Trombolysis agent KGD - prourokinase chimaera, preparing process and application thereof
A kind of technology of prourokinase and thrombolytic agent
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
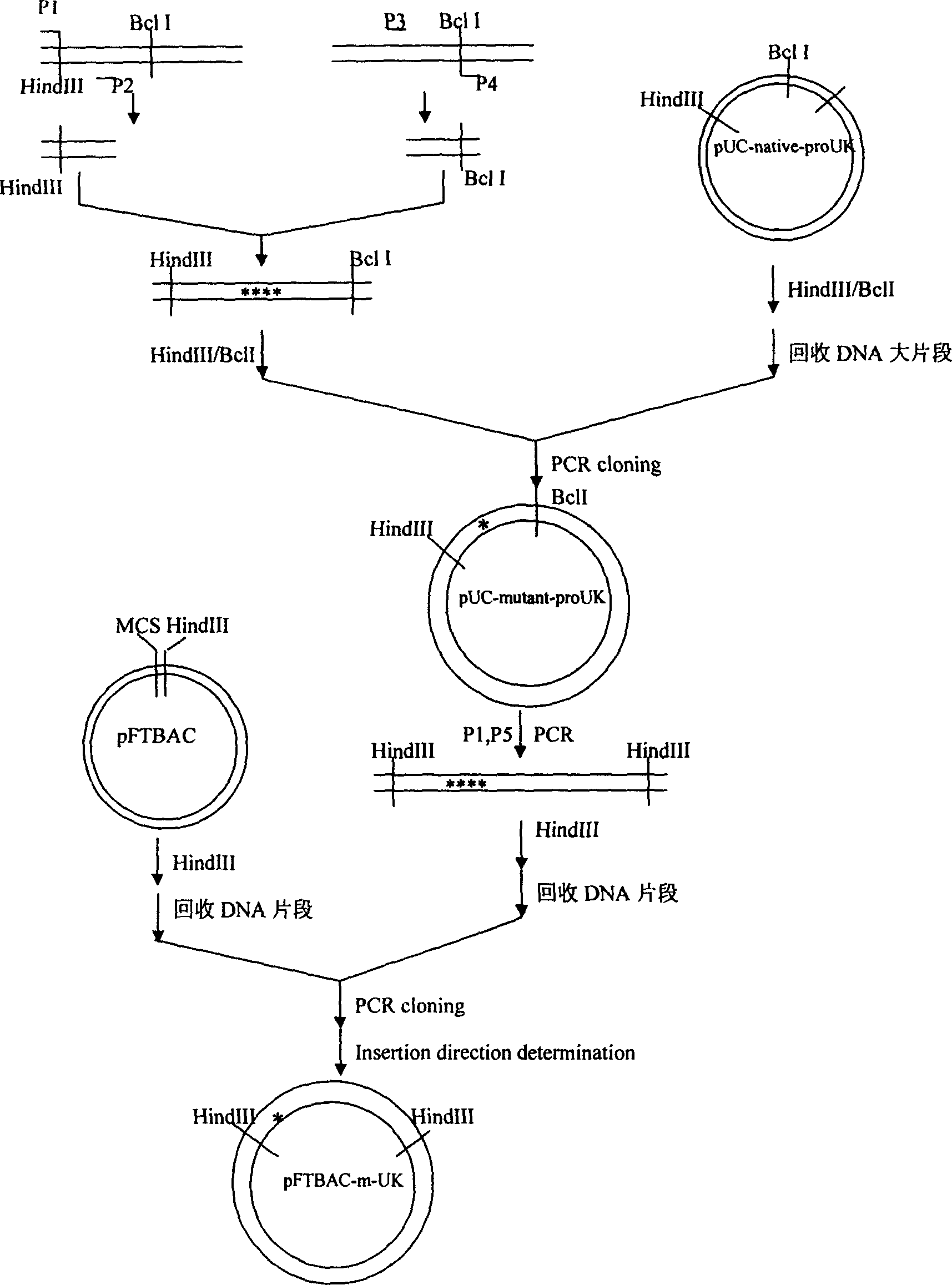
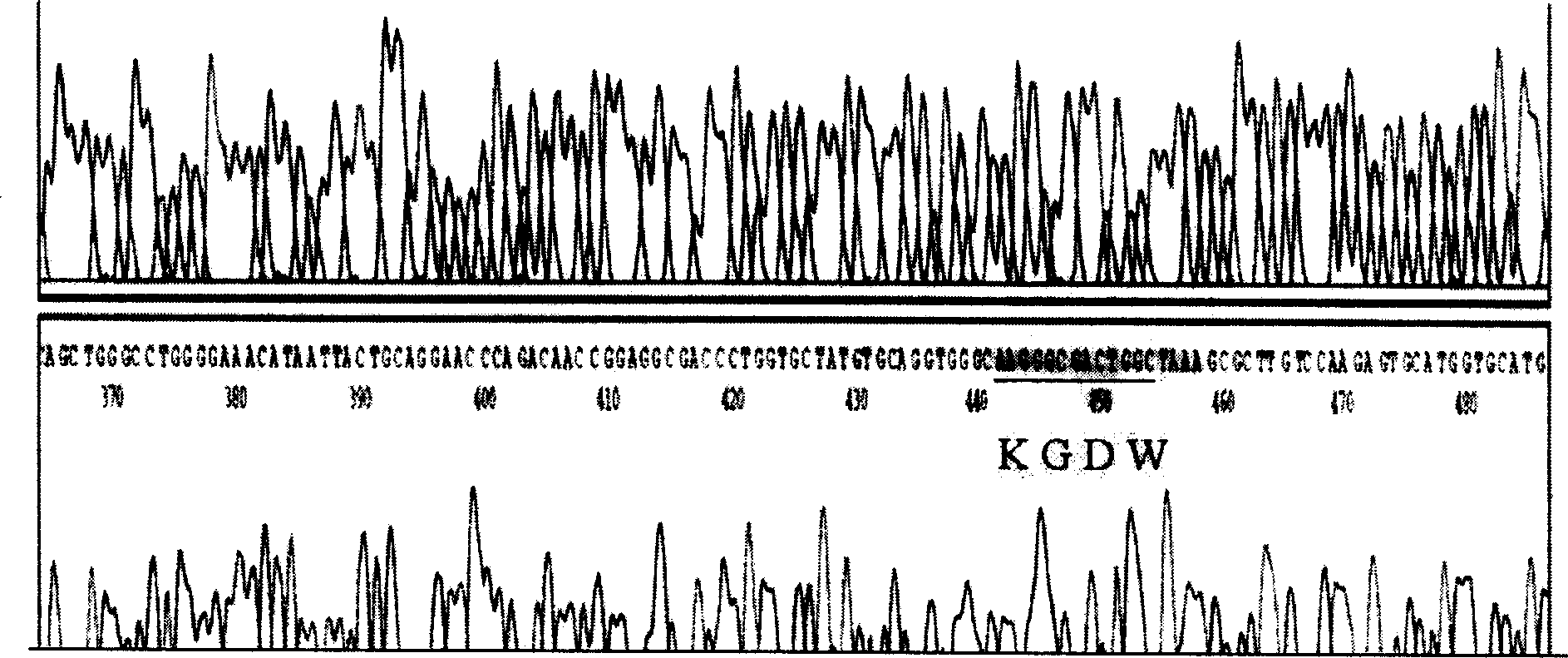
[0038] Example 1. KGDW-prourokinase gene of chimeric KGDW sequence
[0039] Construction and transfection of insect cell Sf9
[0040] Experimental Materials:
[0041] Strains: E.coli DH5α, BL21(DE3), JM109 are all commercially available products, and PUC19-proUK cDNA was constructed in our laboratory. Insect baculovirus expression system Sf9( Spodoptera frugiperda-9 ) cells and Sf21 cells were purchased from Invitrogen, USA.
[0042] Primers: DNA fragments of oligonucleotide primers were synthesized by Beijing Saibaisheng Company. Primer 1: 5'CCCAAG CTT GAT ATC ATG A3'; Primer 2: 5'GACAAGCGGCTTTA GCCAGTCCCCTTTGCCC ACCTGC ACATA 3'; Primer 3: 5'CTA AAG CCG CTT GTC 3'; Primer 4: 5'GTG GCG CTG ATC ACCC 3' .
[0043]Chemical reagents and enzymes: commonly used restriction enzymes, T4 DNA ligase, Klenow fragment, T4 DNA polymerase, T4 polynucleotide kinase, Taq DNA polymerase, RNase, ATP, dNTPs, etc. were purchased from Promega Company and New England Biolab Company , Hua...
Embodiment 2
[0055] Example 2. Insect cell Sf9 transfected with KGDW-prourokinase chimeric gene
[0056] Culture and induced expression of KGDW-prourokinase chimeric protein
