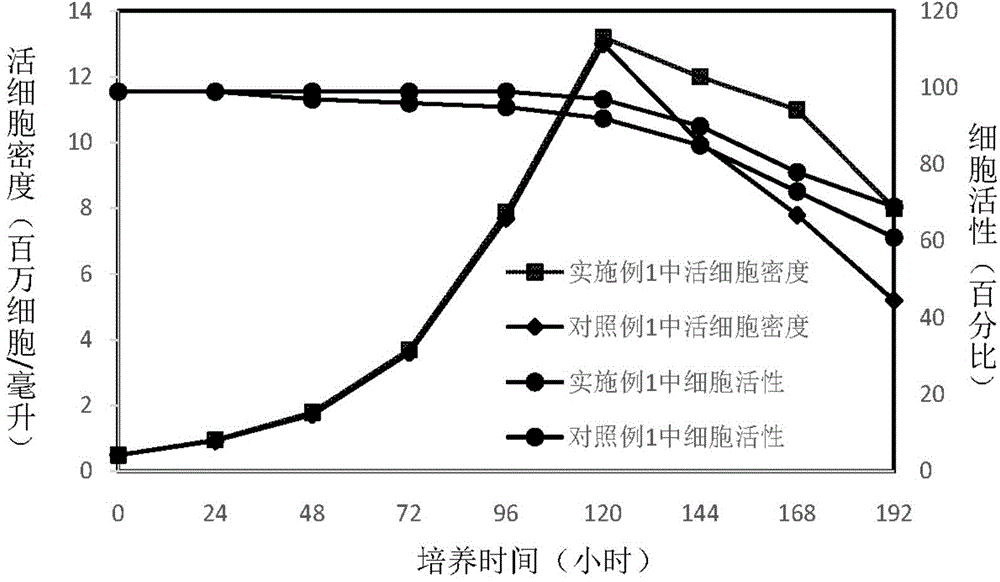
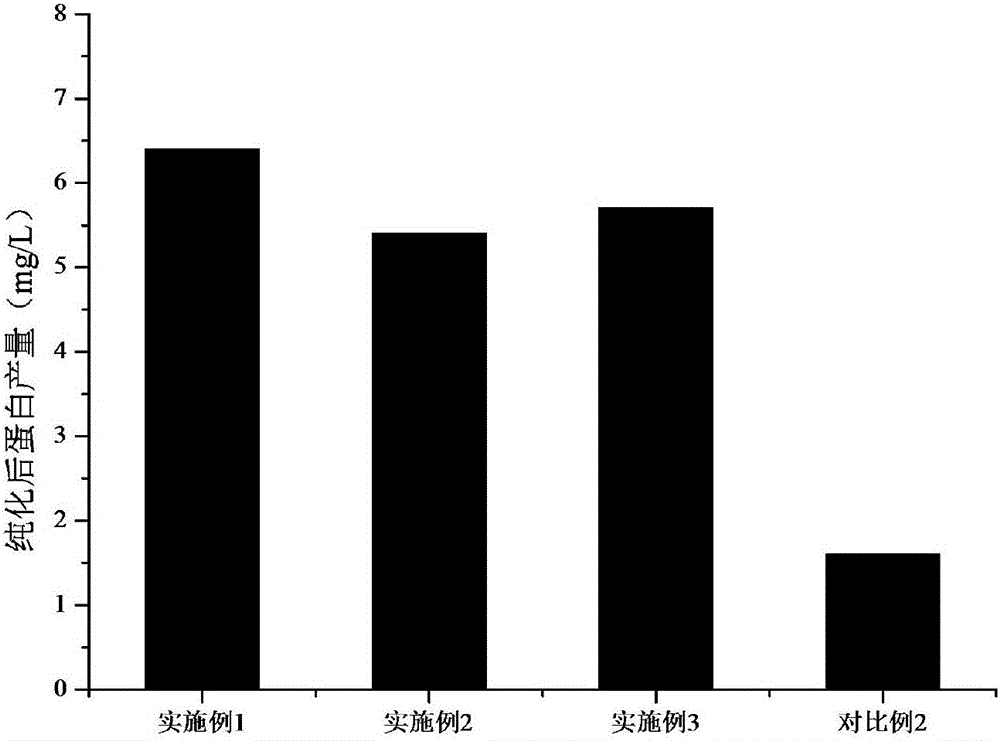
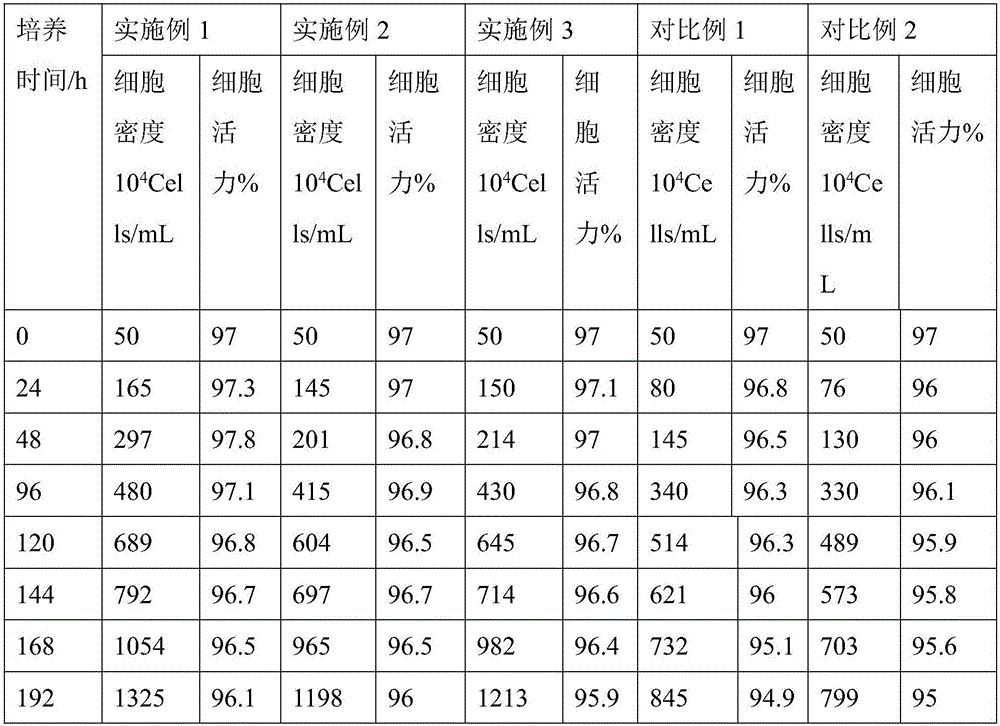
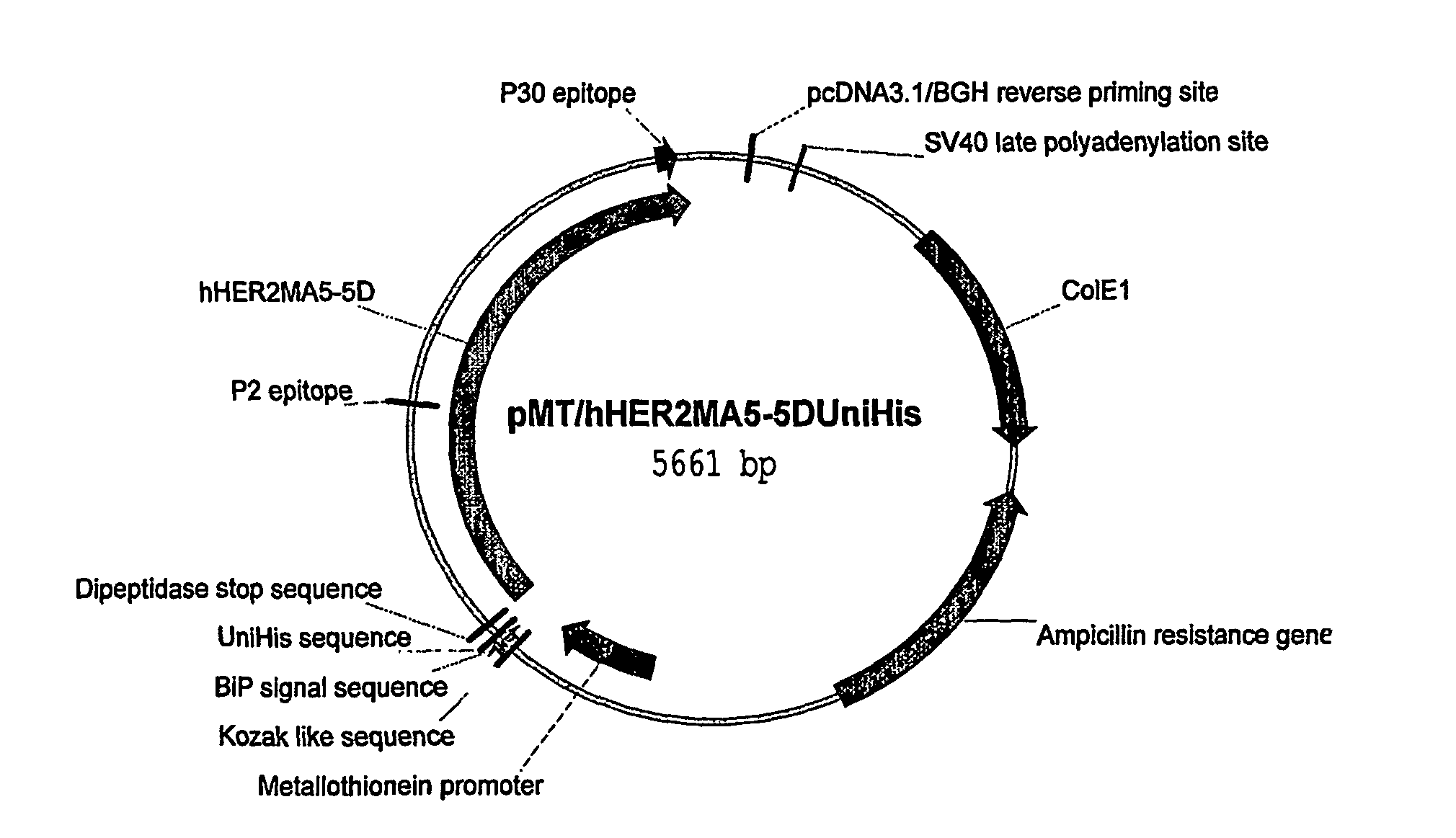
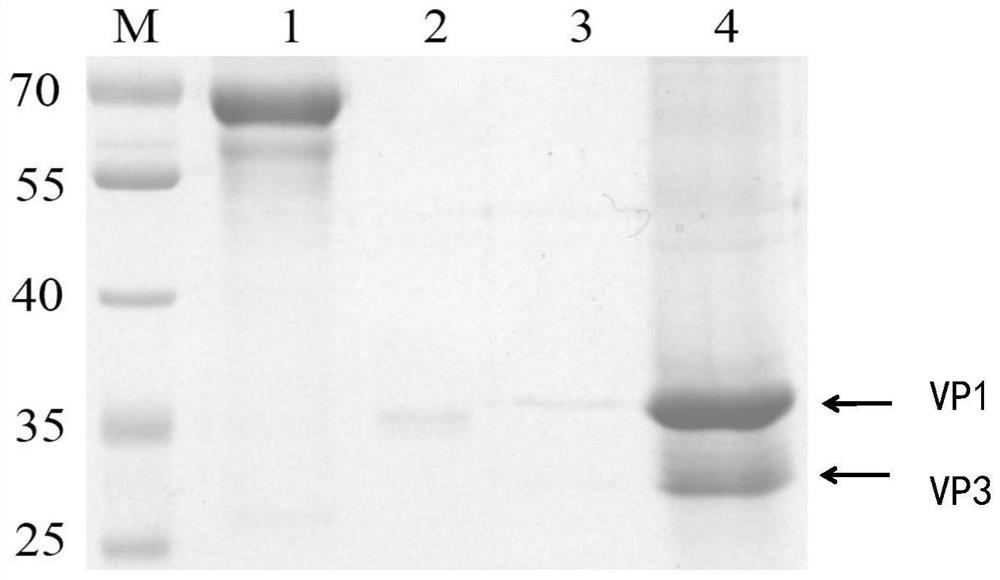
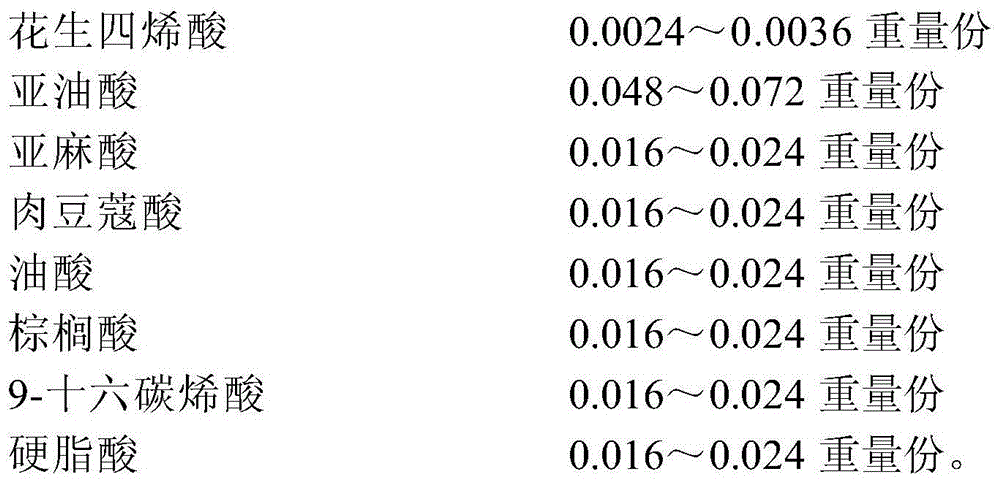Patents
Literature
53 results about "Insect cell culture" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The use of insect cell lines as production hosts is an emerging technology for the production of bio pharmaceuticals. There are currently more than 100 insect cell lines available for recombinant protein production with lines derived from Bombyx mori, Mamestra brassicae, Spodoptera frugiperda, Trichoplusia ni, and Drosophila melanogaster being of particular interest. Insects cell lines are commonly used in place of prokaryotic ones because post-translational modifications of proteins are possible in insect cells whereas this mechanism is not present in prokaryotic systems. The Sf9 cell line is one of the most commonly used lines in insect cell culture.
