Coxsackie virus B5 type virus-like particle as well as preparation method and application thereof
A coxsackie virus and virus-like technology, applied in the fields of genetic engineering and biomedicine, can solve the problems of long production cycle, listing virus-like particle vaccines, and difficulty in containing the outbreak of the epidemic, and achieve the effect of simple production process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] The preparation method of Coxsackievirus B5 virus-like particles in this embodiment includes the following steps:
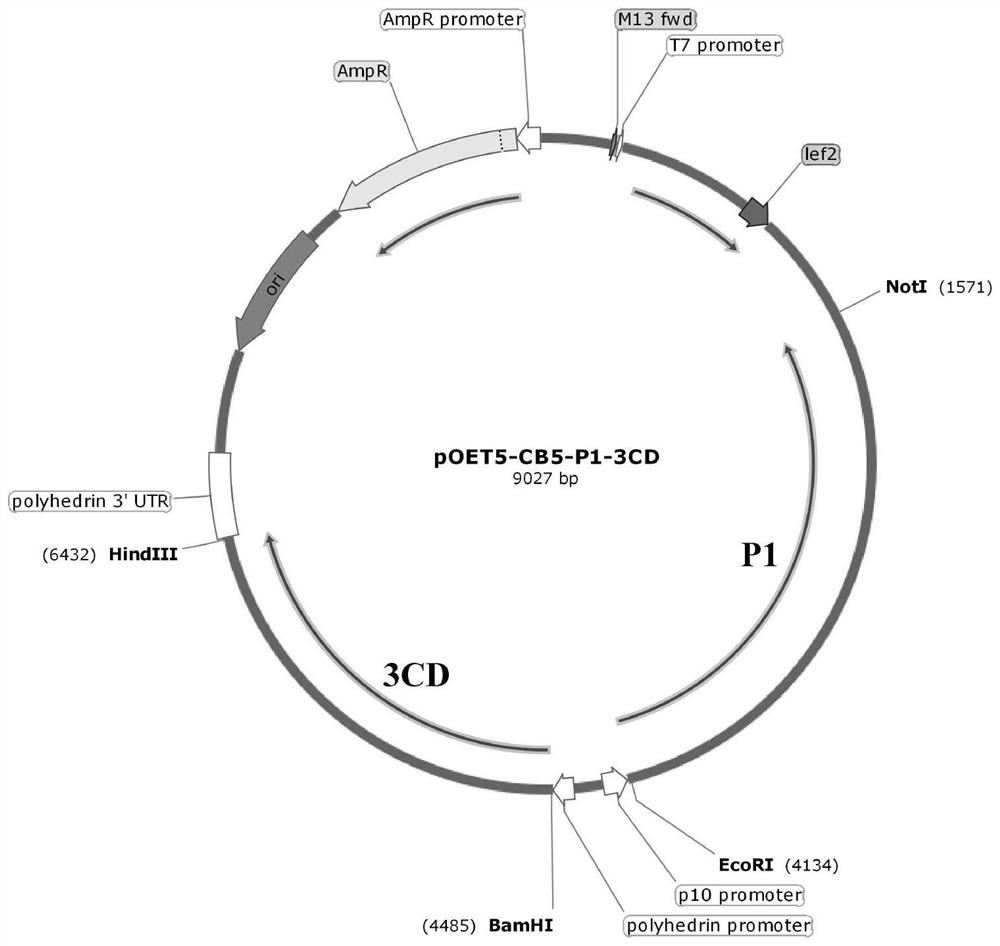
[0074] 1, preparing the recombinant plasmid pOET5-CB5-P1-3CD.
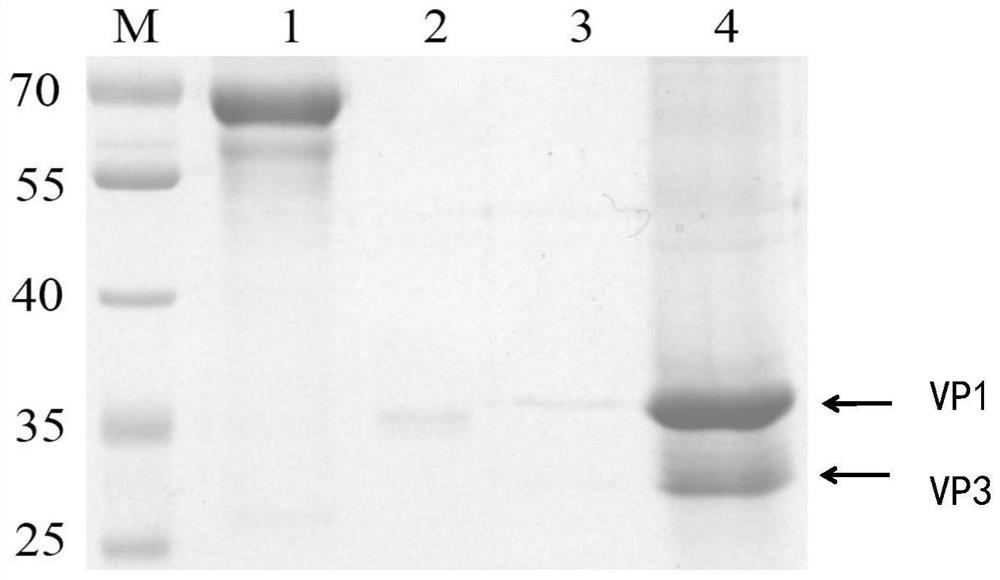
[0075] The capsid protein P1 gene and 3CD protease gene of coxsackievirus B5 with codon optimization were synthesized respectively, and then connected to the EcoRⅠ / NotⅠ restriction site after P10 promoter and the Bamhi / Hindiii restriction site after Ph promoter of shuttle vector pOET5 of insect baculovirus, respectively, to obtain the recombinant plasmid of pOET5-CB5-P1-3CD, such as Figure 1 Shown. The main elements of the plasmid are as follows: P10 promotor is p10 promoter, polyhedrin promotor is Ph promoter, AmpR is ampicillin resistance gene and AmpR promotor is ampicillin resistance gene promoter.
[0076] In which, the nucleotide sequence of the codon optimized capsid protein P1 gene is shown in SEQ ID NO.1; The nucleotide sequence of the optimized 3CD protease gene is shown in SEQ ID NO.2
[...
Embodiment 2
[0093] This embodiment provides a coxsackievirus B5 virus-like particle vaccine. The coxsackievirus B5 virus-like particle vaccine is a liquid injection prepared by mixing 50μg / mL coxsackievirus B5 virus-like particle obtained in Example 1 with Freund's adjuvant in equal volume.
experiment example 1
[0094] Example 1: Animal immunity, determination of serum antibody and virus neutralization reaction
[0095] According to the currently used animal immunization methods of enterovirus. The animals were six-week-old BALB / c female mice without specific pathogen (SPF). The specific operation ELISA as follows: 0.3ml of coxsackievirus B5 virus-like particles (10μg total protein) emulsified with Freund's adjuvant was subcutaneously injected into each BALB / c mouse at week 0, week 2 and week 4. The same dose of coxsackievirus B5 inactivated virus was used as a positive control and PBS group as a negative control, and the mice were cut off at week 0, week 2, week 4, week 6 and week 12.
[0096]The steps of ELISA are as follows: the purified inactivated virus B5 of Coxsackie virus is diluted to 1μg / ml with coating buffer (i.e. 0.05M carbonate buffer with pH 9.6), 100μl is added to each well of 96-well plate, and the coating is carried out overnight at 4℃. Washing: The next day, washing wit...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com