[0057] The KGD-prourokinase chimeric protein was produced by insect cell secreted expression. Firstly, adapt the insect cell Sf9 series under serum-free conditions to obtain insect cells that can grow well in Sf900II serum-free and protein-free medium, and use Sf900II SFM medium for insect cell culture in order to express the target gene Product purification. A sequence before the coding frame of the recombinant KGD-prourokinase gene is responsible for encoding a signal peptide, which assists the expression product of the KGD-prourokinase gene to penetrate the membrane and be secreted into the supernatant of the cell culture medium.
[0058] There are two ways to culture insect cells: adherent culture and suspension culture. Both culture methods can express exogenous genes well. The culture of insect cells re...
Embodiment 3
[0060] Example 3. Separation and purification of KGDW-prourokinase chimeric recombinant protein
[0061] From the perspective of the primary structure, the KGD-prourokinase recombinant protein is basically the same as the wild-type prourokinase, only four amino acid residues are added in the K region of the prourokinase; According to the homology modeling results of the protein structure, KGD-prourokinase basically maintained the three-dimensional structure of the natural prourokinase. The results of Western blotting analysis and antibody neutralization inhibition experiments showed that the KGD-prourokinase recombinant chimera molecule retained the immunodeterminant structure on the prourokinase molecule, and could be recognized and combined by anti-prourokinase antibody. Therefore, using this characteristic, an affinity chromatography column coupled with prourokinase antibody was prepared to purify the target gene expression product KGDW-prourokinase in insect cells by aff...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com