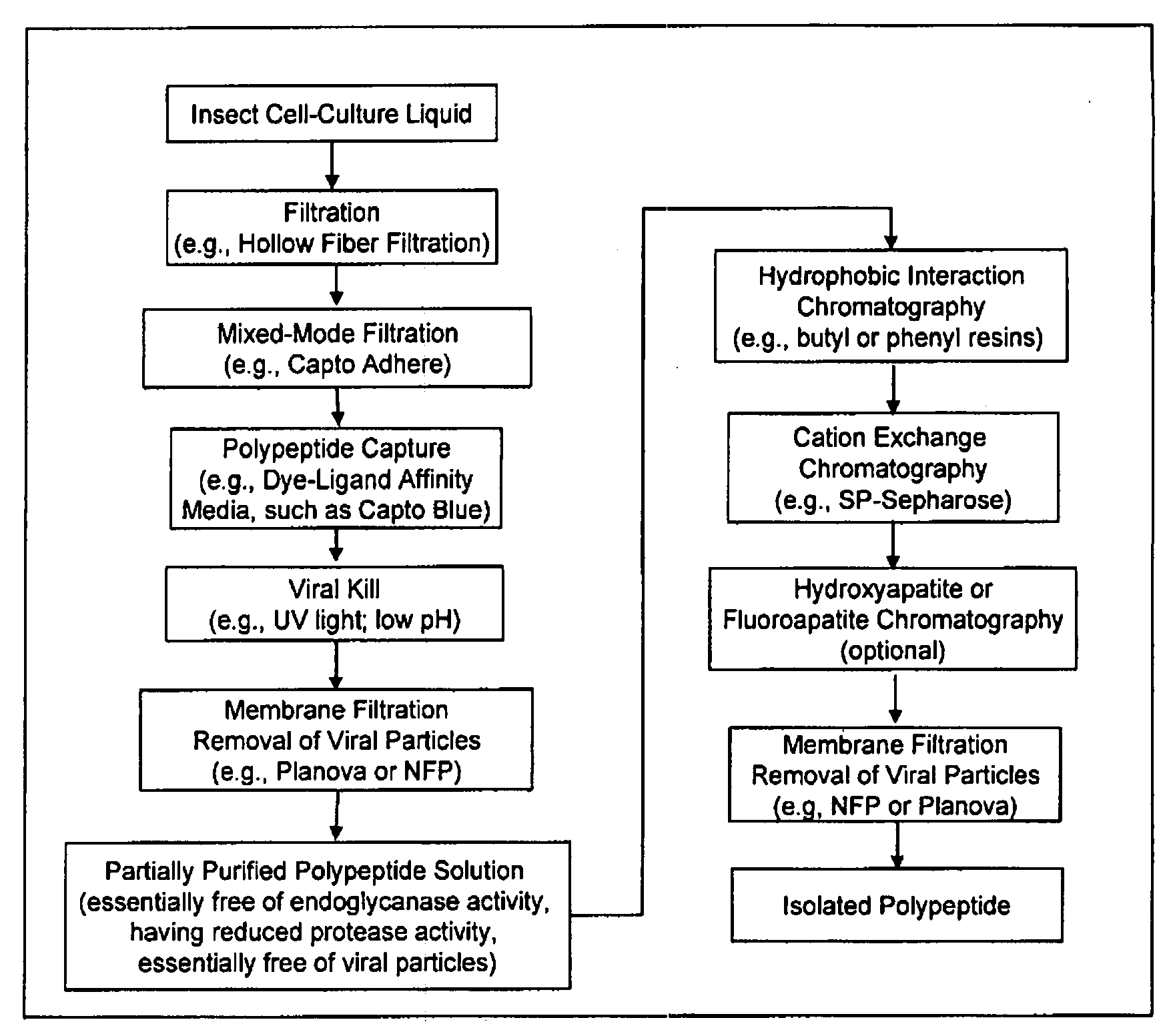
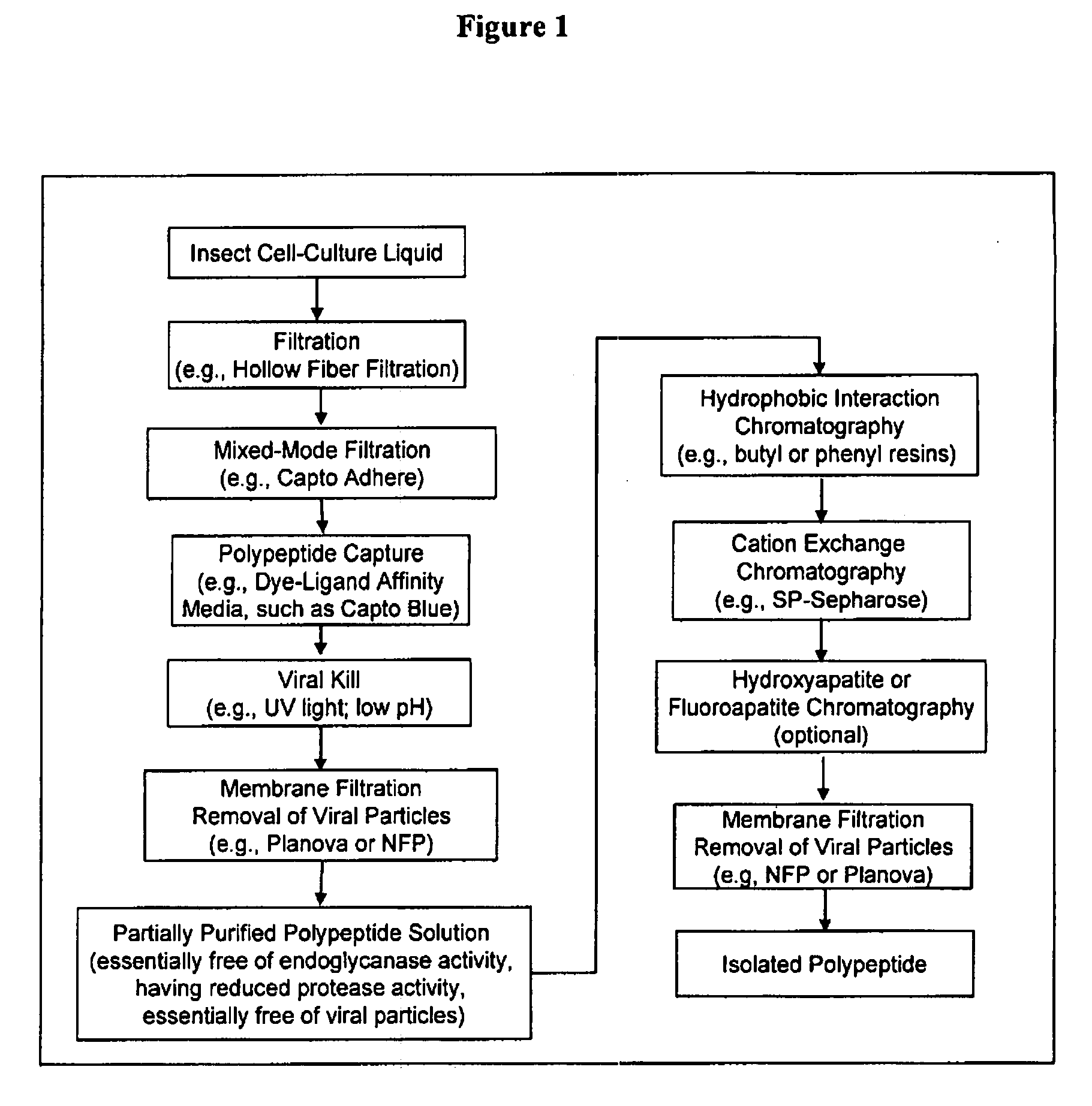
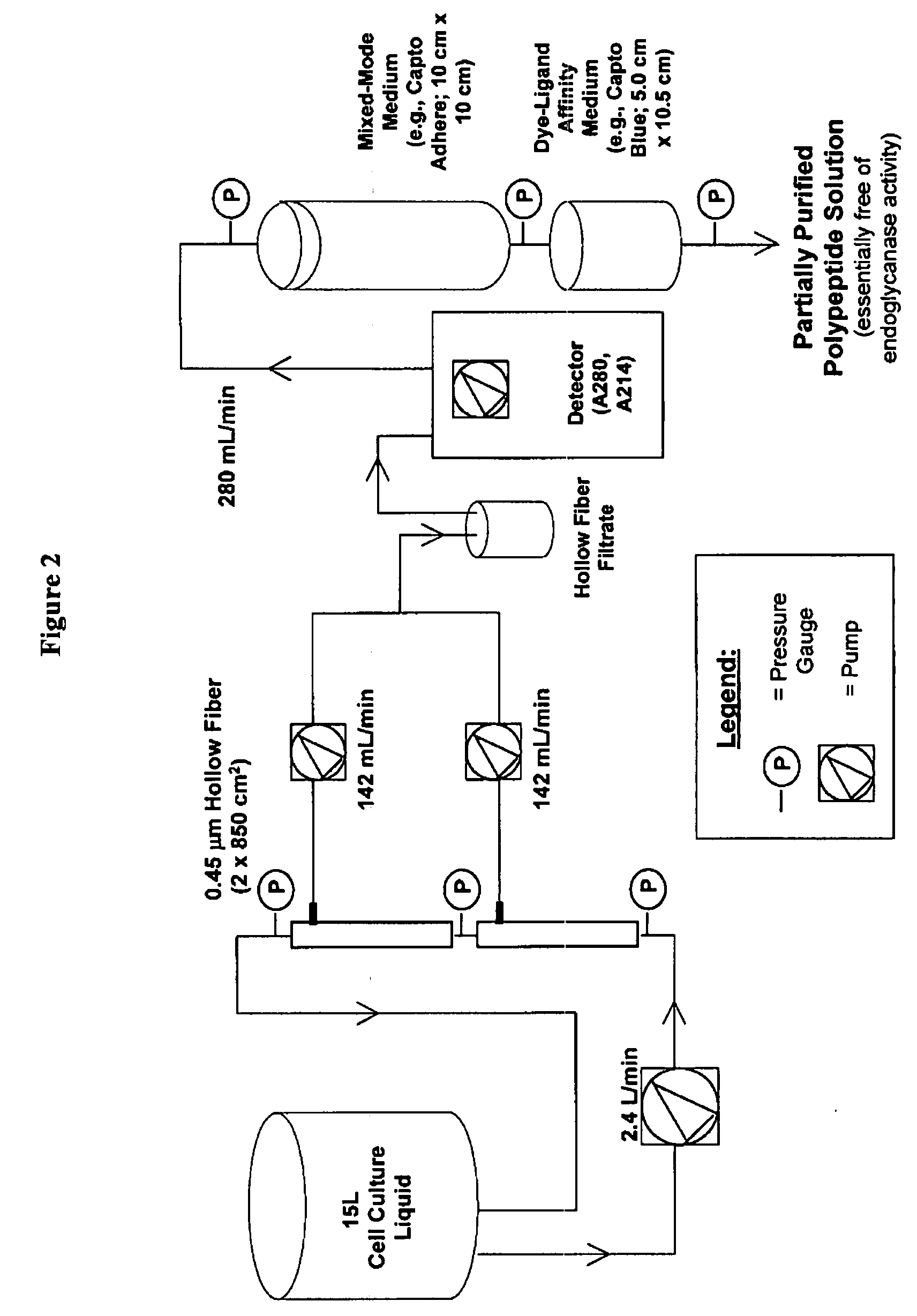
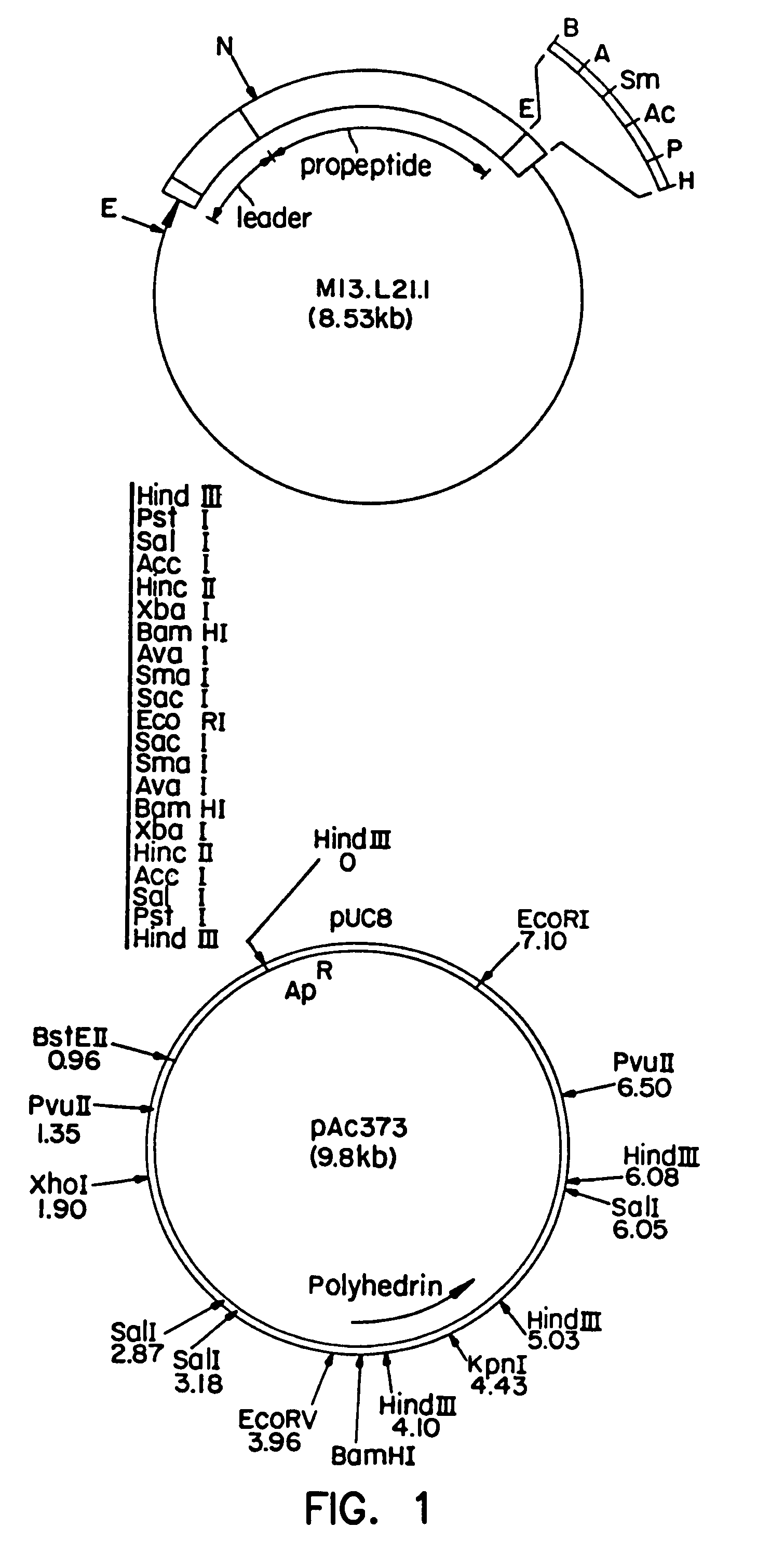
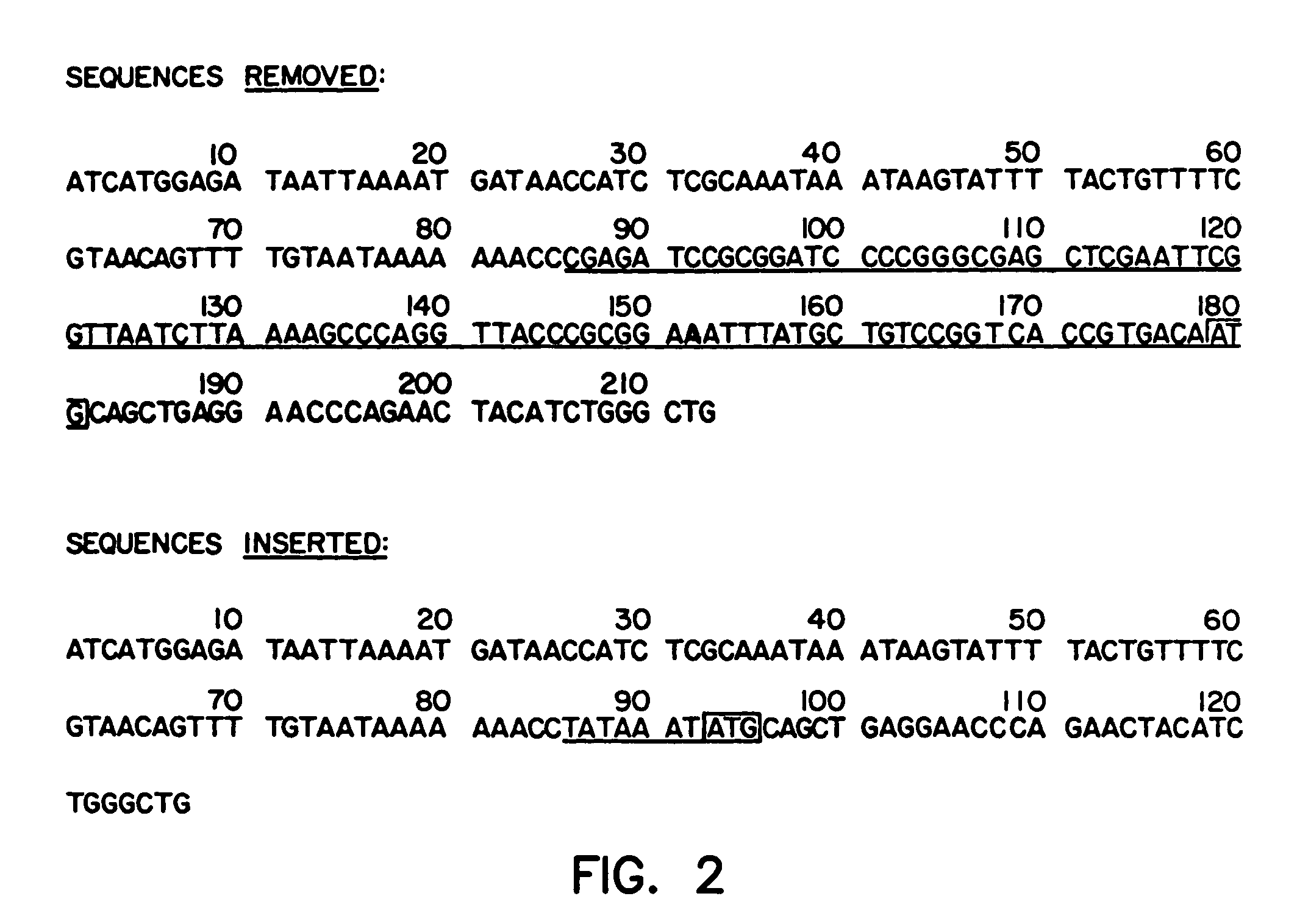
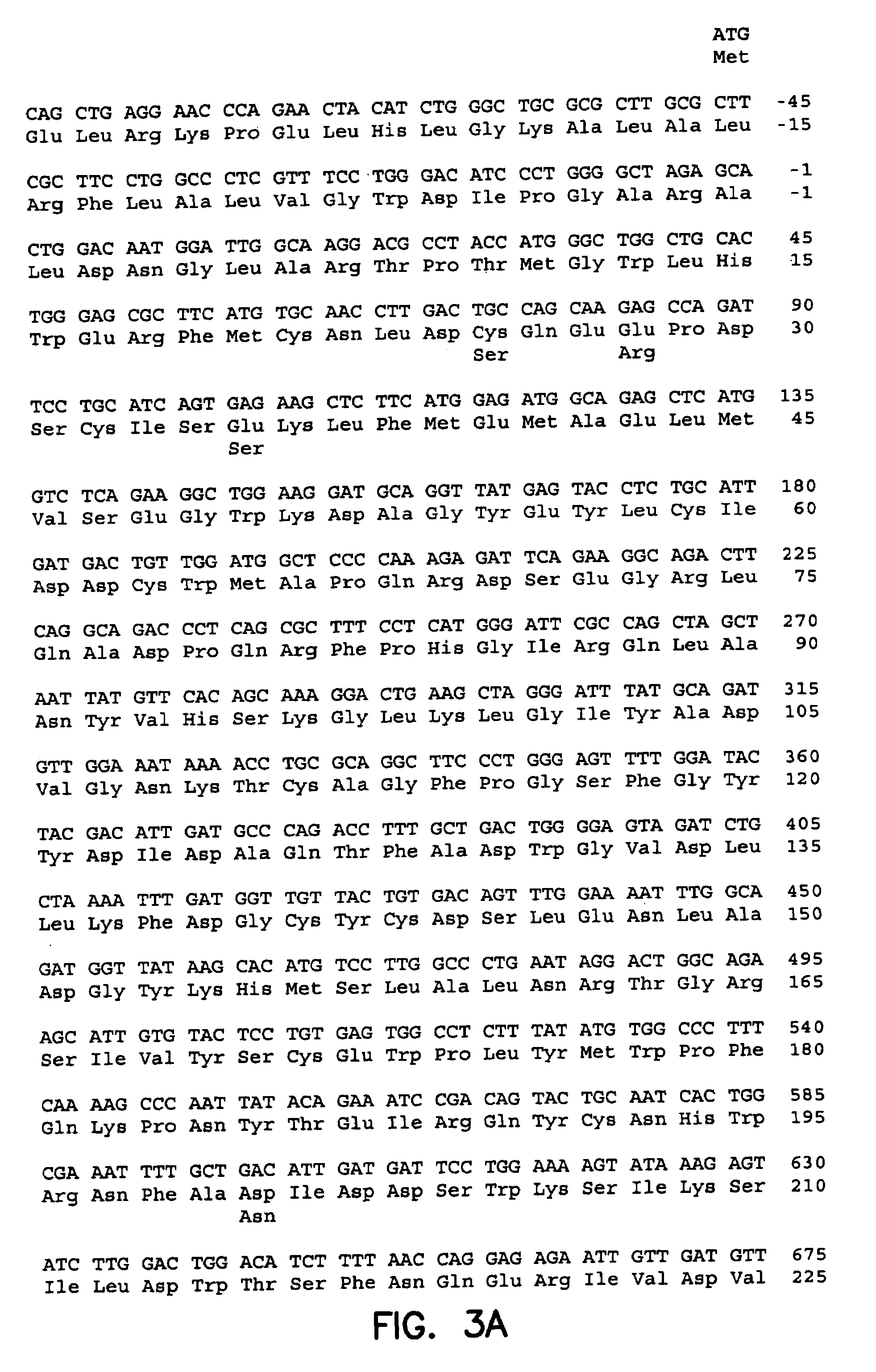
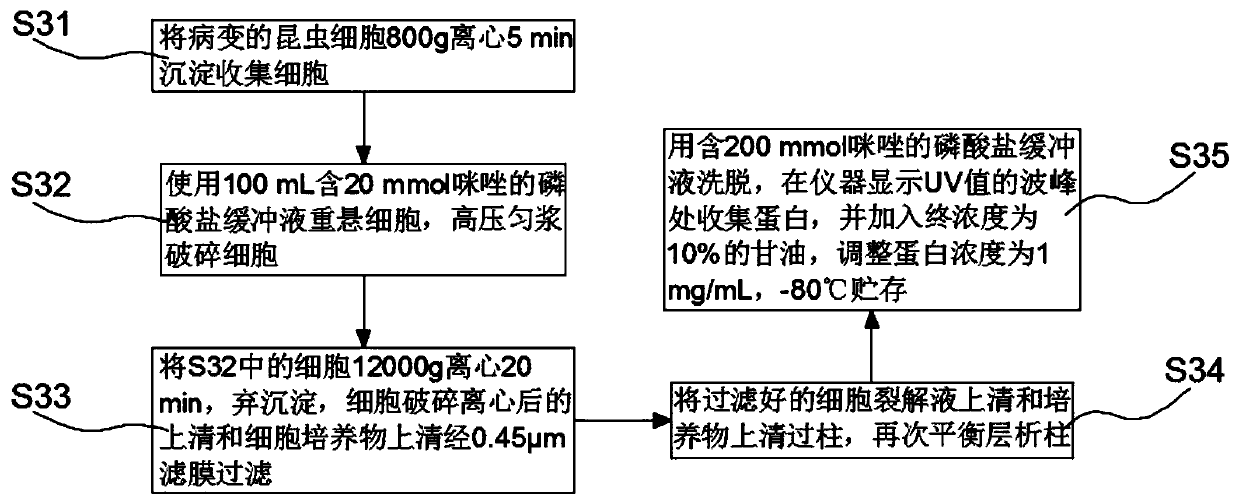
Manufacturing process for the production of polypeptides expressed in insect cell-lines
InactiveUS20080207487A1Promote recoveryReduce manufacturing costPeptide/protein ingredientsDepsipeptidesFiberCulture fluid
The present invention provides a manufacturing method for polypeptides that are produced in insect cells using a baculoviral expression system. In one example, the insect cell culture is supplemented with a lipid mixture immediately prior to infection (e.g., one hour prior to infection). The polypeptides are isolated from the insect cell culture using a method that employs anion exchange or mixed-mode chromatography early in the purification process. This process step is useful to remove insect-cell derived endoglycanases and proteases and thus reduces the loss of desired polypeptide due to enzymatic degradation. In another example, mixed-mode chromatography is combined with dye-ligand affinity chromatography in a continuous-flow manner to allow for rapid processing of the insect-cell culture liquid and capture of the polypeptide. In yet another example, a polypeptide is isolated from an insect cell culture liquid using a process that combines hollow fiber filtration, mixed-mode chromatography and dye-ligand affinity in a single unit operation producing a polypeptide solution that is essentially free of endoglycanase and proteolytic activities. In a further example, the isolated polypeptides are glycopeptides having an insect specific glycosylation pattern, which are optionally conjugated to a modifying group, such as a polymer (e.g., PEG) using a glycosyltransferase and a modified nucleotide sugar.
Owner:NOVO NORDISK AS
Targeting proteins to cells expressing mannose receptors via expression in insect cells
The present invention is based on the discovery that proteins produced in insect cell cultures are glycosylated in a unique manner that causes them to be selectively imported by cells that express mannose receptors on their membranes, particularly macrophages. Proteins that are selectively imported into cells containing mannose receptors are provided, as well as pharmaceutical compositions containing such proteins and methods for producing such proteins. Application of the present invention to produce proteins useful for treating lysosomal storage disorders is also disclosed. Engineering of cells to express mannose receptors so that they will selectively import proteins produced in insect cells is also taught, as well as a protein targeting system using such cells and proteins. Finally, an improved elution buffer for the purification of proteins produced in insect cells from a Concanavalin A column is provided.
