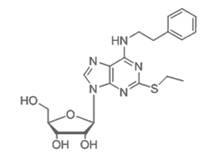
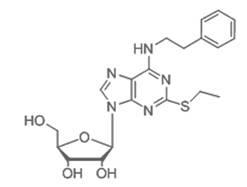
Bi-functional antiplatelet aggregation medicine and application thereof
An anti-platelet aggregation, dual-function technology, applied in the field of medicine, can solve the problems that have not yet been
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Example 1. Inhibition of Phosphodiesterase Activity by BF061
[0021] The purpose of this example is to demonstrate the anti-phosphodiesterase activity of BF061.
[0022] In this example, phosphodiesterase was purified from human platelets, and the enzyme reaction was performed in 200 μL of PBS buffer (137 mM NaCl, 2.7 mM KCl, 8.8 mM NaCl 2 HPO 4 , 1.5 mM KH 2 PO 4 , 1 mM CaCl 2 , 1 mM MgCl 2 , 10 μM cAMP, pH 7.4), the substrate is 25 μM cAMP (sigma company), the system contains 10 μL of phosphodiesterase, and the phosphodiesterase is calculated by measuring the residual substrate concentration after 30 minutes of enzyme reaction activity. The residual substrate concentration was determined by high-pressure liquid chromatography with a C18 column (Kromasil 4.6 × 150 mm, Eka-chemicals, Bohns, Sweden). The results are shown in Table 1, showing that BF061 has obvious inhibitory effect on phosphodiesterase activity.
[0023]
[0024] Table 1: BF061 inhibits phosp...
Embodiment 2
[0026] Example 2. P2Y of BF061 12 receptor antagonism
[0027] The purpose of this example is to prove that BF061 has P2Y 12 Receptor antagonism.
[0028] In this example, the ligands ADP and P2Y 12 The binding force between the receptors was determined by atomic force microscopy (Asylum Research, Santa Barbara, CA). The probe was pre-coated in ADP (500 ng / μL, 20 μL) overnight, and finally ADP was coupled to the probe. P2Y 12 The receptors are expressed in CHO-K1 cells. The conditions for the determination of the binding force between the probe and the cell surface receptors are: pressure velocity 3 μm / s, contact time 0.2 s, contact force 100 pN, cantilever correction coefficient about 20 pN / nm. The results are shown in Table 2. After BF061 treatment, the ligands ADP and P2Y 12 The binding force between receptors is significantly weakened, showing that BF061 has obvious P2Y 12 Receptor antagonism.
[0029]
[0030] Table 2: BF061 antagonizes P2Y 12 Receptors b...
Embodiment 3
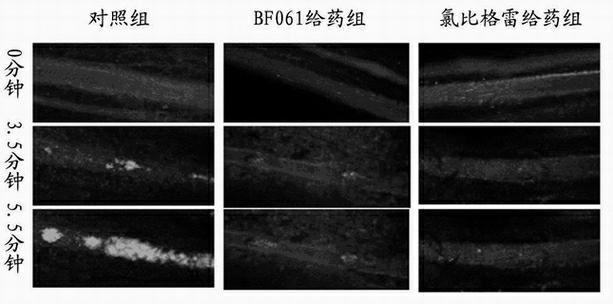
[0032] Example 3. Inhibitory effect of BF061 on ADP-induced human platelet aggregation
[0033] The purpose of this example is to prove that BF061 can inhibit ADP-induced human platelet aggregation
[0034] Preparation of platelets: Platelets were obtained from healthy volunteers who signed the informed consent form. Volunteers had not taken any antiplatelet drugs such as aspirin and clopidogrel within 20 days before blood collection. Anticoagulant ACD (85 mmol L -1 sodium citrate, 71.38 mmol L -1 citric acid, and 27.78 mmol L -1 glucose). Centrifuge at 300 x g for 20 minutes to obtain platelet-rich plasma, take the supernatant and centrifuge at 900 x g for 10 minutes to obtain platelets, and finally resuspend in Tyrode’s buffer (138 mmol L -1 NaCl, 2.7 mmol L -1 KCl, 2 mmol L -1 MgCl 2 , 0.42 mmol L -1 NaH 2 PO 4 , 5 mmol L -1 glucose, 10 mmol L -1 HEPES, 0.2% bovine serum albumin, and 0.02 unit mL -1 apyrase, pH 7.4), to obtain washed platelets, the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com