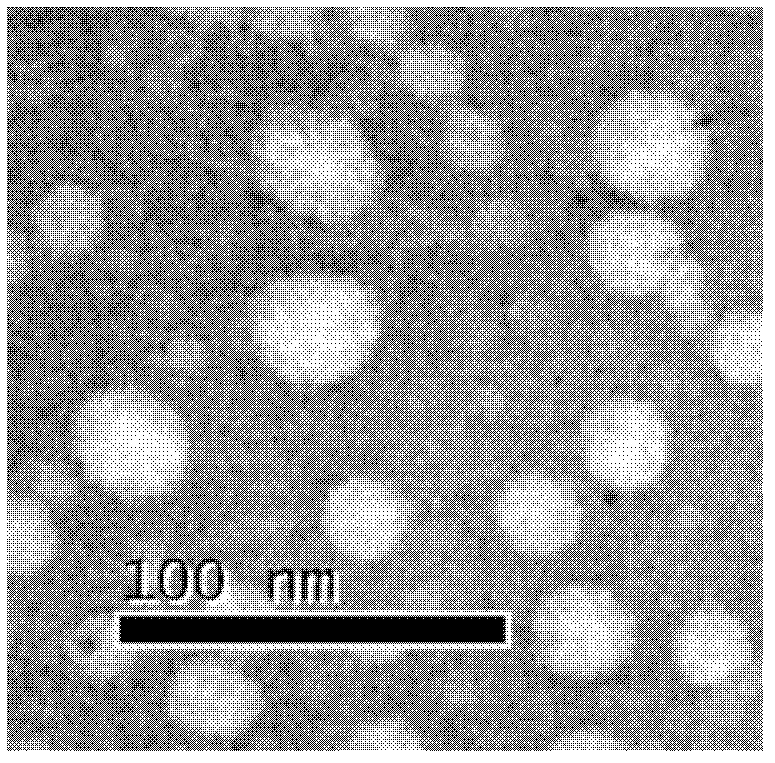
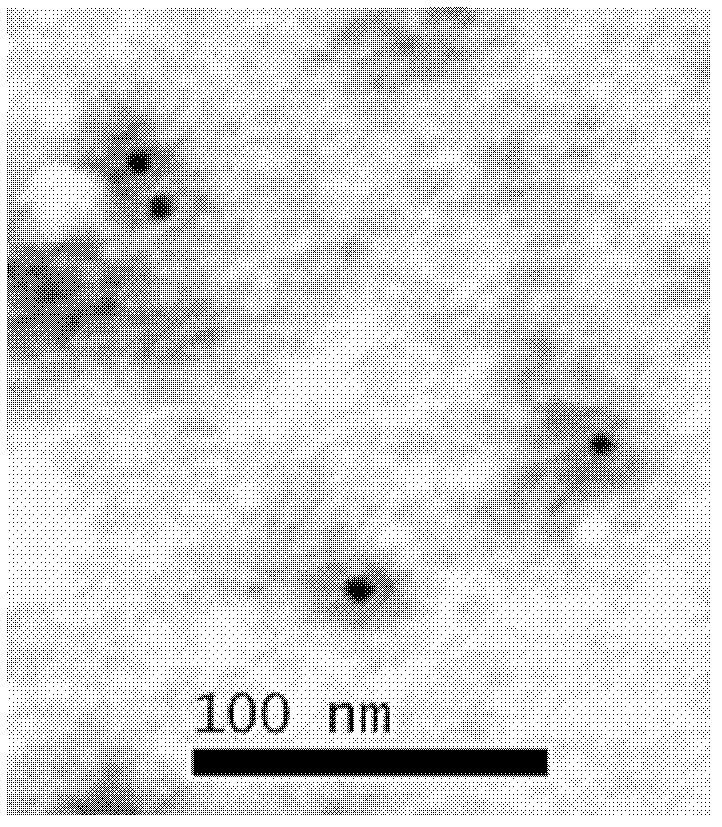
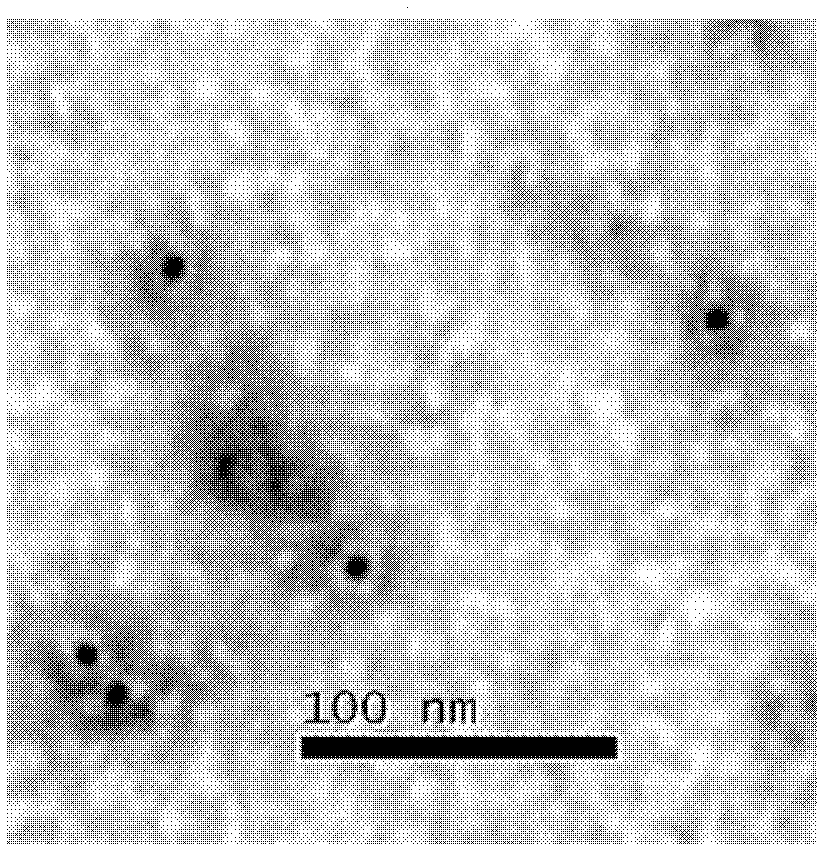Preparation method for human papillomaviral empty capsid particles, and products thereof
A technology of human papillomavirus and empty capsid particles, which is applied in the field of preparation of human papillomavirus empty capsid particles, and can solve the problems of high production cost, high energy consumption, and poor safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Expression and immune experiment of embodiment 1 human papillomavirus (HPV) type 16 main capsid protein gene L1 in silkworm bioreactor
[0033] 1 Experimental method
[0034] 1.1. About the configuration of solution and medium
[0035] Solution I: 50mmol / L glucose, 25mmol / L Tris-HCl (pH8.0), 10mmol / L EDTA.
[0036] Solution II: 0.2mol / L NaOH, 1% SDS (prepared and used immediately).
[0037] Solution III: 100mL system, 5mol / L potassium acetate 80mL, glacial acetic acid 12mL, ddH 2 O8mL.
[0038] TAE (50×): 242g Tris base, 57.1mL glacial acetic acid, 100mL 0.5mol / L EDTA (pH8.0), dilute to 1000mL with sterile water.
[0039] TER solution: Pancreatic RNAse (RNAse A) was dissolved in 10mM Tris-HCl, 15mMNaCl, made into a 10mg / mL storage solution and stored at -20°C, then diluted with 1×TE buf to a 20μg / mL working solution and stored at 4°C .
[0040] PPt Buffer: isopropanol 22mL; 5mol / mL KAc 1mL; ddH 2 02mL.
[0041]PEG solution: Weigh a certain amount of NaCl to prep...
Embodiment 2