Owner:ST JUDE CHILDRENS RES HOSPITAL INC
Recombinant alpha-galactosidase A therapy for Fabry disease
InactiveUS7011831B2Peptide/protein ingredientsGenetic material ingredientsBaculovirus expressionInsect cell culture
Fabry disease results from an X-linked deficiency in the enzyme α-galactosidase A. The present invention is directed to recombinant human α-galactosidase A and provides baculovirus expression vectors and recombinant virus that provide stable expression of extracellular and intracellular levels of this enzyme in an insect cell culture. The recombinant-derived enzyme can be used in enzyme replacement therapy to treat Fabry patients. Composition useful in therapeutic administration of α-galactosidase A are also provided.
Owner:SHELBYZYME
High-rate perfusion bioreactor
InactiveUS20090280565A1Prevent extractionStable settlementBioreactor/fermenter combinationsBiological substance pretreatmentsPerfusion bioreactorMetabolite
The present invention relates to a novel perfusion bioreactor allowing continuous medium feed and extraction of metabolites or other desired products from cells. The invention is useful for plant cell cultures but may also be used for mammalian cell cultures, insect cell cultures and bacterial cell cultures. The design of the reactor includes sedimentation columns mounted inside the bioreactor to separate single cells and cell aggregates from the culture medium at a very low shear stress. The operating conditions allow a stable cell / medium separation by maintaining the medium upward velocity equal to or slightly lower than the cell sedimentation velocity.
Owner:CORP DE LECOLE POLYTECHIQUE MONTREAL
E2 subunit vaccine comprising recombinant pestivirus E2 protein
InactiveUS6919085B2Increased and improved yieldProduce improveSsRNA viruses positive-senseViral antigen ingredientsCell culture mediaProtein C
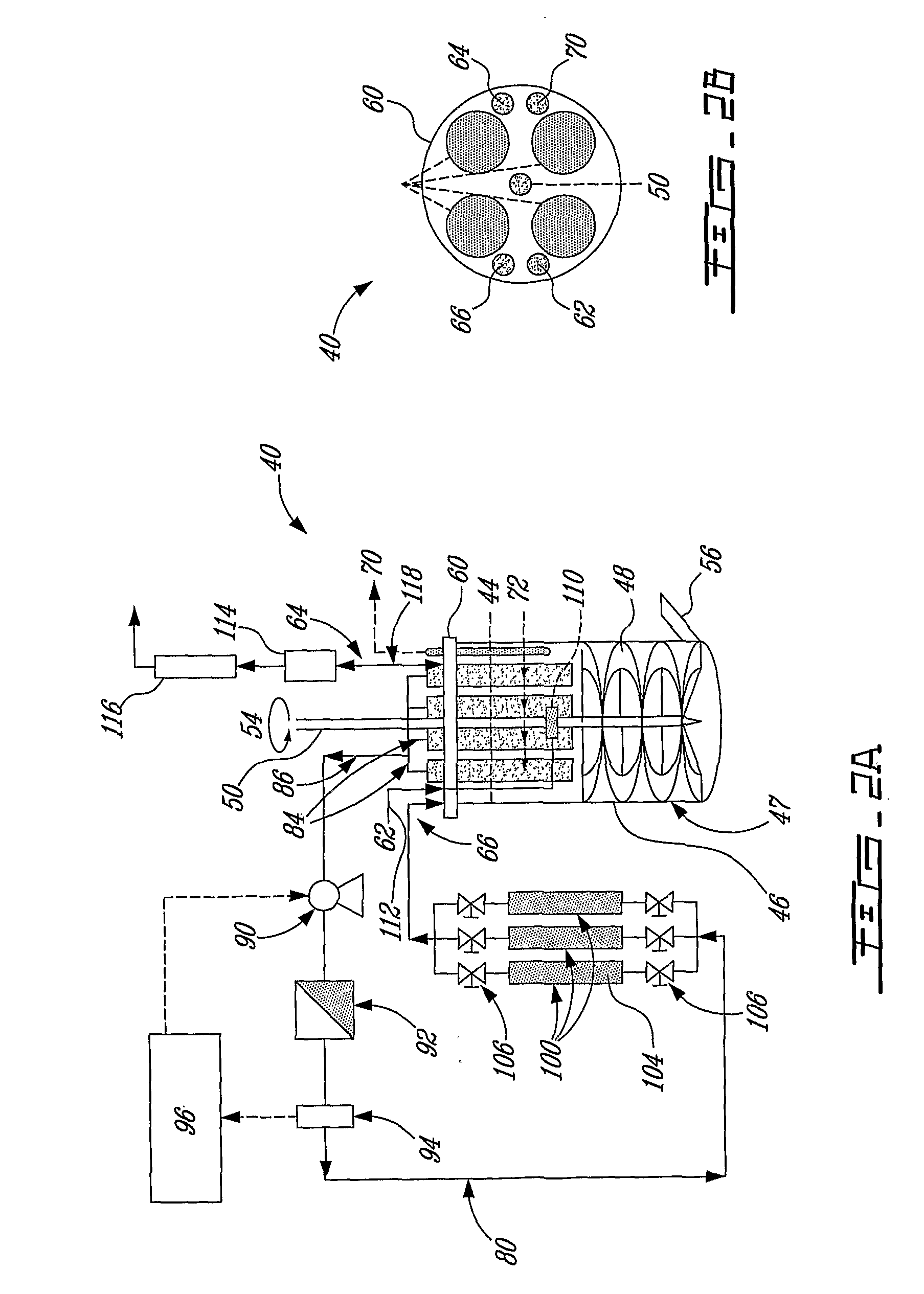
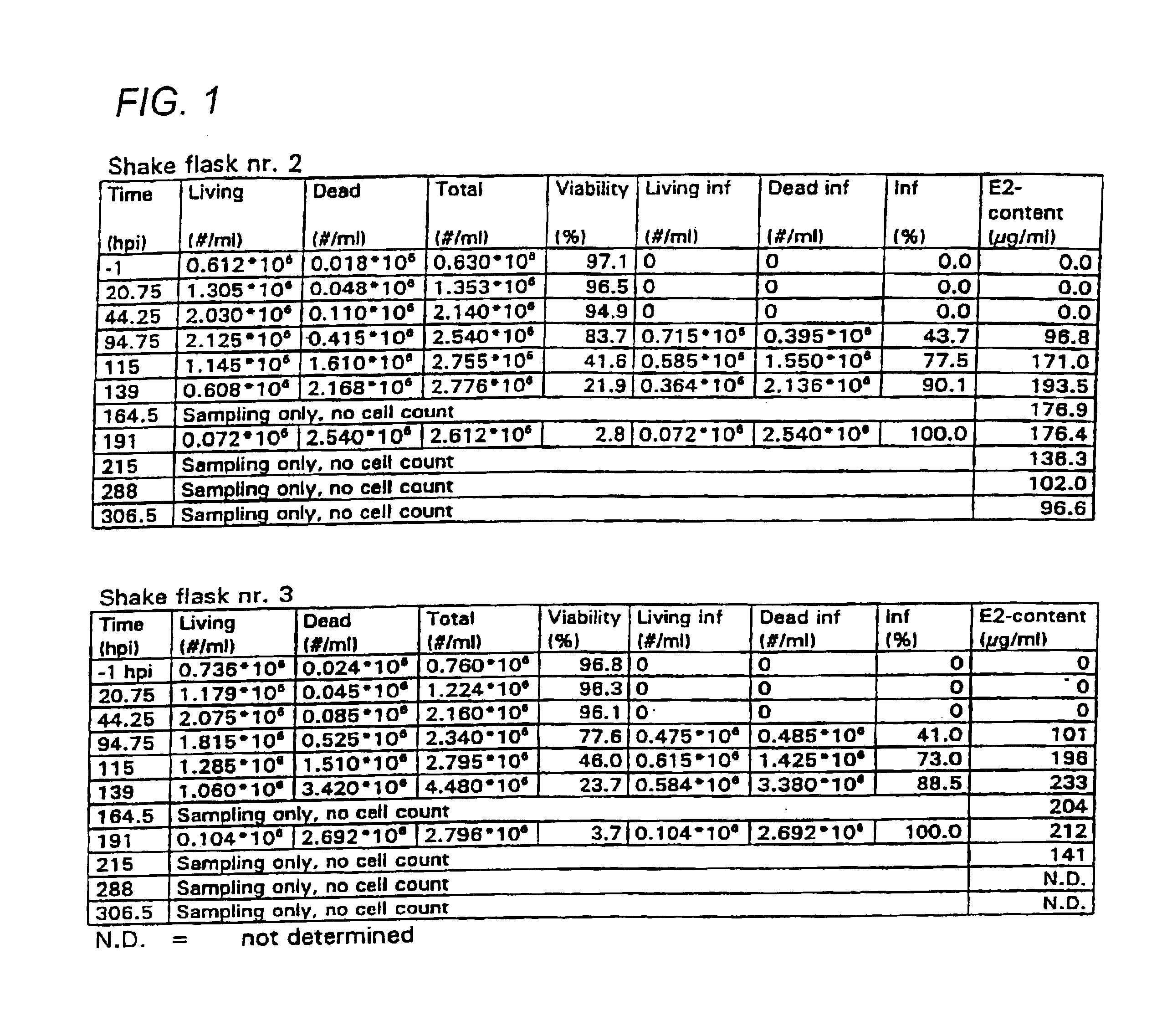
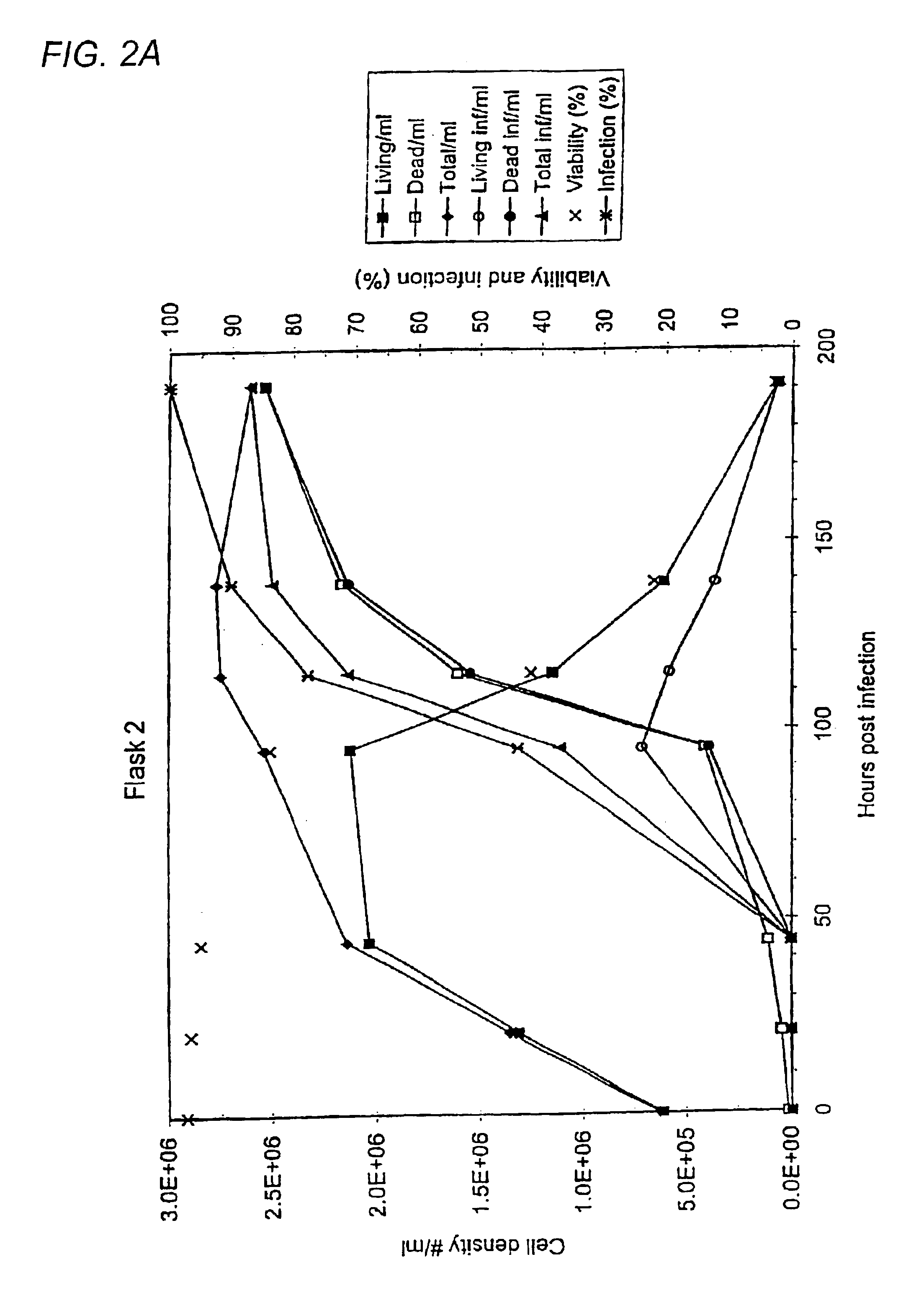
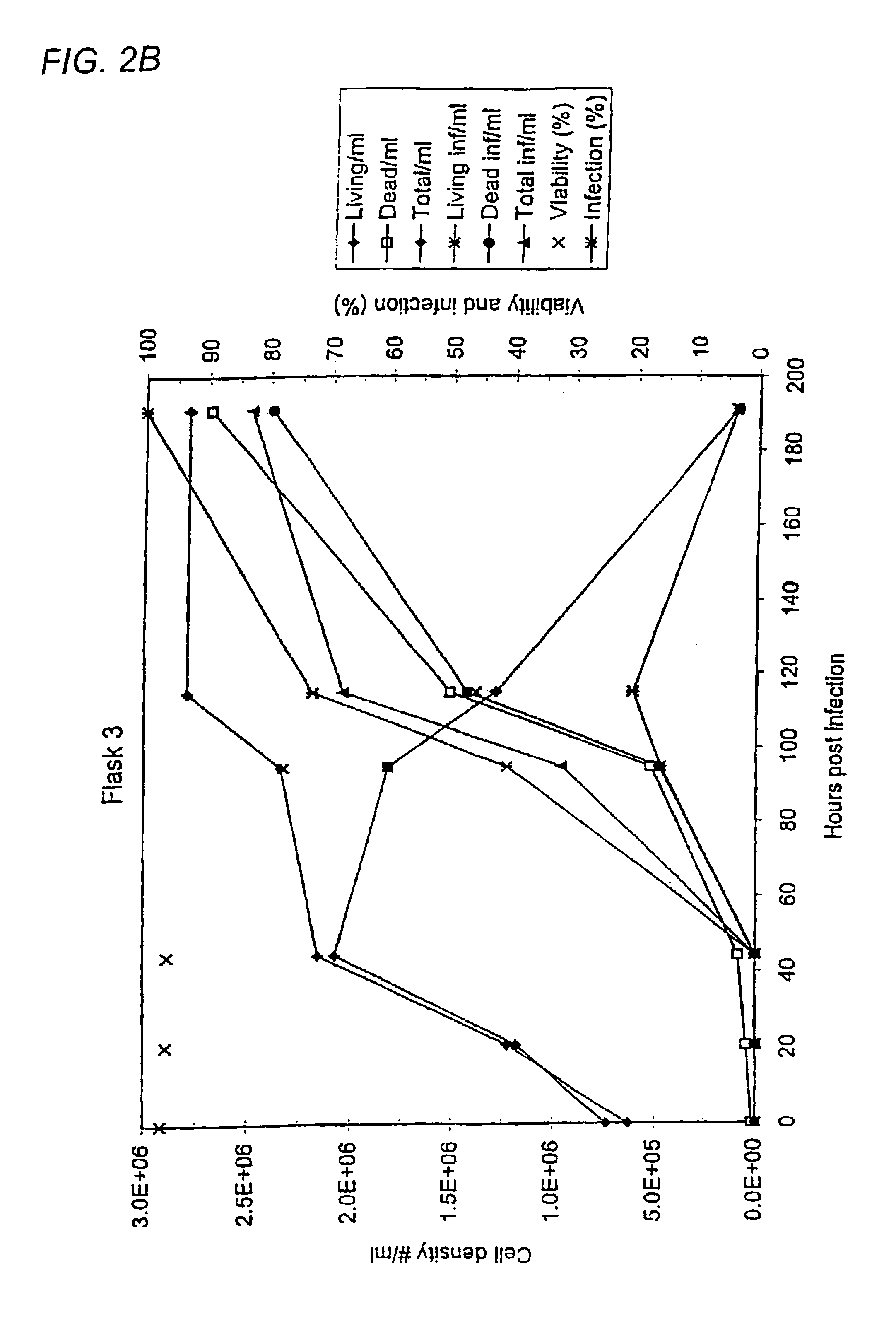
The invention relates to a method of increasing protein expression in baculo vector virus expression systems. The invention provides a method to produce a recombinant protein in insect cell culture which comprises selecting a recombinant baculovirus expressing said protein, growing insect cells in growth medium in a culture vessel and infecting the cells with an inoculum of at least one baculovirus at a cell density of 1×105 to 5×106 cells / ml with an m.o.i of <0.01. The invention also provides a method to produce recombinant pestivirus E2 or Em9 protein or fragments thereof in insect cell culture characterized by a final concentration of the protein fragments in the growth medium at harvest of at least 100 μg / ml. The invention also provides a method of producing recombinant FSH, α-units and / or β-units, and complexes and fragments thereof, at a concentration in the growth medium at harvest of at least 15 μ / ml.
