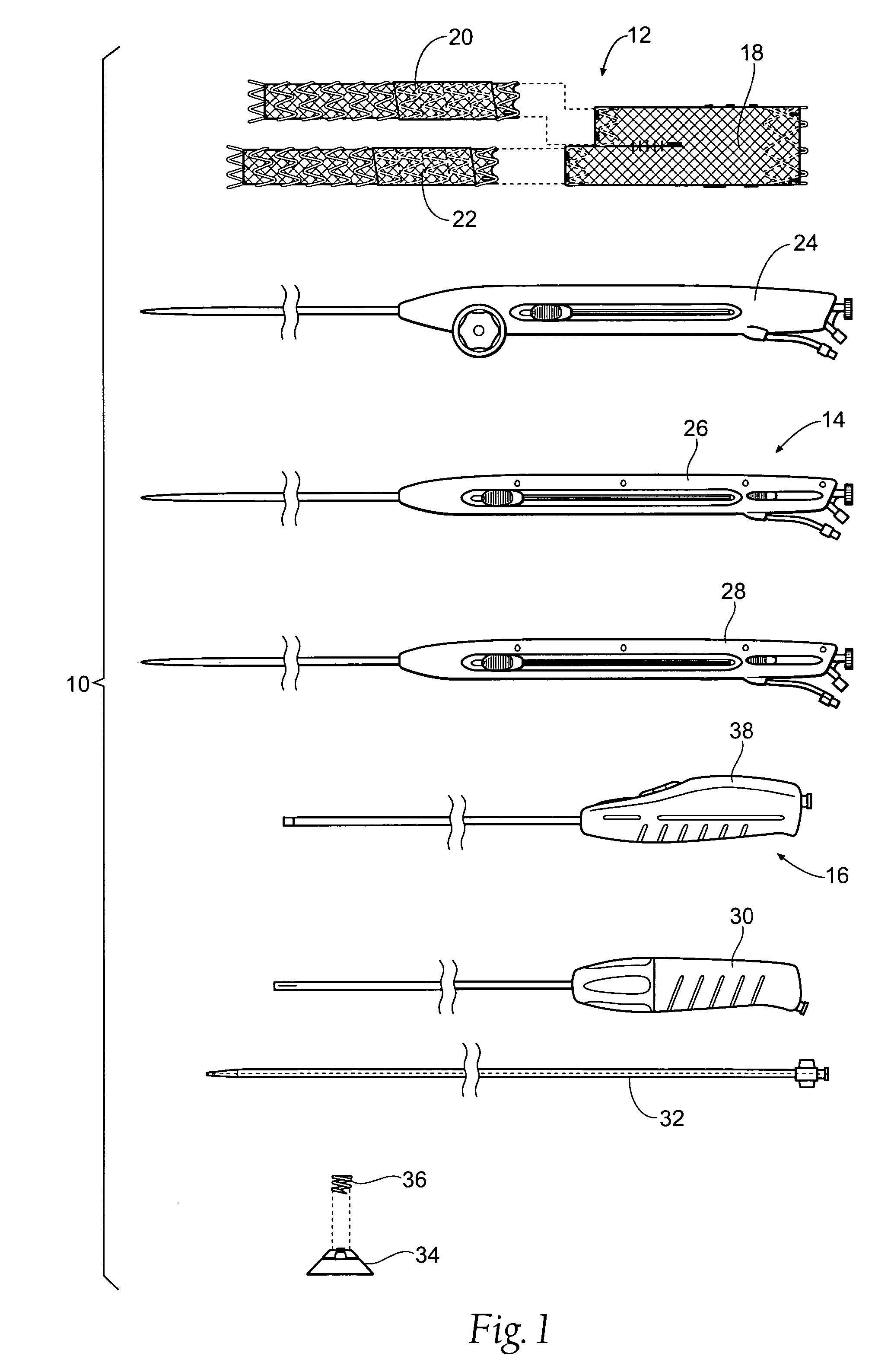
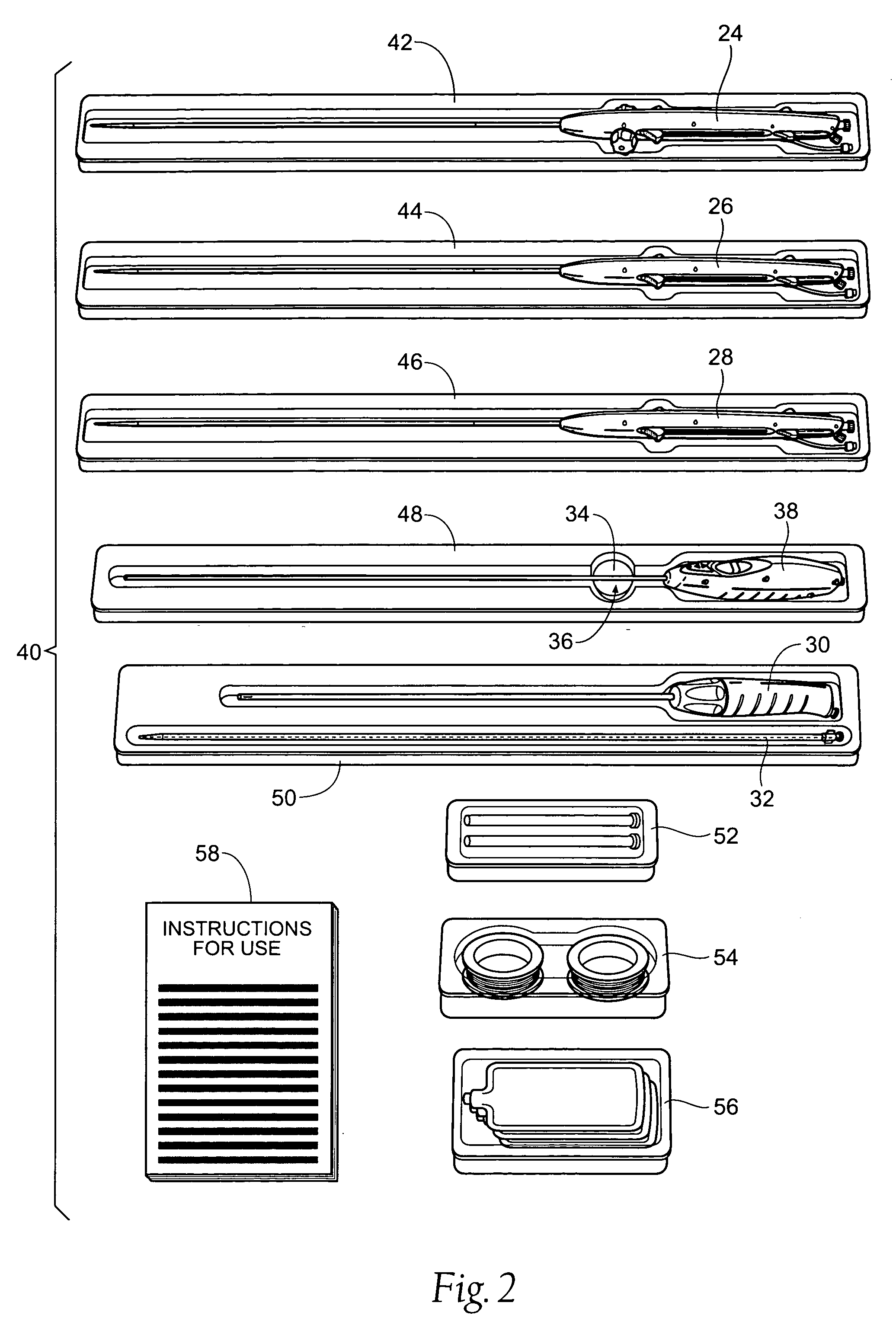
[0044] This Specification discloses various catheter-based devices, systems, and methods for delivering and implanting radially expandable prostheses in the body lumens. For example, the various aspects of the invention have application in procedures requiring the repair of diseased and / or damaged sections of a hollow body organ and / or blood vessel. The devices, systems, and methods that embody features of the invention are also adaptable for use with systems and surgical techniques that are not necessarily catheter-based.
[0045] The devices, systems, and methods are particularly well suited for treating aneurysms of the aorta that primarily occur in the abdominal region, usually in the infrarenal area between the renal arteries and the aortic bifurcation, as well as aneurysms that also occur in the thoracic region between the aortic arch and renal arteries. For this reason, the devices, systems, and methods will be described in this context. Still, it should be appreciated that the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com