Owner:STICHTING INST VOOR DIERHOUDERIJ & DIERGEZONDHEID +1
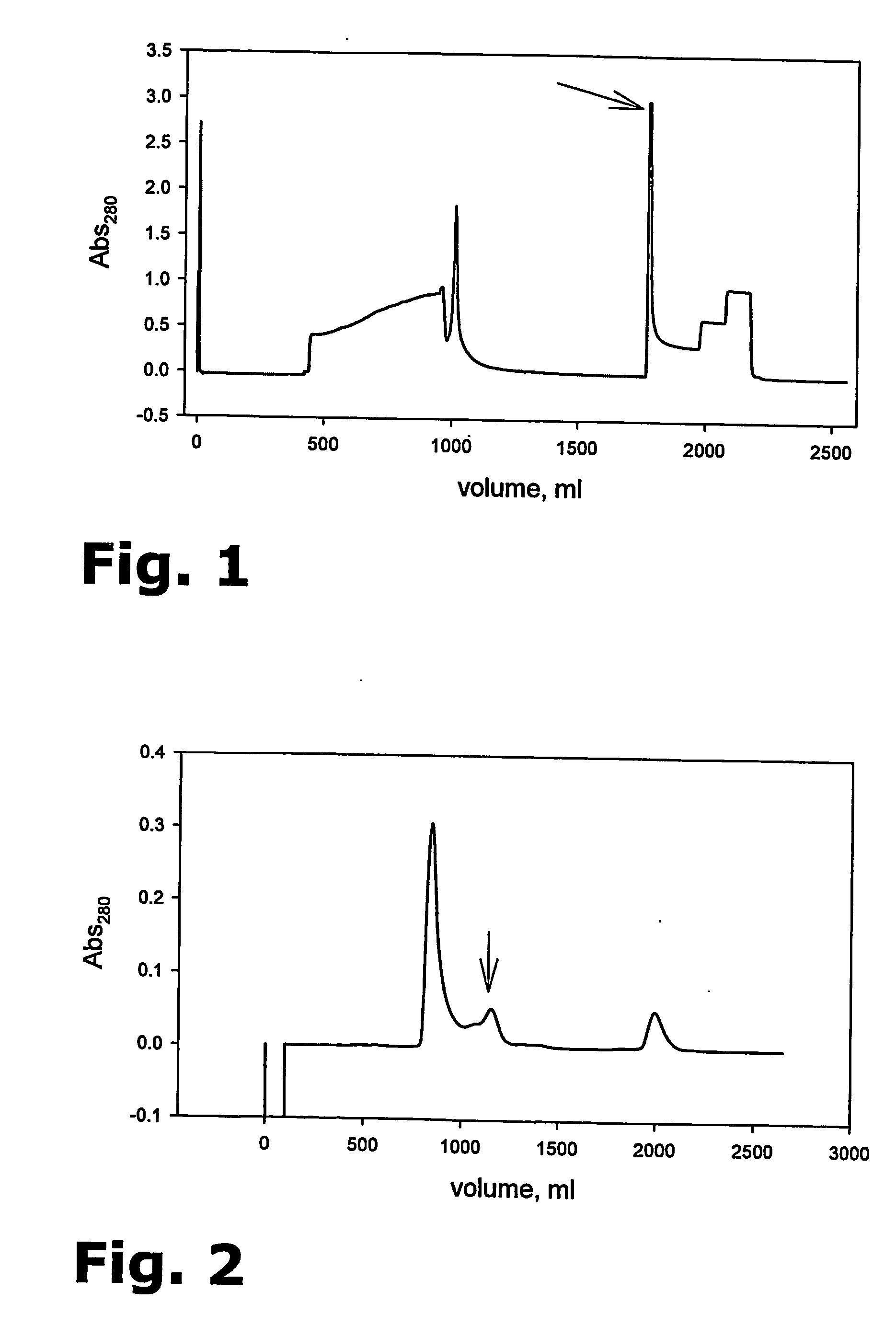
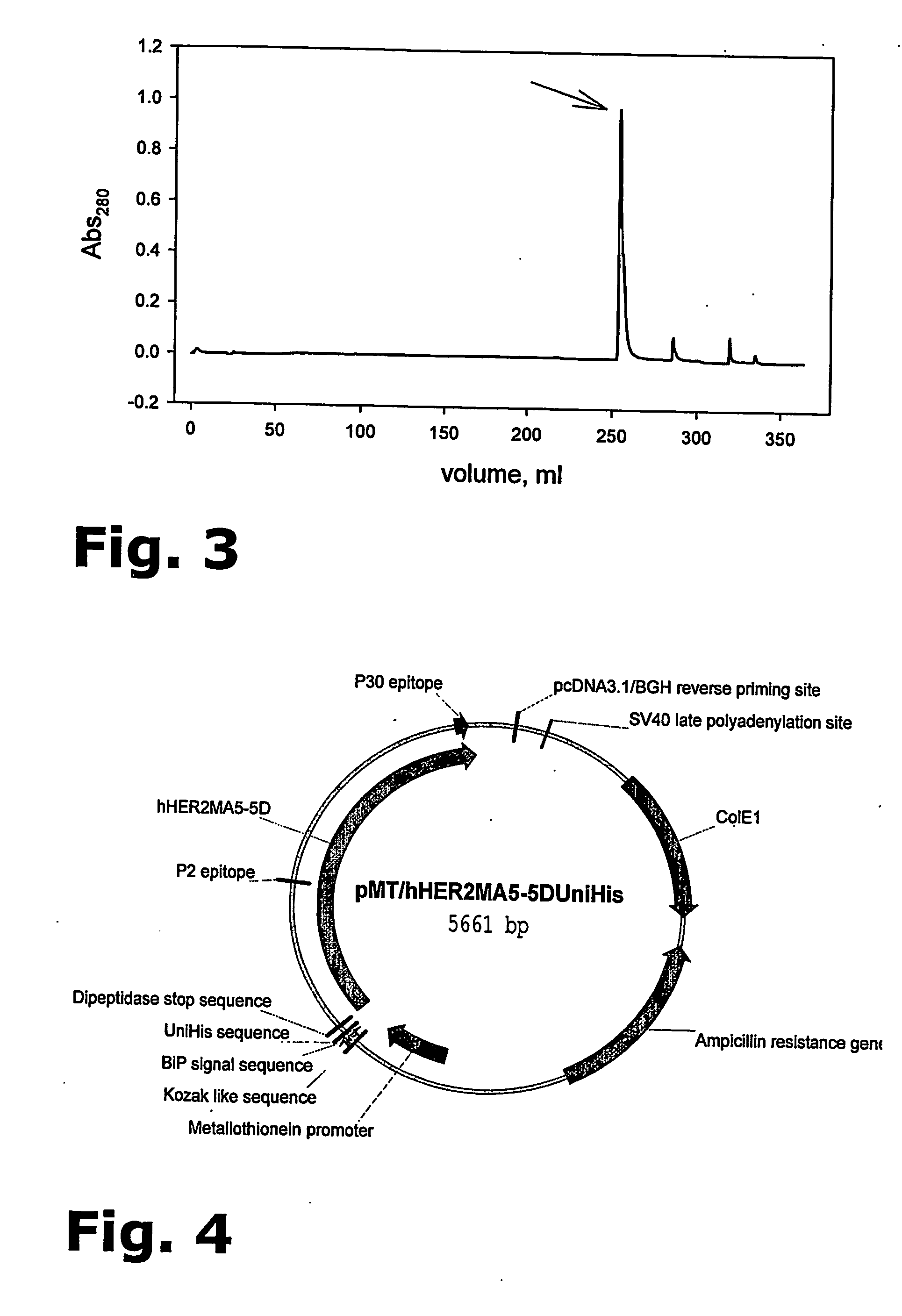
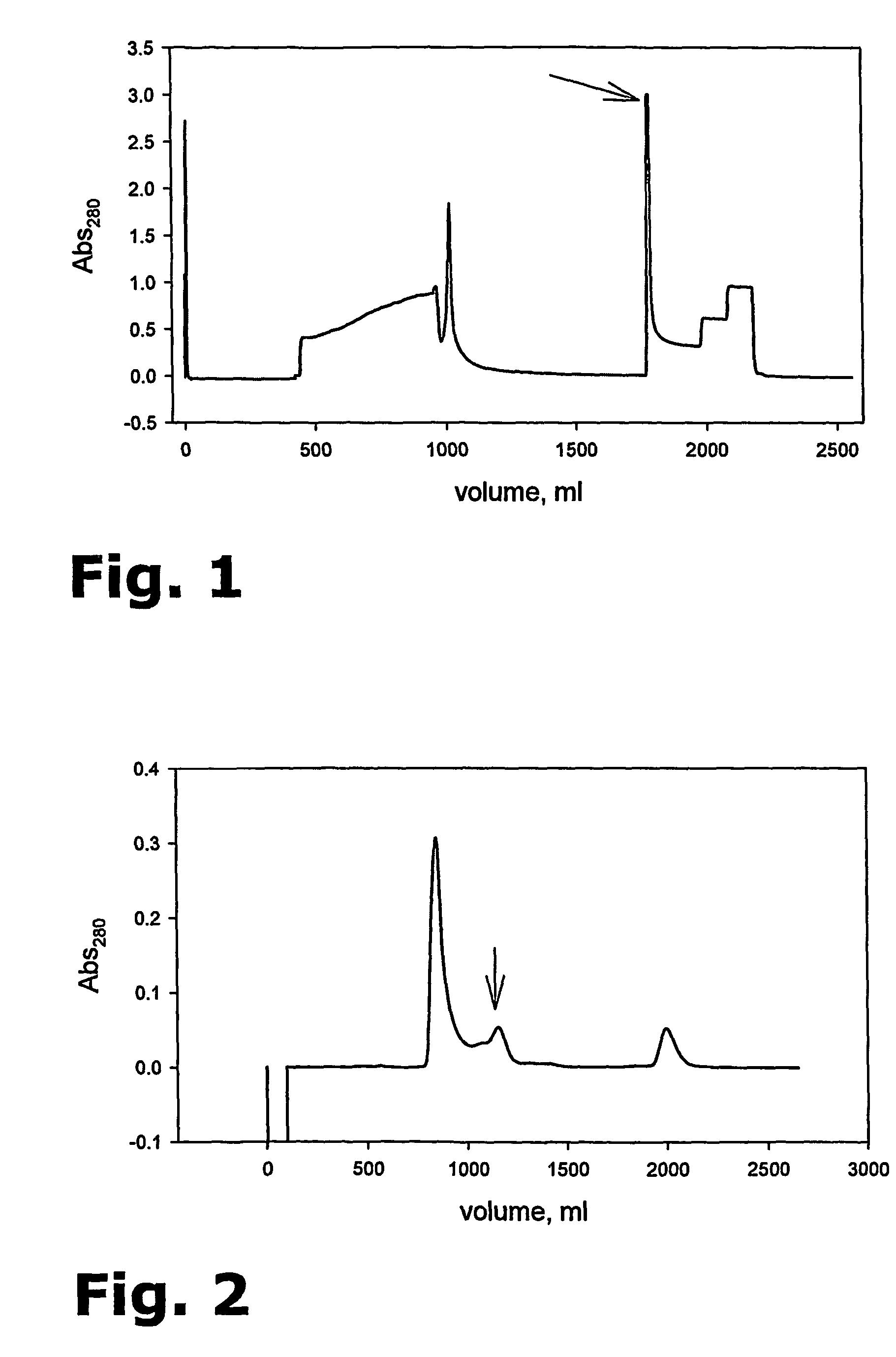
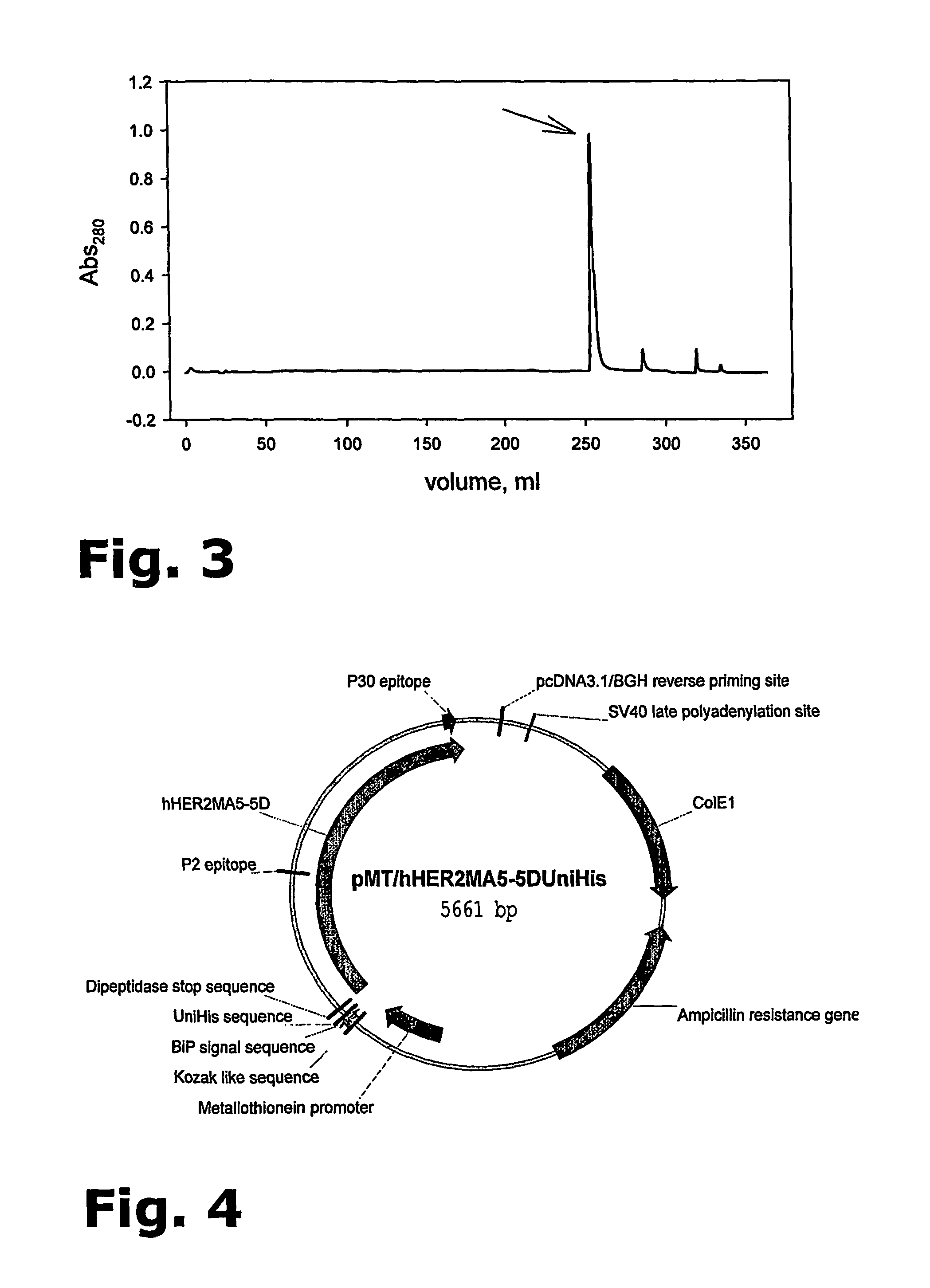
Purification of her-2 variants
The present invention provides for a novel method for purification of EGFR family proteins obtained from cultures of insect cells. The process comprises subsequent steps of a) diafiltration and exchange of culture medium with buffer, b) immobilized metal affinity chromatography (IMAC), c) size exclusion chromatography (SEC), and d) anion exchange chromatography (AIE). The method also provides for an immunogenic variant of HER-2 protein which for which the purification process has been especially adapted, as well as means for the preparation of the variant.
Owner:BAVARIAN NORDIC AS
Insect cell serum-free culture medium and application thereof
ActiveCN104593316AImprove cultivation efficiencyThe components are simple and clearAnimal cellsFermentationLithium chlorideDiethylenetriamine
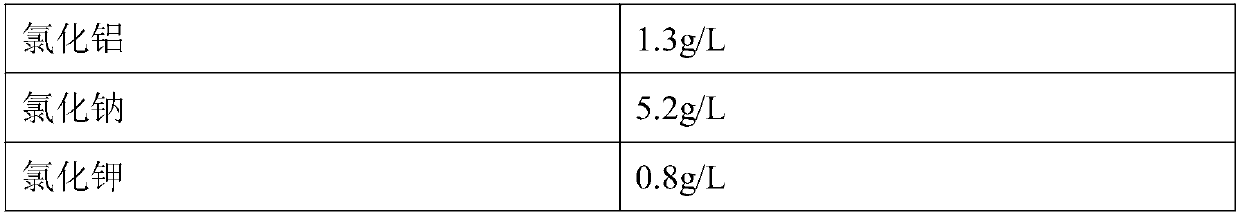
The invention discloses an insect cell serum-free culture medium and application thereof. The culture medium comprises amino acid, inorganic salt, vitamin and carbohydrate and further comprises 0.5-15mg / L of diethylenetriamine dioleoyl phosphatidylcholine and / or 0.1-10mg / L of distearoyl phosphatidyl choline; and particularly, further comprises 0.001-0.1mg / L of barium chloride dihydrate, 0.005-0.02mg / L of lithium chloride and 0.05-7mg / L of nickel chloride. The insect cell serum-free culture medium is simple and clear in components and low in cost and is easily prepared; by the insect cell serum-free culture medium, the insect cell culture efficiency and recombinant protein expression efficiency can be significantly increased and the large-scale culture and large-scale preparation of insect cells are well achieved.
Owner:苏州沃美生物有限公司
Non-serum non-animal-origin-additive insect cell culture medium
The invention relates to the field of cell culture medium, and in particular to a non-serum non-animal-origin-additive insect cell culture medium. The medium comprises mainly the following components: basic culture medium, glucose, inorganic salt, vitamins, L-arginine, L-agedoite, L-glycocoll, L-histidine, L-isoleucine, L-leucine, L-lysine, L-methionine, L-threonine, L-tryptophan, L-valine, L-proline, L-glutamine, yeast extracts, recombulin, malic acid, allomaleic acid, cholesterol, linoleic acid, granulesten, putrescine, glutathione, glycerol and fructosan. The culture medium can promote the growth of insect cell and is suitable for the large scale breeding of insect cell and the expression of recombination protein.
Owner:严志海
Method for constructing cell line by using insect egg
The invention relates to a method for establishing a cell line of insect eggs, which comprises the following steps: 1) dipping an insect oosperm sheet which has been laid for 90 to 100 hours in a sodium hypochlorite solution or a formaldehyde solution, and then disinfecting and washing the oosperm sheet; 2) pouring the treated eggs into an insect cell culture solution, and extruding the eggs one by one to release embryos; 3) cutting the embryos into tissue blocks to be cultured in an incubator; 4) adding the cell culture solution into the incubator to culture the tissue blocks continuously; 5) replacing the culture solution periodically until the proliferating cells are full of the whole culture bottle; 6) sucking out half of the cell culture solution containing newly proliferated single cells, putting the cell culture solution into a new culture bottle which is added with the same amount of a new cell culture solution, and putting the new culture bottle back to the incubator to culture the cells continuously; and 7) repeating the step 5) and the step 6) periodically to cause the cells to begin to passage so as to establish the cell line successfully, wherein the whole process is performed under a sterile condition. The insect eggs can be lepidoptera insect eggs. The method shortens the time from the prior 1.5 to 2 years to 1 to 3 months, thereby having quite obvious superiority and strong repeatability.
Owner:INST OF FOREST ECOLOGY ENVIRONMENT & PROTECTION CHINESE ACAD OF FORESTRY
Method for producing proteins suitable for treating lysosomal storage disorders
Owner:ST JUDE CHILDRENS RES HOSPITAL INC
Purification of HER-2 variants
The present invention provides for a novel method for purification of EGFR family proteins obtained from cultures of insect cells. The process comprises subsequent steps of a) diafiltration and exchange of culture medium with buffer, b) immobilized metal affinity chromatography (IMAC), c) size exclusion chromatography (SEC), and d) anion exchange chromatography (AIE). The method also provides for an immunogenic variant of HER-2 protein for which the purification process has been especially adapted, as well as means for the preparation of the variant.
Owner:BAVARIAN NORDIC AS
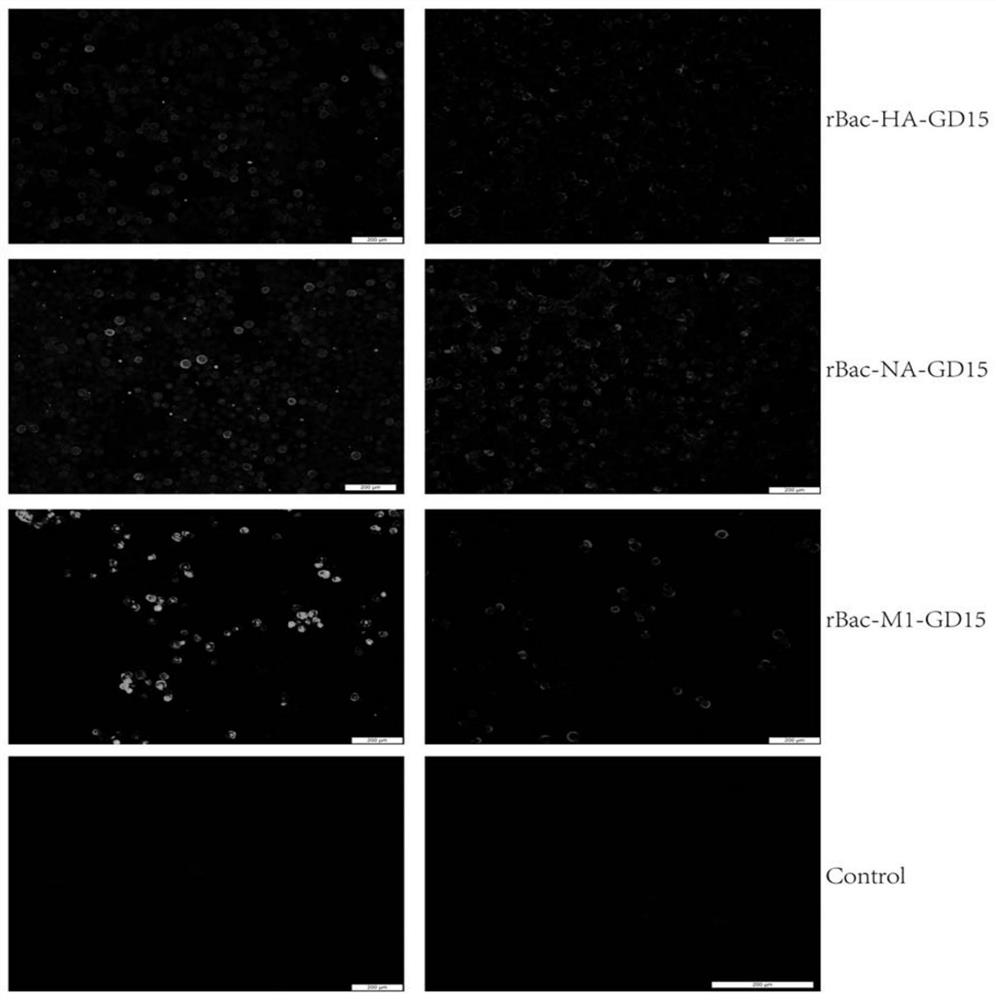
Recombinant H7N9 subtype avian influenza virus-like particle, and preparation method and application thereof
ActiveCN112079904AGood cross reactivityMild diseaseSsRNA viruses negative-senseViral antigen ingredientsAdjuvantImmunogenicity
The invention discloses a recombinant H7N9 subtype avian influenza virus-like particle, and a preparation method and application thereof. Recombinant baculoviruses for expressing HA protein, NA protein and M1 protein of an H7N9 highly pathogenic avian influenza virus are respectively constructed on the basis of a recombinant baculovirus insect cell culture system. The three recombinant baculovirusstrains are used for co-infecting suspended insect cells, so that the H7N9 subtype avian influenza virus-like particle capable of being self-assembled in the cells can be obtained. After the concentration and purification with cane sugar with different gradient concentrations through an ultrafiltration tube, the H7N9 subtype avian influenza virus-like particle is mixed and emulsified with an adjuvant to prepare a vaccine. When a chicken is immunized by the prepared vaccine, the body can be induced to generate a specific antibody; and the advantages of high immunogenicity, good safety, high hereditary stability and the like are realized. After the attack by a lethal dose of H7N9 subtype highly pathogenic avian influenza viruses, the complete clinical protection can be provided, and virus expelling of the chicken is obviously inhibited. The invention provides a novel method for preventing H7N9 subtype avian influenza virus infection, and also lays a foundation for the development of a novel influenza virus vaccine.
Owner:YANGZHOU UNIV
Conotoxin and biological preparation method and application thereof
InactiveCN102876683AImprove expression efficiencyComplete post-processingNervous disorderPeptide/protein ingredientsBiologyDrug biological activity
The invention discloses conotoxin and a biological preparation method and application thereof. The preparation method includes the steps of firstly, cloning conotoxin gene to an expression cassette of a baculovirus carrier to obtain a recombination transfer expression carrier; secondly, subjecting the recombination transfer expression carrier and baculovirus DNA (deoxyribonucleic acid) to co-transfection so as to obtain recombination baculovirus; and thirdly, infecting insect hosts or insect cells with the recombination baculovirus, culturing expression conotoxin of the infected insect hosts, and collecting and purifying the expression products. The invention further provides optimized conotoxin gene which is evidently improved in expression efficiency in insect hosts compared with original gene. Bioactive conotoxin is expressed efficiently and safely in individual insect bioreactor by the aid of baculovirus expression systems, safety is quite high, and expression products can be directly applied to analgesia drugs and the like. The biological preparation method is high in expression efficiency, complete in post-processing, fine in bioactivity, low in production cost, available for scale production and the like.
Owner:THE INST OF BIOTECHNOLOGY OF THE CHINESE ACAD OF AGRI SCI
Serum-free insect cell culture medium and preparation method and application thereof
InactiveCN106148268ASimple compositionReduce manufacturing costAnimal cellsBiotechnologyLipid formation
The invention provides a serum-free insect cell culture medium and a preparation method and application thereof. The serum-free insect cell culture medium is prepared from basic components, protein components, lipid components and auxiliaries, wherein the basic components include, by weight, 9-14 parts of inorganic salt, 3.3-5 parts of amino acid or salt, 0.17-0.26 part of water soluble vitamins, 0.0016-0.0024 part of hormones and 0.0024-0.0036 part of microelements; the protein components include, by weight, 1.6-2.4 parts of animal protein hydrolysate, 2.4-3.6 parts of plant protein hydrolysate and 2.4-3.6 parts of a yeast extract; the lipid components include, by weight, 0.14-0.22 part of fatty acid, 0.028-0.042 part of lipid-soluble vitamins and 0.24-0.36 part of cholesterol; the auxiliaries include, by weight, 7.2-10.8 parts of a defoaming agent and 2.6-4 parts of a surface active agent. The serum-free insect cell culture medium is low in cost, good in long-term storage stability, high in cell density during insect cell culture and capable of achieving large-scale production and application easily.
Owner:中生天信和(无锡)生物科技有限公司
Preparing method for serum-free and animal-source-free culture medium additive suitable for growth of insect cells
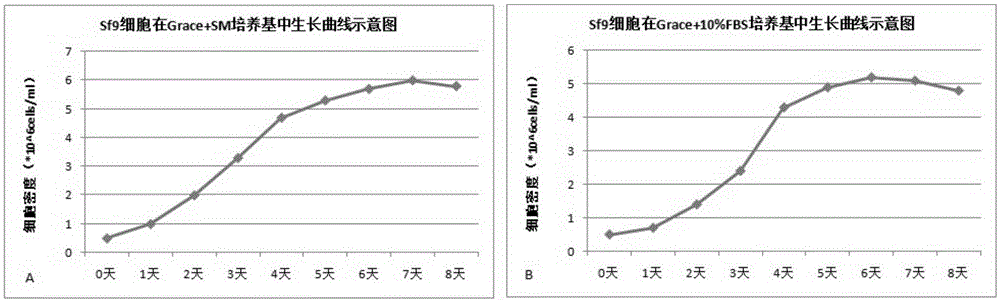
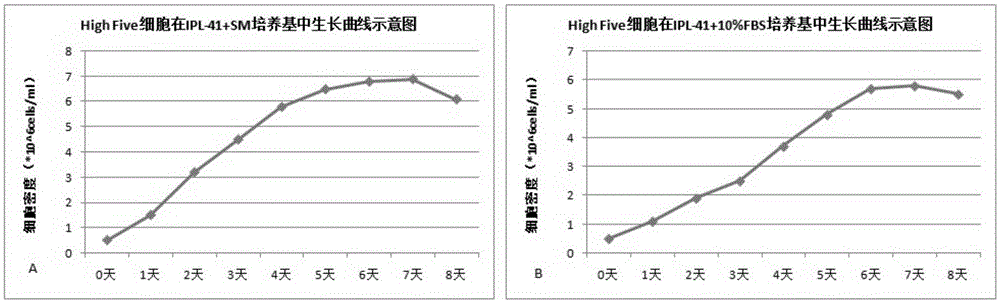
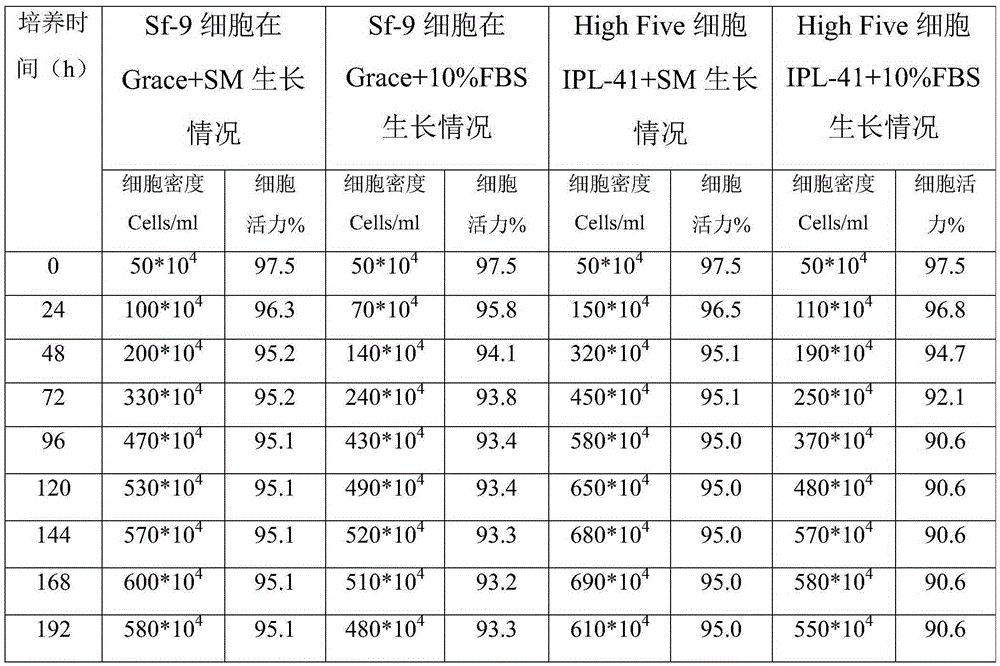
The invention relates to the technical field of biological pharmacy, in particular to development of a serum-free and animal-source-free culture medium additive suitable for insect cells mainly including Sf9 and High Five cells. A traditional culture medium Grace or IPL-41 serves as a basis, the additive beneficial for the growth of the insect cells is added, in the insect cell culture process, the additive has a function of replacing serum, price is low, the insect cells can grow for 240 h continuously, and cell activity can keep over 95%.
Owner:内蒙古金源康生物工程股份有限公司
Method for improving transient transfection of insect cell and stably expressing protein expression quantity
ActiveCN107236761AHigh expressionGood lifting effectVector-based foreign material introductionAnimal husbandryCellular respirationProtein target
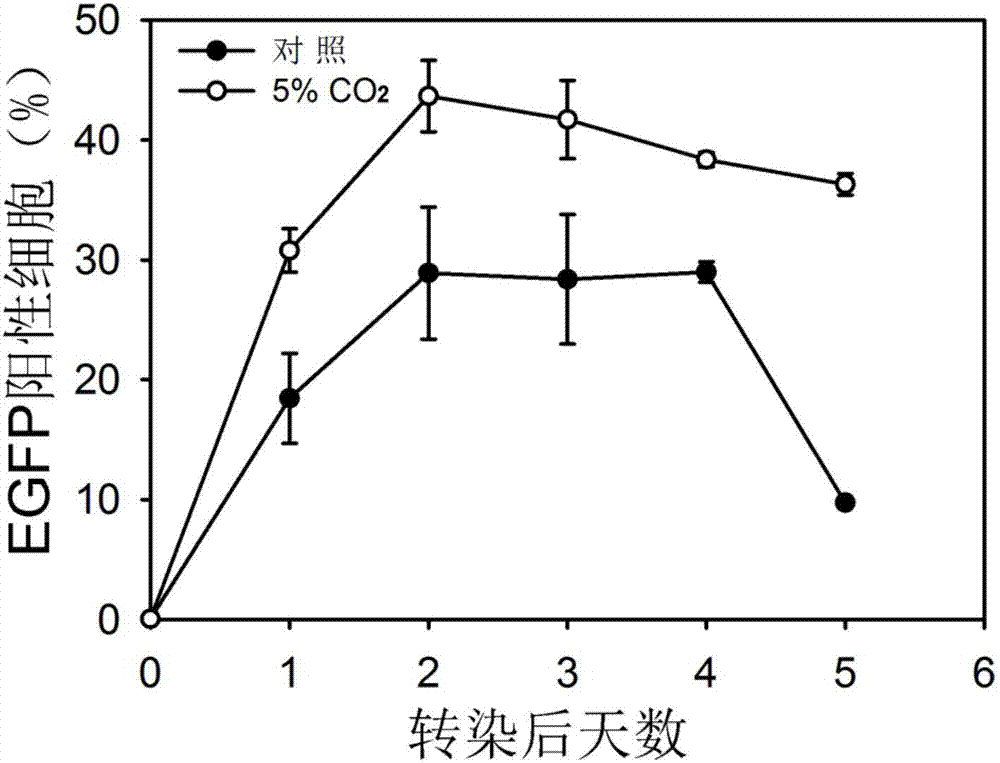
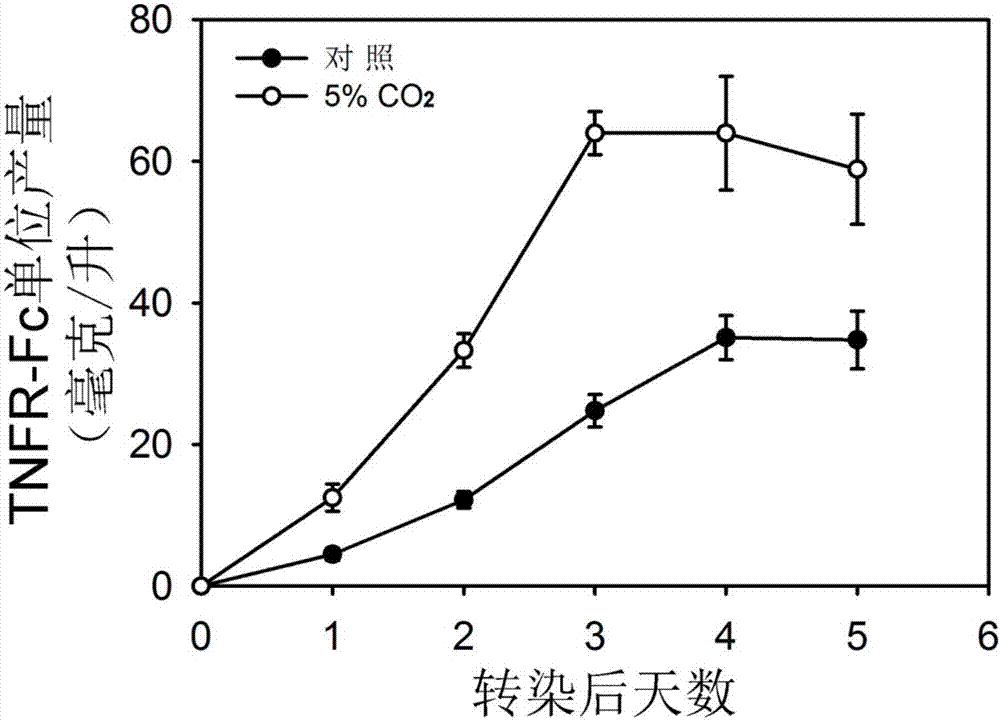
The invention discloses a method for improving transient transfection of insect cell and stably expressing protein expression quantity. A traditional insect cell cultivation method adopts a phosphate buffer system mostly, CO2 is not required to add during the cultivation process, and the expression quantity of a target protein is relatively low. The method creatively finds that the protein expression quantity can be improved by adding CO2 in a cultivating box or changing an air permeable cover to be a sealing cover after transfection to increase CO2 concentration in a bottle upon the self-breathing effect of insect cells regardless of transient transfection or protein expression and production process of a stable cell pond when the insect cell is cultivated; moreover, the lifting effect is significant. The CO2 is added in the cultivation box during the insect cell cultivation process, or the cultivation bottle cover is changed to be a sealing cover, the method is simple and easy to practice, and the cost is low; the method is extensive in use and capable of significantly improving the protein expression quantity of the insect cells.
Owner:FOSHAN CANTON BIOLOGICS CO LTD +1
Preparation method of silkworm decellularization hemolymph liquid
The invention relates to a preparation method of silkworm decellularization hemolymph liquid. The preparation method comprises a series of steps of disinfecting the body surfaces of silkworms, preparing the hemolymph liquid of the silkworms, carrying out antioxidant treatment and cell extraction on the liquid, removing bacteria from the liquid and the like. The method takes the hemolymph liquid of the silkworms as materials to prepare a nutrient substance applied to the cell culture of insects. Vitamin C is used for inhibiting the activity of tyrosine oxidase in the hemolymph liquid, so that the hemolymph liquid does not get black by oxidation when being contacted with the outside, the composition and the content of main nutrient substances in the hemolymph liquid are not changed, and the cell culture is not influenced. Compared with serum, the hemolymph liquid of the silkworms is more suitable for the cell culture of the insects, and is low in price.
Owner:SOUTHWEST UNIVERSITY
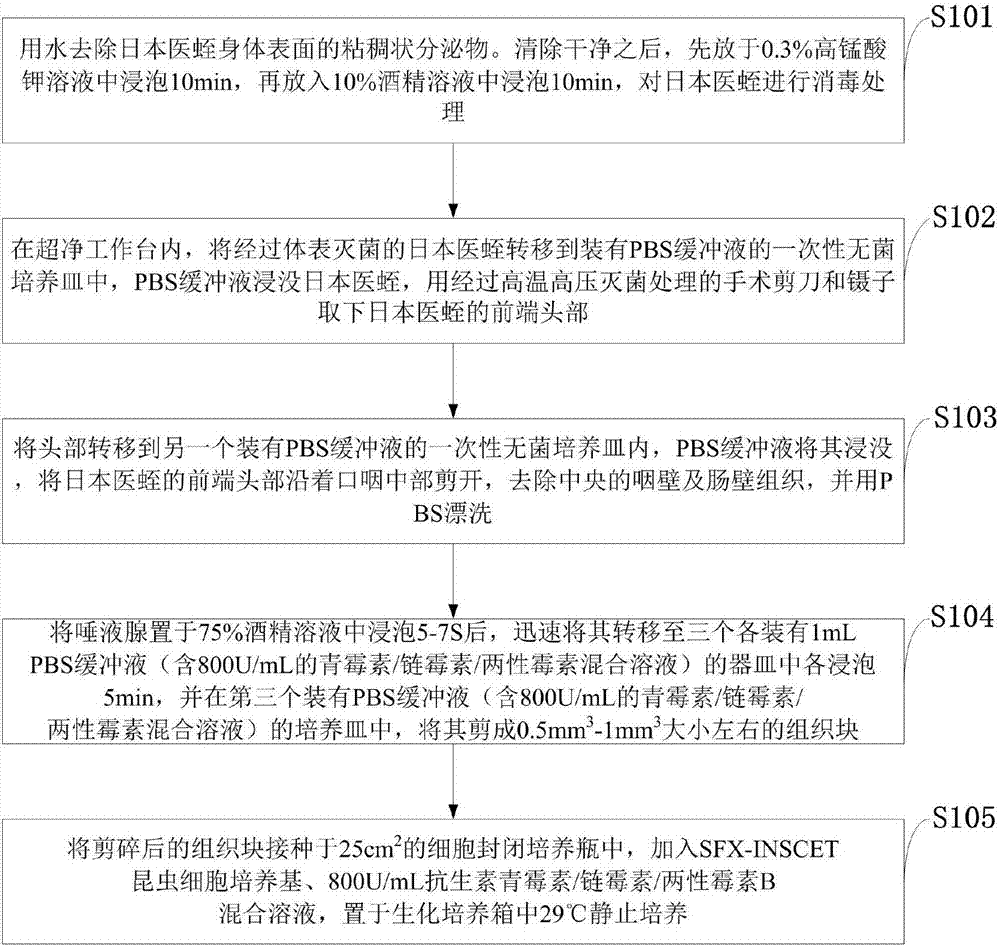
Primary culture method for salivary gland cells of Hirudo nipponia
ActiveCN107129962AIncrease productionImprove protectionInvertebrate cellsAnimal husbandryGermplasmBio engineering
The invention discloses a primary culture method for salivary gland cells of Hirudo nipponia, belonging to the field of bioengineering technology. The method comprises the following steps: inoculating an enclosed cell culture flask having a volume of 25 cm<2> with scissored tissue blocks; adding 5 mL of a SFX-INSCET insect cell culture solution; and placing the flask in a biochemical incubator for static culture at a temperature of 29 DEG C. The method provided by the invention substantially increases the output of natural hirudin; and a great number of salivary gland cells can be obtained after in-vitro cell culture of a tiny number of salivary gland cells of wild Hirudo nipponia, and a great amount of natural hirudin is separated and extracted, so wild Hirudo nipponia populations are protected, deep development and utilization are realized, and the germplasm resources of animals are substantially protected. The method is rapid, effective and repeatable, improves culture of leech cells and provides reliable test data and foundation for research on primary culture of leech cells.
Owner:CHINA JILIANG UNIV
Insect cell culture medium
The invention belongs to the technical field of in vitro cell culture, and relates to an insect cell culture medium. The insect cell culture medium is prepared from carbohydrate, amino acid, inorganic salt, vitamins, yeast extract, lactoalbumin hydrolysate, cholesterol, linoleic acid, malic acid, fumaric acid, soyabean lecithin, glutamine, putrescine and 0.1 to 1 g / L of glycerinum. The insect cell culture medium is scientific in matching of various components, so that the living time of insect cells can be effectively prolonged, the cell can still have sufficient nutrition under the condition that the culture medium is not replaced for a long time, and the metabolic balance is maintained; and in addition, the culture medium contains fewer types of components and is simple in preparation and low in cost.
Owner:严志海
Preparation method and application of recombinant human leucocyte interleukin 27
InactiveCN103409465AIncrease productionImprove biological activityPeptide/protein ingredientsAntiviralsEscherichia coliHepatitis B immunization
The invention discloses a preparation method and application of recombinant human leucocyte interleukin 27. The preparation method comprises the following steps: amplifying EBI3 segment and P28 segment of human leucocyte interleukin 27, and cloning onto a vector pFASTbac-dual; recombining the obtained vector transformation Escherichia coli DH10Bac and a Bacmid plasmid into a recombinant transposition plasmid rBacmid, transfecting rBacmid into sf9 insect cell under the mediation of liposome to obtain recombinant baculovirus; infecting the recombinant baculovirus into the sf9 insect cell; and carrying out sf9 insect cell culture, and purifying the supernatant through a nickel affinity chromatography column and a gel chromatography column to obtain the recombinant human leucocyte interleukin 27. The human leucocyte interleukin 27 has the function of resisting hepatitis B virus infection, and can be used for preparing medicines for resisting hepatitis B virus infection. The recombinant human leucocyte interleukin 27 obtained by the method is more approximate to a natural state, and has high bioactivity; and the method has the advantages of low production cost and high yield.
Owner:WUHAN UNIV
Coxsackie virus B5 type virus-like particle as well as preparation method and application thereof
ActiveCN112048004ASimilar Appearance StructureSimilar in sizeSsRNA viruses positive-senseViral antigen ingredientsImmunogenicityGenetic engineering
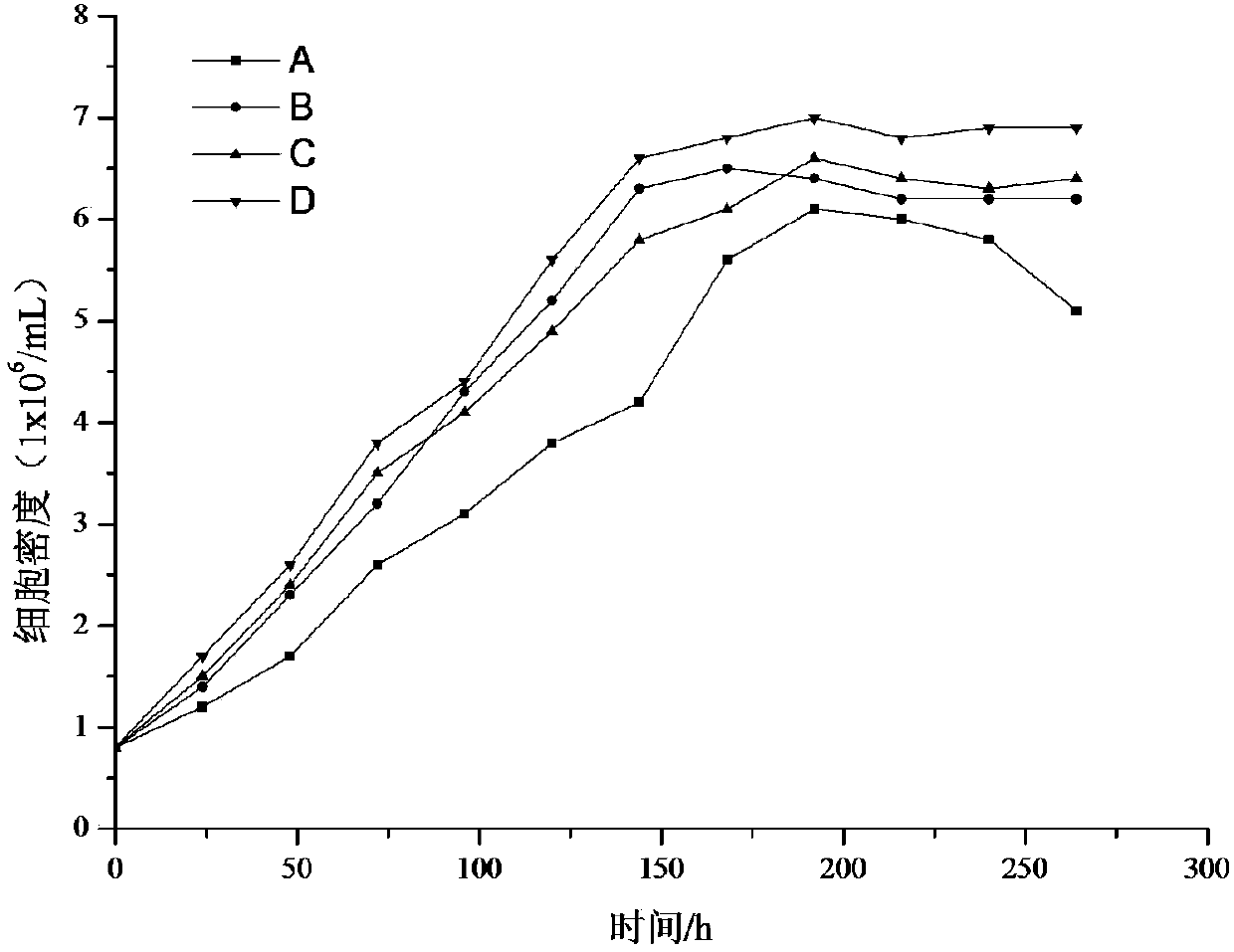
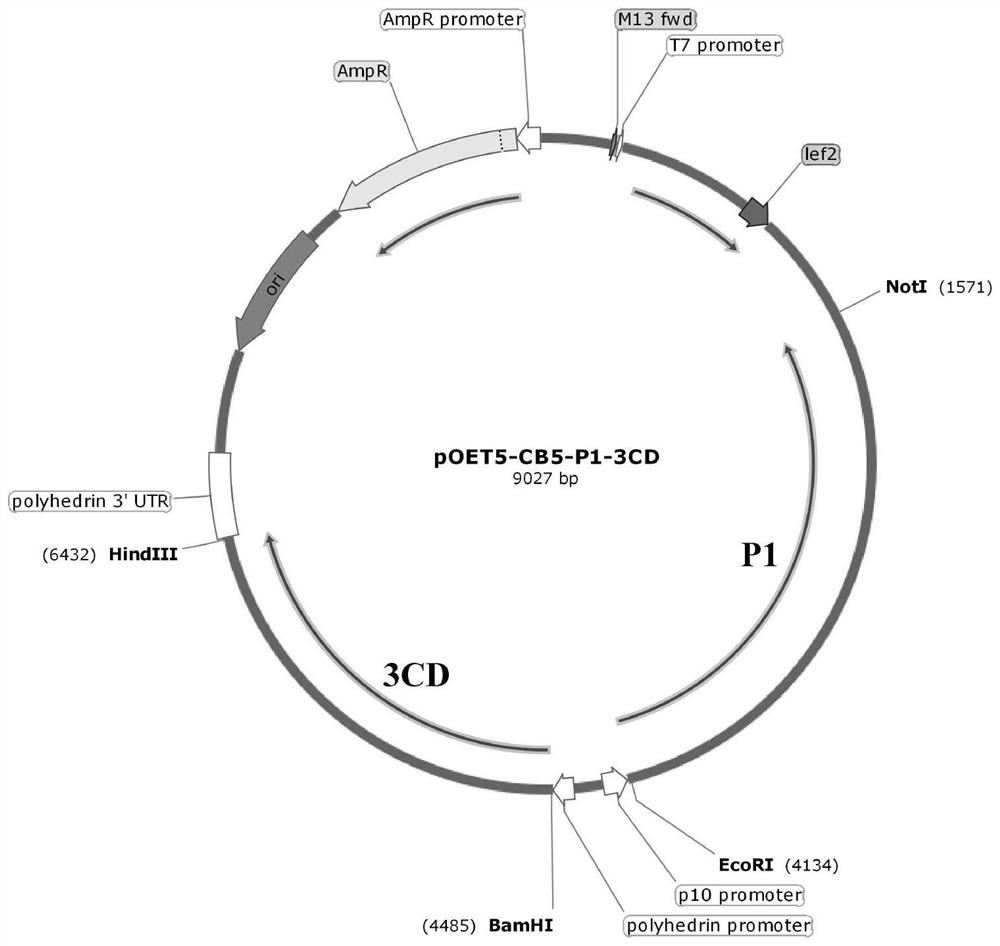
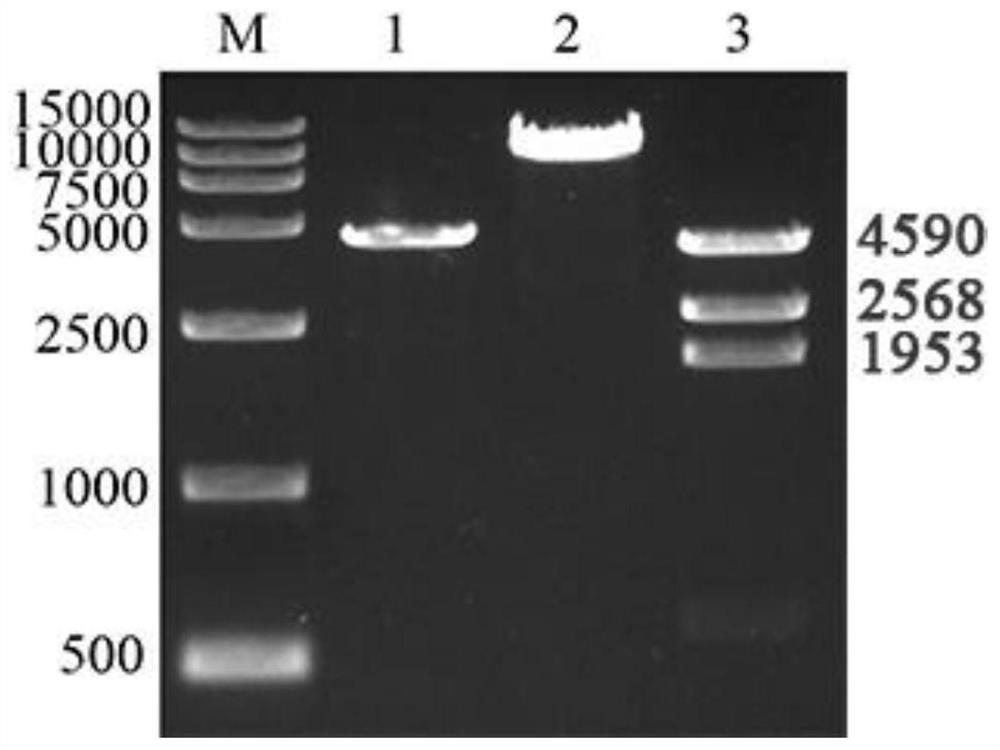
The invention discloses a coxsackie virus B5 type virus-like particle as well as a preparation method and application thereof, and belongs to the technical field of gene engineering and biological medicines. The coxsackie virus B5 type virus-like particle is obtained by infecting Sf9 insect cells with recombinant baculovirus for expressing P1 capsid protein gene and 3CD protease gene of coxsackievirus B5 type, and culturing until more than 90% of the Sf9 insect cells are infected with lesions. The invention further discloses a preparation method and application of the coxsackie virus B5 typevirus-like particle. The coxsackie virus B5 type virus-like particle and a wild virus particle have similar appearance structures and sizes, the immunogenicity reaches or even exceeds the degree of the wild inactivated virus, and the coxsackie virus B5 type virus-like particle can be used for preparing a coxsackie virus B5 type virus-like particle vaccine.
Owner:GUILIN MEDICAL UNIVERSITY
Preparation method for human papillomaviral empty capsid particles, and products thereof
ActiveCN102321635AImprove securityReduce manufacturing costViral antigen ingredientsInactivation/attenuationAntigenCapsid
The present invention discloses a preparation method for human papillomaviral (HPV) empty capsid particles, and products thereof. The preparation method comprises: (1) inserting main capsid protein gene of the human HPV empty capsid particles or a combination coexpressed from the main capsid protein gene of the human HPV empty capsid particles and the secondary capsid protein gene of the human HPV empty capsid particles into baculovirus genome to construct recombinant baculovirus; (2) transfecting an insect host or insect cells through the constructed recombinant baculovirus; (3) culturing the transfected insect host or the transfected insect cells, collecting and purifying the expressed antigen to obtain the human HPV empty capsid particles. According to the preparation method, the baculovirus expression system is adopted for safely and efficiently producing the human HPV empty capsid particles in a bombyx mori bioreactor; with adopting the method provided by the present invention, the production cost for the human HPV empty capsid particles can be significantly reduced; the method has advantages of safety, high performance, less energy consumption, low cost, and the like.
Owner:THE INST OF BIOTECHNOLOGY OF THE CHINESE ACAD OF AGRI SCI
Universal influenza vaccine
ActiveUS11351241B2SsRNA viruses negative-senseViral antigen ingredientsAdjuvantAntiendomysial antibodies
Immunogenic compositions for inducing a universal immune response to influenza, and particularly influenza A, by eliciting anti-neuraminidase antibodies which provide protection against heterologous influenza infection. Compositions comprising recombinant baculovirus expression vectors expressing neuraminidase in cultured insect cells dispersed in a pharmaceutically-acceptable carrier comprising insect cell culture media, and optional adjuvant. Methods of inducing immune responses against influenza, and particularly influenza A, by eliciting anti-neuraminidase antibodies in a host animal susceptible to infection.
Owner:CAMBRIDGE TECH LLC
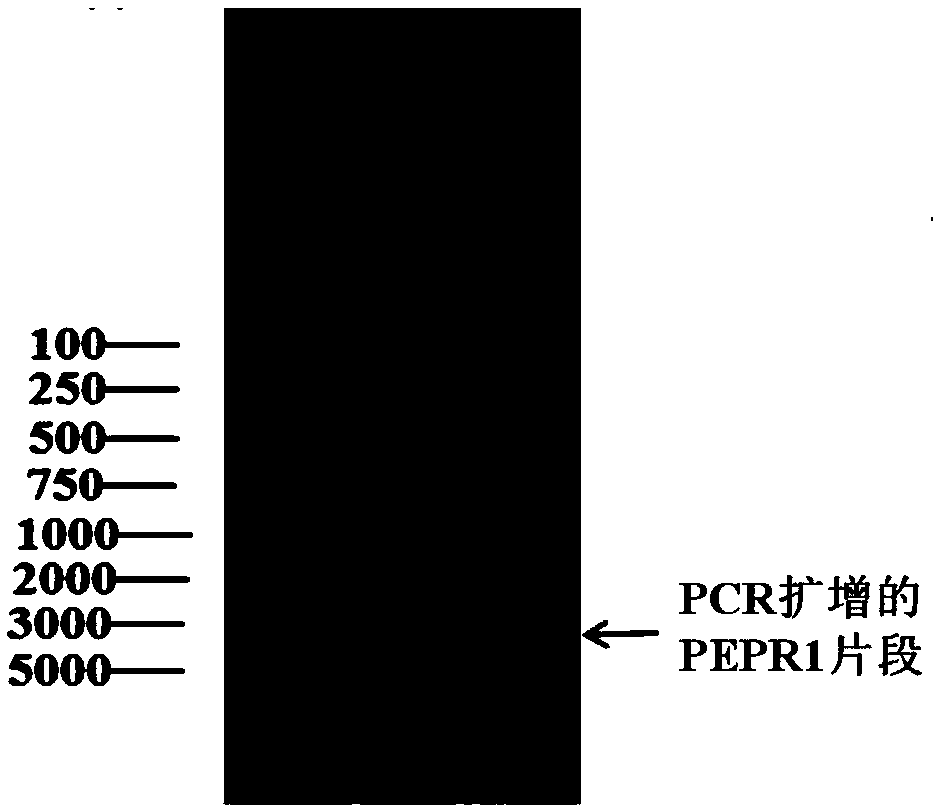
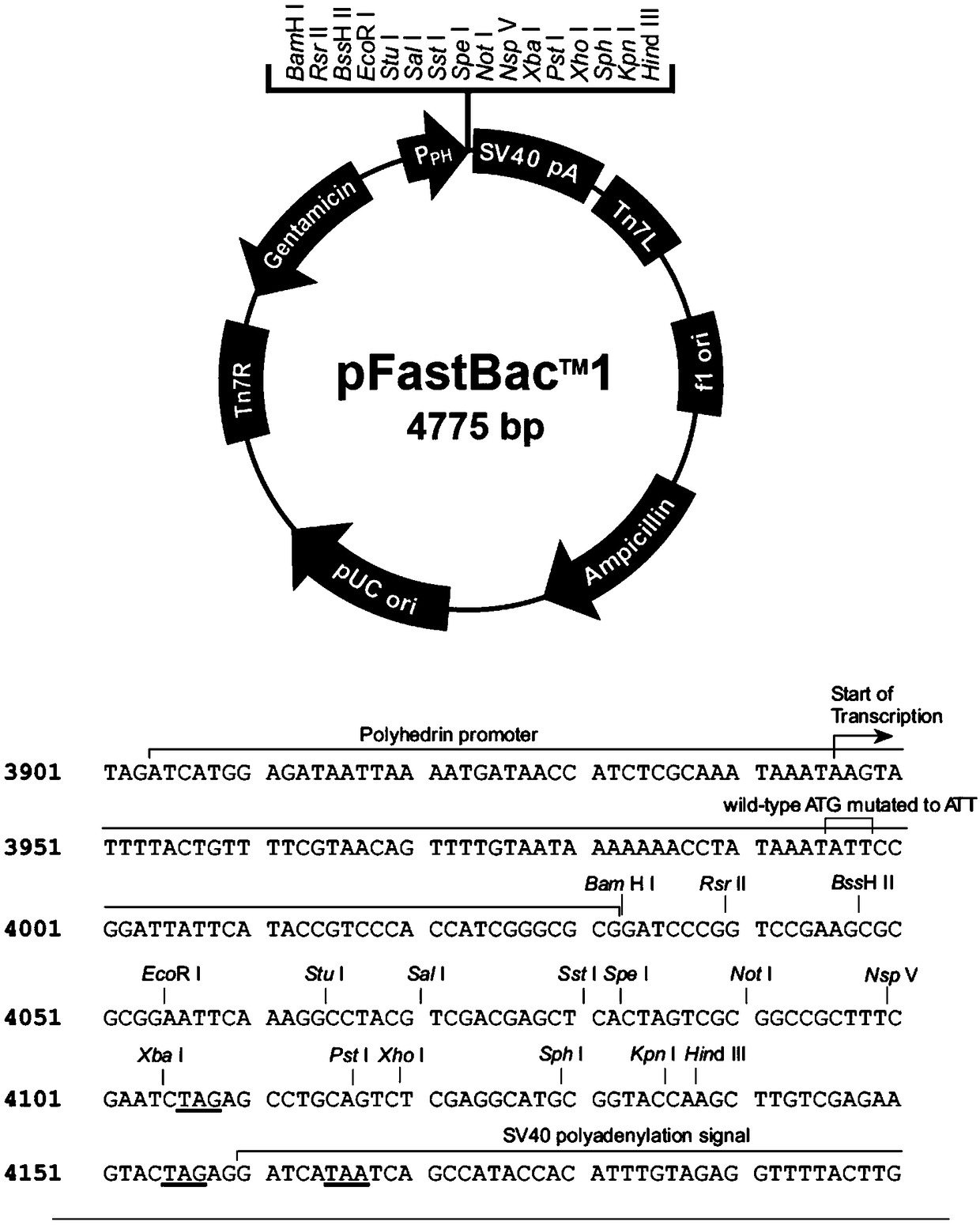
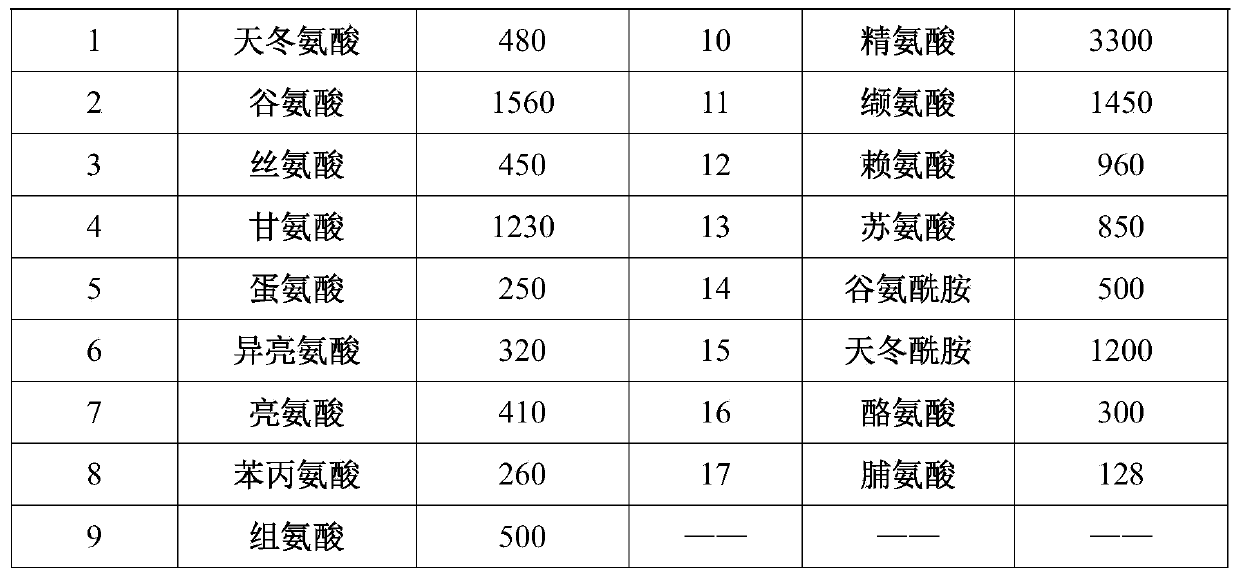
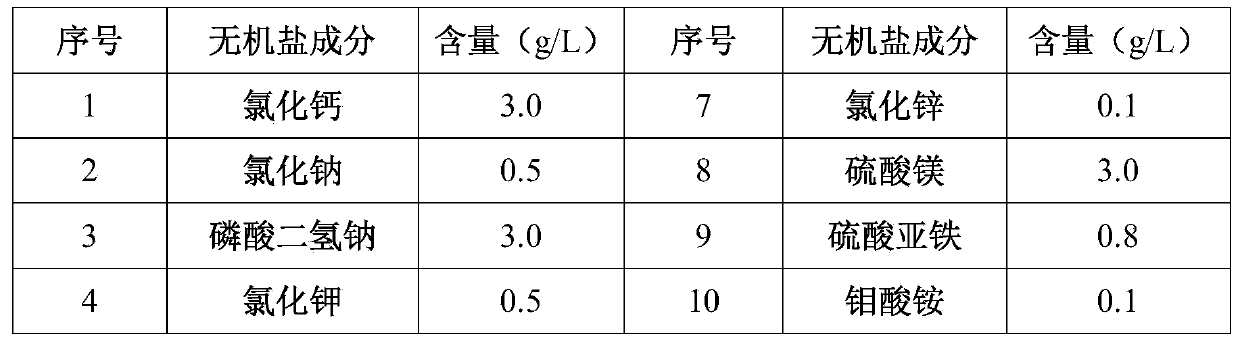
Method for heterogenous expression of PEPR1 protein
InactiveCN109402173AContribute to successful secretionHelp expressMicrobiological testing/measurementTransferasesSequence signalProkaryotic expression
The invention provides a method for heterogenous expression of PEPR1 protein. The method comprises the following steps of adding Hemolin signal peptides in front of a BamH1 digestion site of pFastBac<TM>1 to obtain a pFastBac<TM>Hem vector; performing digestion on a PEPR1 gene by BamH1 and Sal1; performing digestion on the pFastBac<TM>Hem vector by using BamH1 and Xho1; performing connection afterthe digestion; building an heterogenous expression vector; transfecting an insect cell by the heterogenous expression vector; after the cell is cultured, harvesting the cells and supernatant; performing chromatography and purification on the supernatant to obtain the PEPR1 protein with the correct conformation. The expression quantity reaches 2.24 mg / L. The method overcomes the defect that in theprior art, the PEPR1 protein with the correct conformation cannot be obtained by using a prokaryotic expression system, or when other eukaryotic expression systems are used, the expression quantity of the PEPR1 protein is low. The expression quantity of the PEPR1 protein with the correct conformation is improved; the cost is reduced.
Owner:BEE RES INST CHINESE ACAD OF AGRI SCI
Insect cell serum-free culture medium and preparation process thereof
PendingCN110564670AEasy to grow on a large scaleImprove cultivation efficiencyInvertebrate cellsCulture processSerum free mediaLipid formation
The invention discloses an insect cell serum-free culture medium and a preparation process thereof. The insect cell serum-free culture medium comprises 11-16 g / L of amino acid, 5-15 g / L of carbohydrates, 7-12 g / L of inorganic salt, 0.01-0.2 g / L of vitamin, 3-10 g / L of a protein hydrolysate, 1-5 g / L of yeast powder and 15-35 g / L of a lipid emulsifier. The insect cell serum-free medium provided by the invention is clear in components, low in vitamin content, relatively low in cost and simple in preparation process, is beneficial to large-scale culture of insect cells, can greatly improve the insect cell culture efficiency, and has a good commercial prospect.
Owner:广州今成生物科技有限公司
Three Phase Partitioning (TPP) Method for Virus Purification
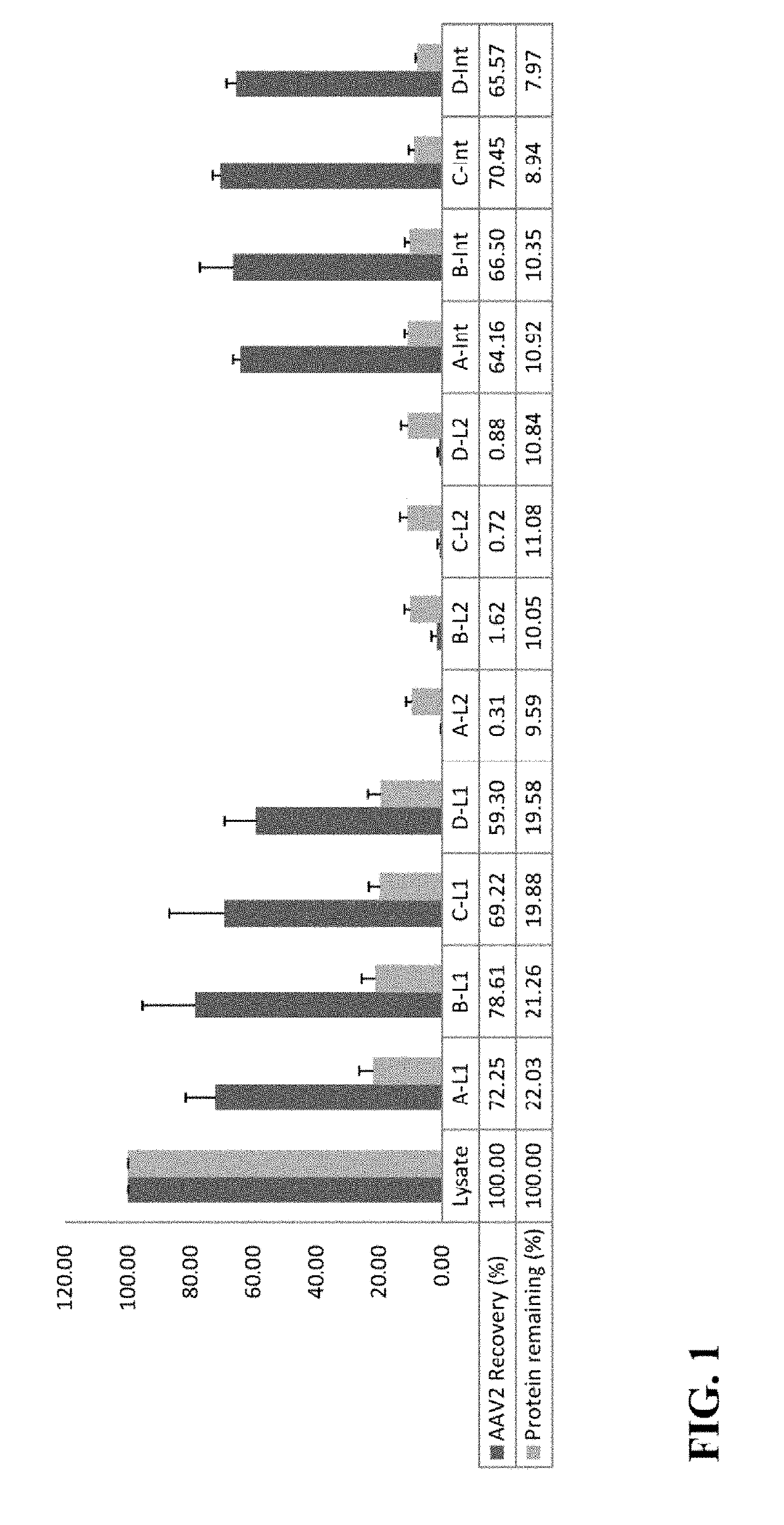
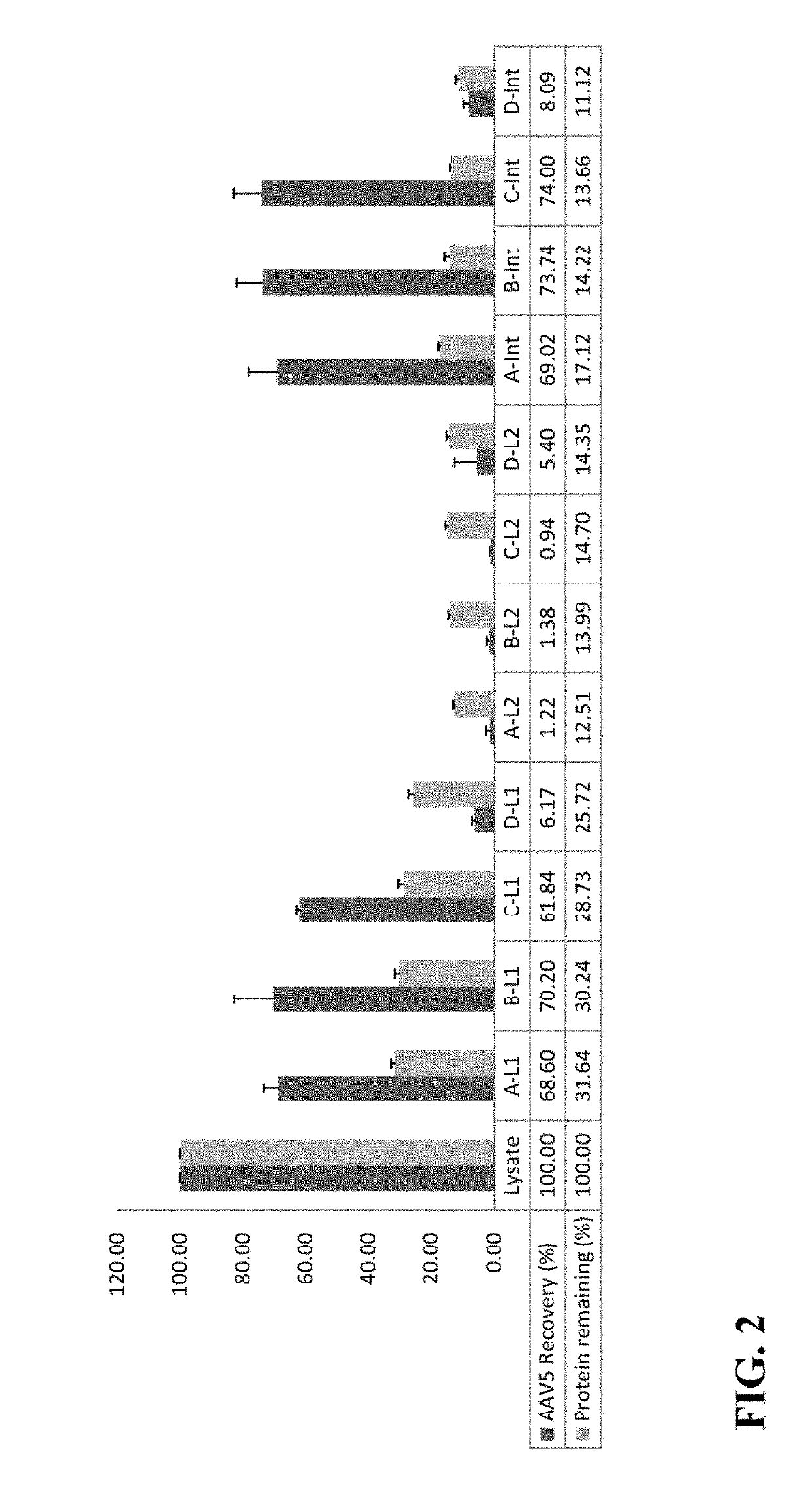
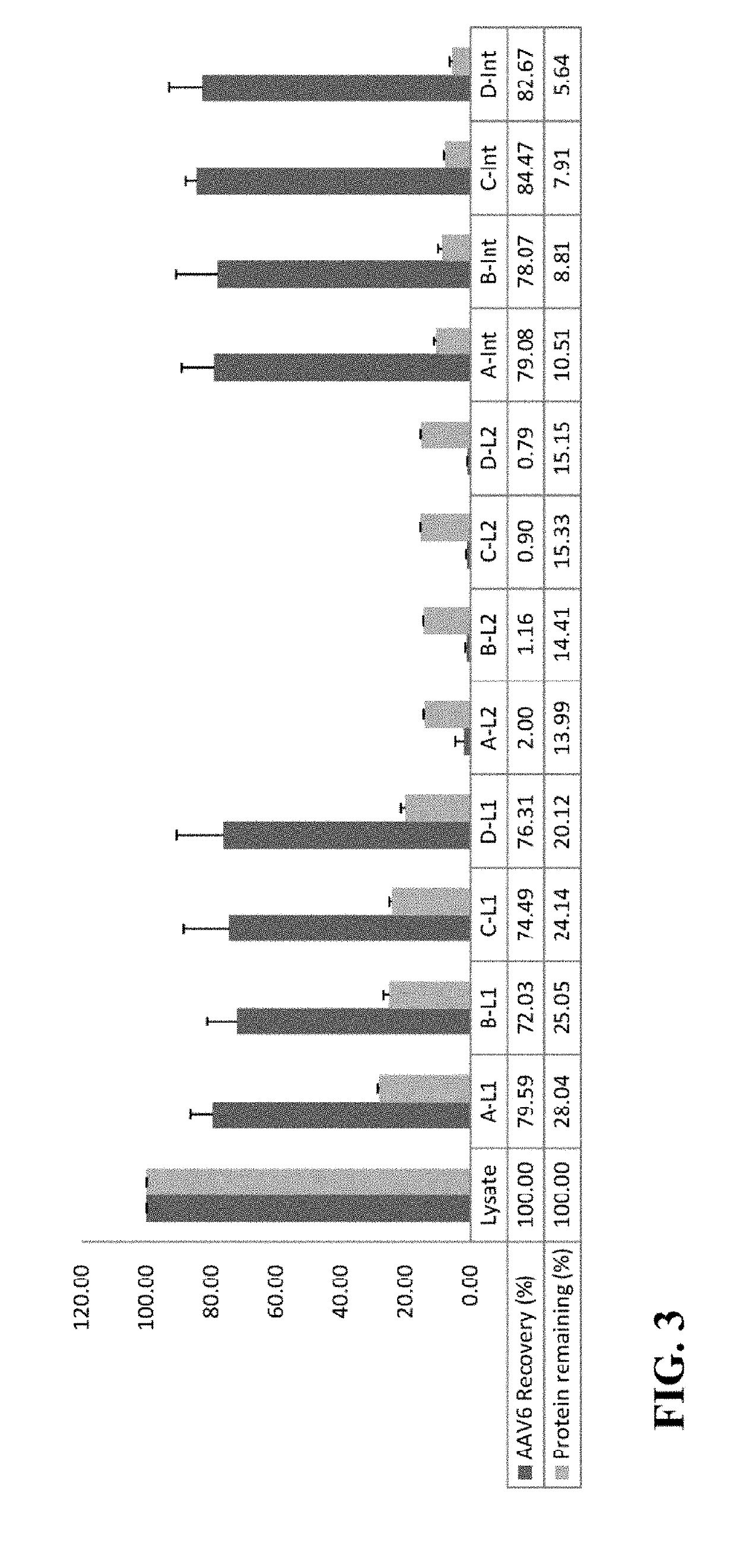
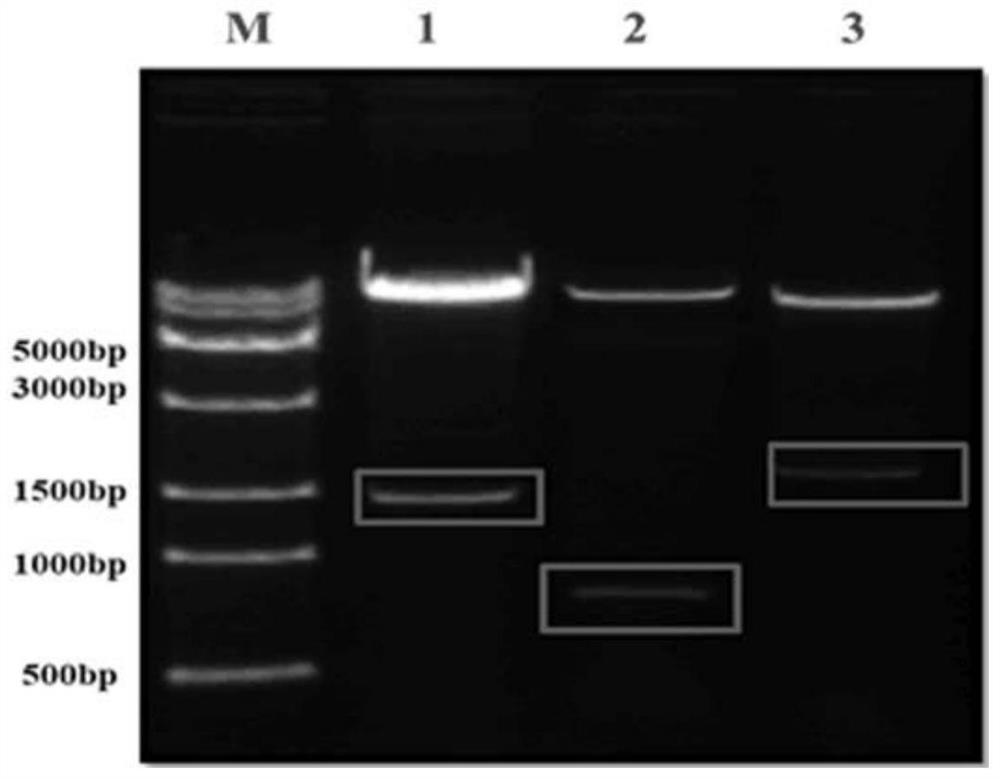
Methods of purifying a virus from a virus-infected cell lysate using three-phase partitioning (TPP) are disclosed. The methods comprise a first round of TPP, including mixing a cell lysate comprising a virus with ammonium sulfate and t-butanol, and separating the mixture, thereby forming a first aqueous phase, a first organic phase, and a first interphase. The first aqueous phase can comprise the virus, which can be subjected to a second round of TPP, resulting in a second aqueous phase, a second organic phase, and a second interphase. The second interphase can comprise highly purified virus. The methods can comprise subjecting a first aqueous phase to further purification by column chromatography or density gradient centrifugation. Purification of AAV, including AAV2, AAV5 and AAV6, from lysates of infected insect cell cultures is demonstrated. TPP-purified AAV particles infect at least as well as those prepared by standard methods.
Owner:VIROVEK
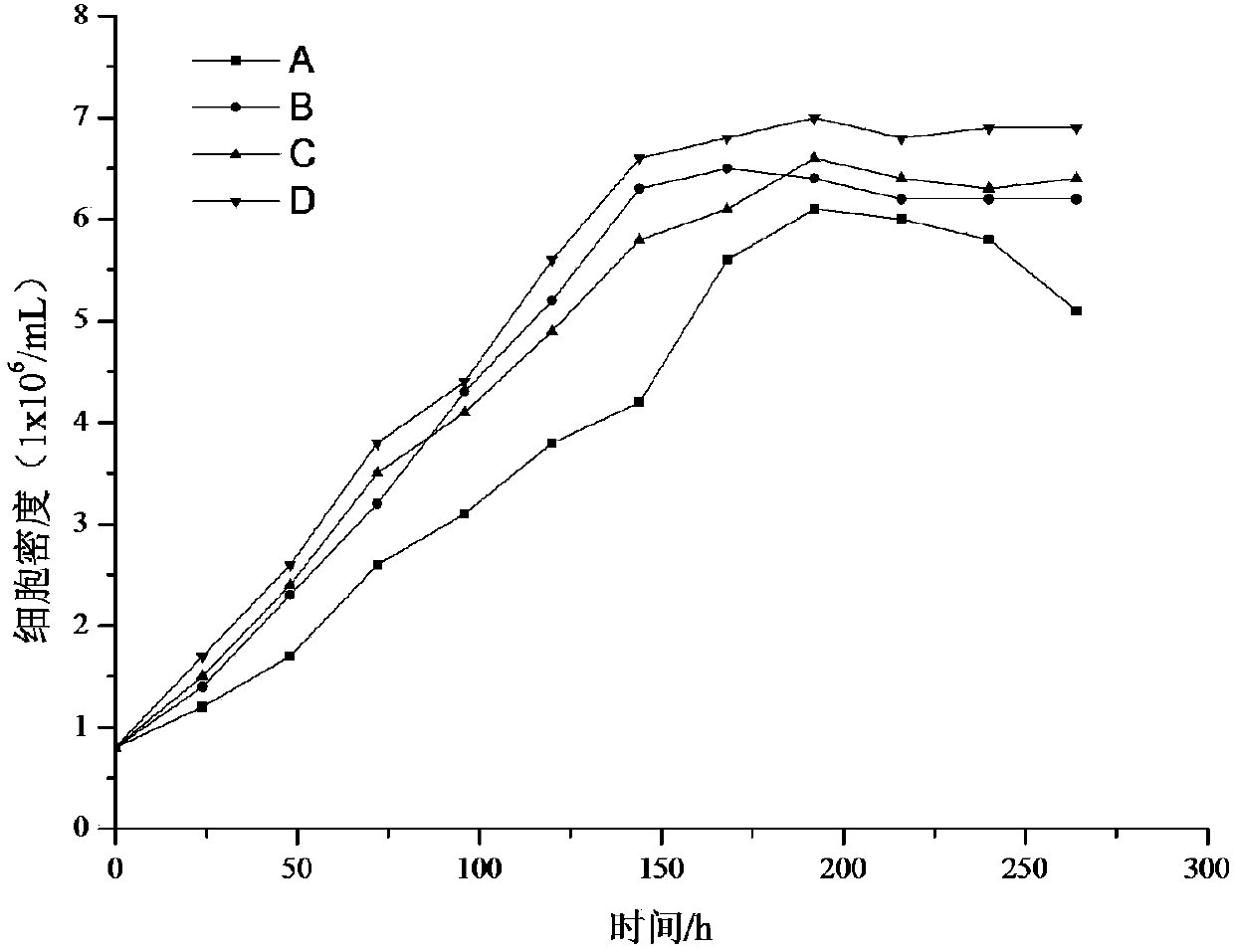
Leech cell in-vitro culture medium and culture method thereof
ActiveCN108265021AQuick successAccurate and reliable experimental basisInvertebrate cellsCulture processRecovery methodInsect cell culture
The invention discloses a leech cell in-vitro culture medium and a culture method thereof. According to the method, sheared leech tissue blocks are inoculated into a cell sealed culture bottle; an Insect-CCulture insect cell culture medium is added; still standing culture is performed in a cell culture box; then, a tissue block recovery method is matched for separation culture; finally, purified leech cells are obtained through a trypsin digestion method for further culture. Compared with other insect cell culture media, the method has the advantages that the obtaining of the leech purified body cells can be successfully obtained at a higher speed; the preparation is made for further performing the leech cell in-vitro subculture; in addition, a set of in-vitro primary culture leech cell system is built.
Owner:长握生物科技(江苏)有限公司
A kind of recombinant h7n9 subtype avian influenza virus-like particle and its preparation method and application
ActiveCN112079904BGood cross reactivityMild diseaseSsRNA viruses negative-senseViral antigen ingredientsAdjuvantImmunogenicity
The invention discloses a recombinant H7N9 subtype avian influenza virus-like particle and its preparation method and application. Based on the recombinant baculovirus insect cell culture system, the expression of H7N9 highly pathogenic avian influenza virus HA protein and NA protein is respectively constructed. , M1 protein recombinant baculovirus. Three strains of recombinant baculoviruses were co-infected in suspended insect cells, and H7N9 subtype avian influenza virus-like particles self-assembled in the cells could be harvested. After being concentrated and purified by ultrafiltration tubes and different gradient concentrations of sucrose, it is mixed with adjuvant and emulsified to prepare the vaccine. The prepared vaccine immunized chickens can induce the body to produce specific antibodies, and has the advantages of strong immunogenicity, good safety, high genetic stability and the like. After challenge with a lethal dose of highly pathogenic avian influenza virus of H7N9 subtype, it can provide complete clinical protection and significantly inhibit the shedding of chickens. The invention provides a new method for preventing H7N9 subtype avian influenza virus infection, and also lays a foundation for the development of new influenza virus vaccines.
Owner:YANGZHOU UNIV
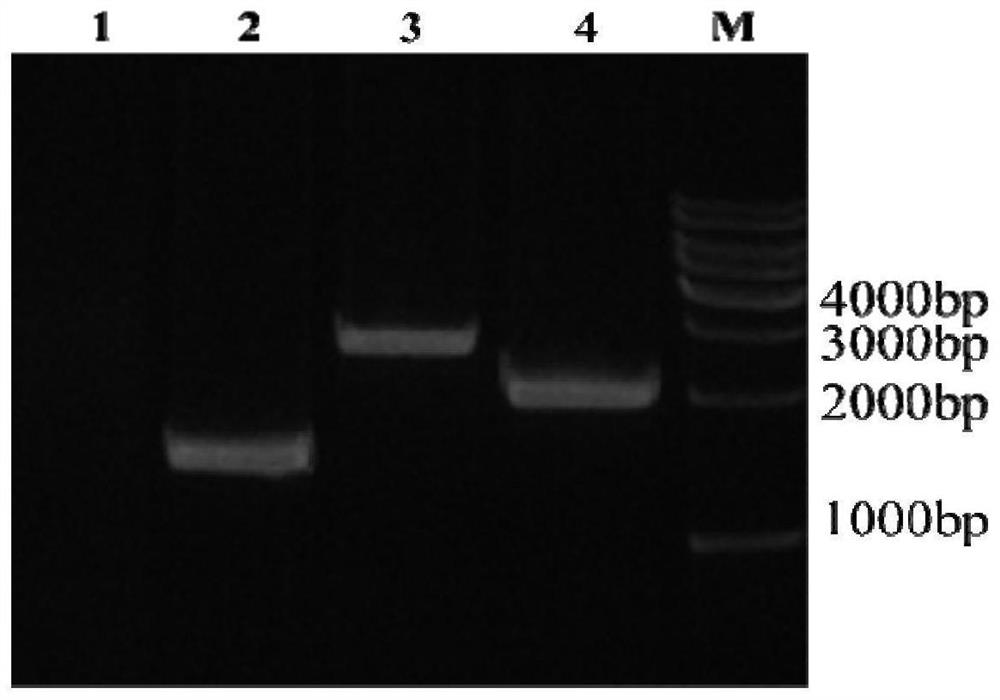
Preparation method of antigen protein for detecting rabies virus antibody, and kit
PendingCN109828109ASolve the problem that it cannot accurately reflect the true level of effective protective antibodies in the bodyAvoid Biosecurity RisksMaterial analysisViral antibodyNeutralizing antibody
The invention relates to the technical field of in-vitro diagnostic reagents, in particular to a preparation method of antigen protein for detecting a rabies virus antibody and a kit. The method comprises the steps: employing a recombinant baculovirus containing a rabies virus G protein expression cassette for infecting insect cells to obtain infected insect cells; culturing and propagating the infected insect cells; extracting antigen protein of the rabies virus antibody from insect cells; and preparing the kit through the antigen protein. The problem that rabies virus whole-virus particles are used as coating antigens in a traditional method due to a fact that the antibody detection result contains a neutralizing antibody and a non-neutralizing antibody is solved. The method can be usedfor large-scale production and detection of other infectious disease positive serum, has no cross reaction, has the advantages of strong specificity, high sensitivity, good repeatability and wide linear range, avoids biological safety risks, and has very strong creativity.
Owner:WUHAN LIFE TECH
Adapted lepidopteran insect cells for the production of recombinant proteins
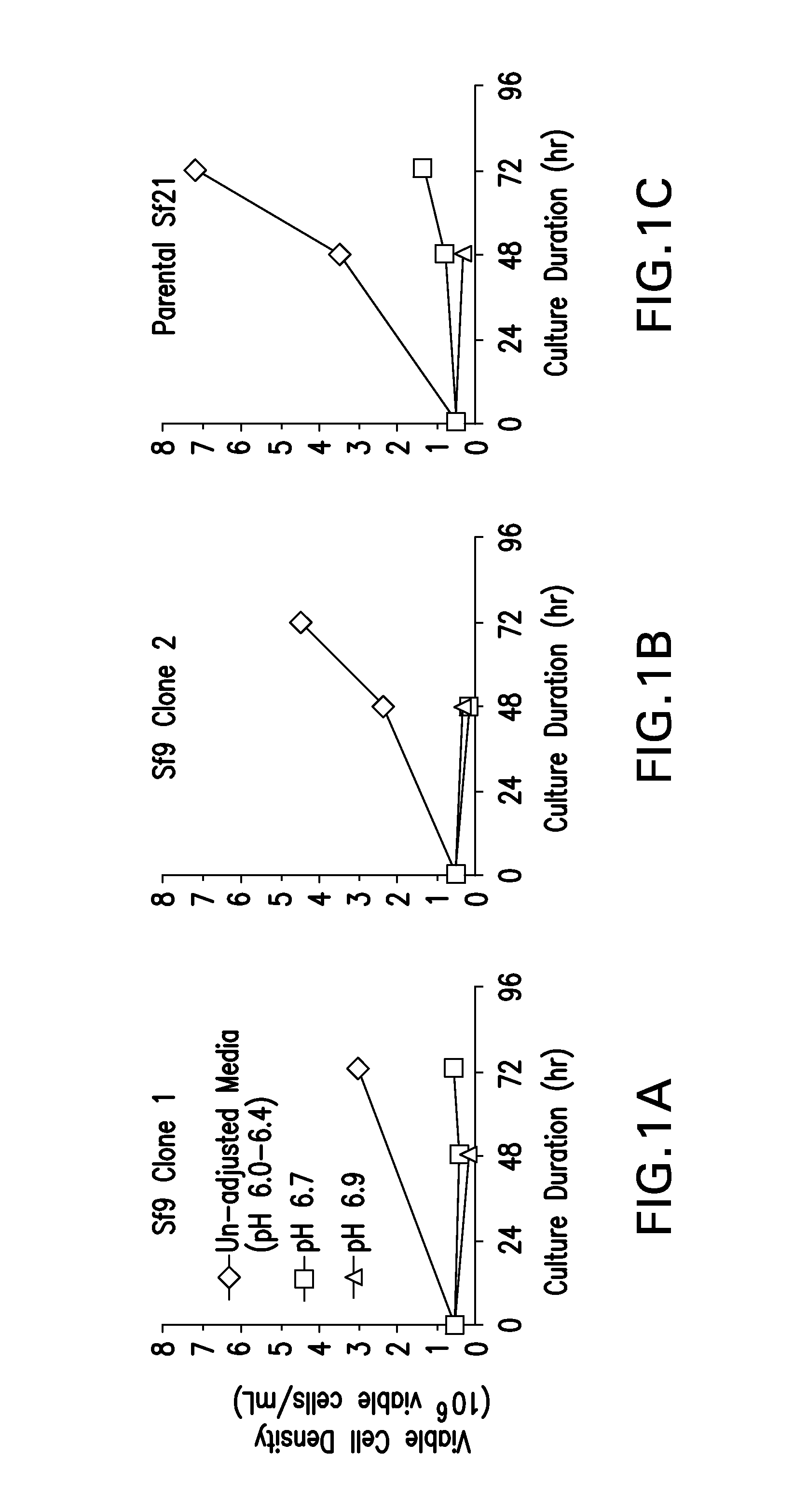
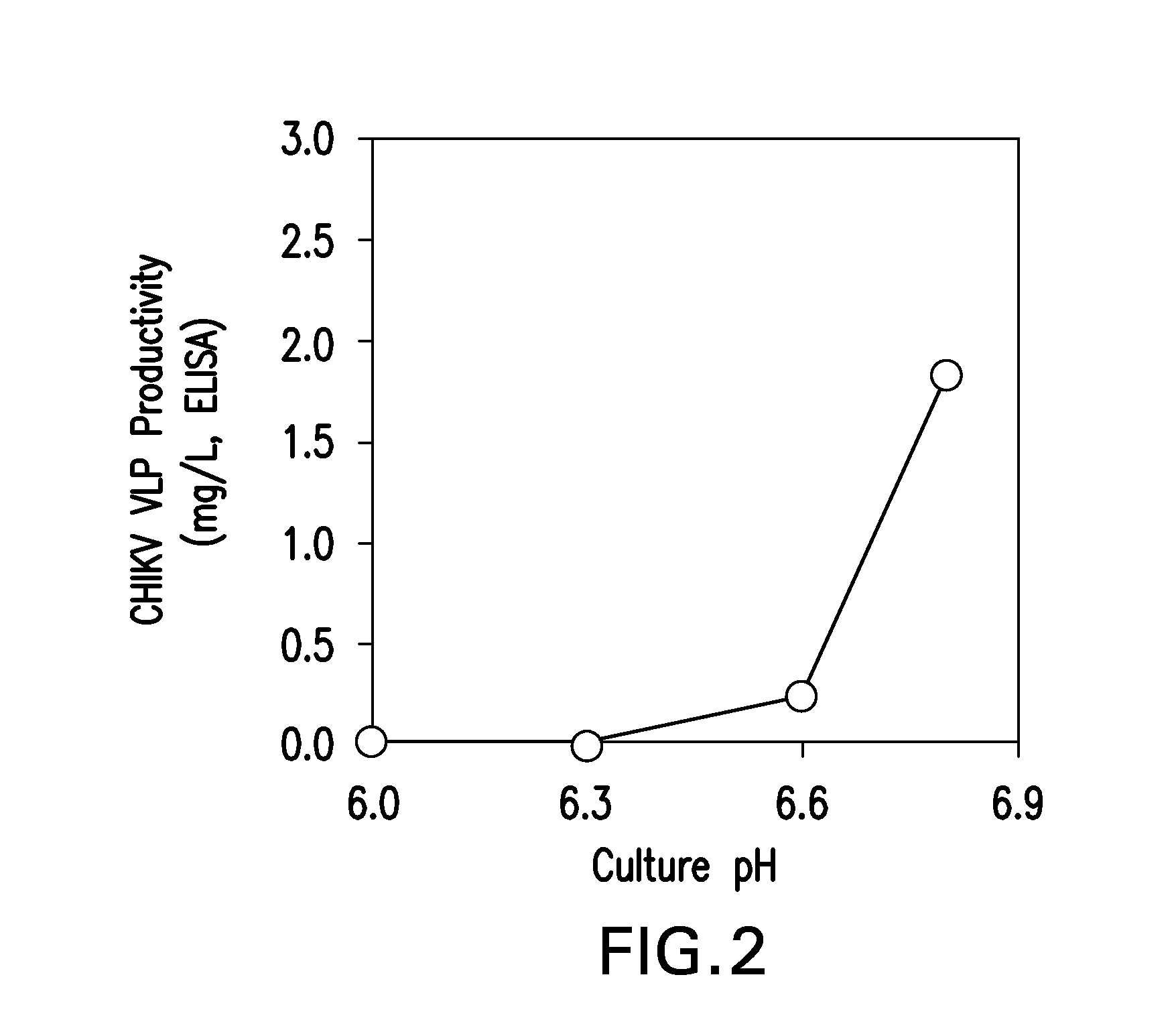
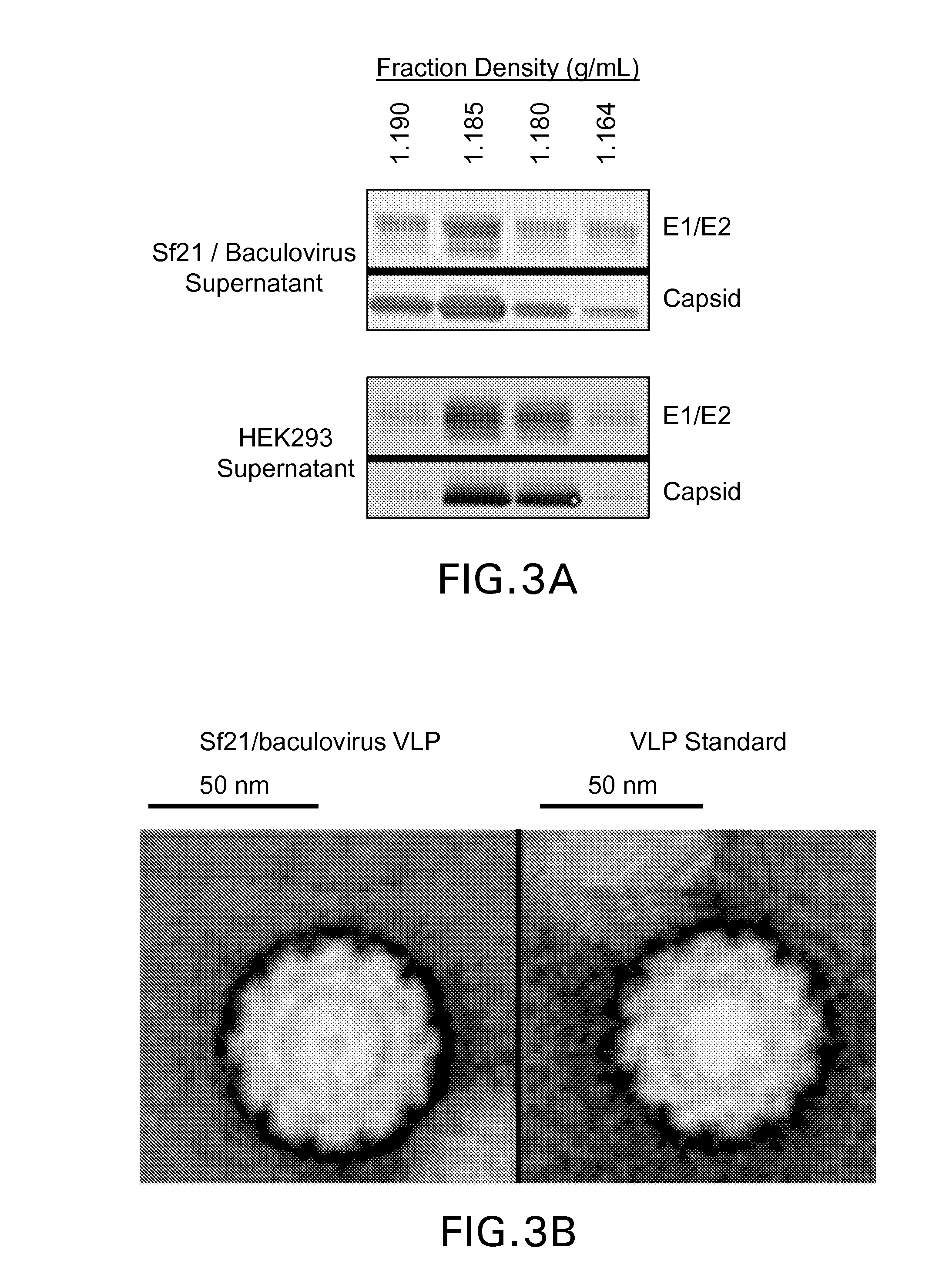
ActiveUS20160040124A1SsRNA viruses positive-senseInvertebrate cellsInsect cell cultureVirus-like particle
The present invention relates to the use of increased culture pH, relative to standard insect cell culture conditions, during baculovirus infection of lepidopteran insect cells to enable production of recombinant chikungunya (CHIKV) virus like particles (VLPs). The invention further relates to adapted insect cell lines derived from insect cells such as Sf21, which can grow robustly at elevated culture pH, the use of said cell lines to recombinantly produce pH sensitive proteins in the correct conformation and increase expression of recombinant proteins relative to standard insect cell lines. In some embodiments of the invention, the cells are useful for recombinant production of CHIKV VLPs. The invention also relates to a method for the production of a pH-adapted lepidopteran insect cell line. In some embodiments of said method, the cell line is produced and / or maintained in reduced phosphate serum-free insect cell media.
Owner:MERCK SHARP & DOHME LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com