[0167] Embodiment Two papillomavirus (HPV) type 16 capsid protein gene L1-L2 combined expression and immune experiment in silkworm bioreactor
[0168] 1 Experimental method
[0169] 1.1. About the configuration of solution and medium
[0170] The routine reagent preparation of this embodiment is the same as embodiment one.
[0171] 1.2. Acquisition of the main capsid protein gene L2 and IRES gene of human papillomavirus (HPV) type 16.
[0172] 1.21 DNAiso (TaKaRa company) reagent method to extract genomic DNA
[0173] The preparation of human papillomavirus DNA in this example is the same as in Example 1.
[0174] 1.2.2 Design and synthesis of primers for the target gene
[0175] Primers were designed to amplify the human papillomavirus type 16 minor capsid protein L2 gene (SEQ ID NO: 3) by PCR. The amplification primers of the designed human papillomavirus type 16 minor capsid protein L2 gene are:
[0176] HPV16L2 upstream:
[0177] 5′-GG AGATCT TCAACATGCGACACAAACGTT...
Embodiment 3
[0322] Embodiment three human papillomavirus (HPV) type 18 main capsid protein gene L1 expression in the silkworm bioreactor and immune experiment
[0323] 1 Experimental method
[0324] 1.1. About the configuration of solution and medium
[0325] The routine reagent preparation of this embodiment is the same as embodiment one.
[0326] 1.2. Acquisition of the main capsid protein gene L1 of human papillomavirus (HPV) type 18.
[0327] 1.2.1 DNAiso (TaKaRa company) reagent method to extract genomic DNA
[0328] The preparation of human papillomavirus DNA in this example is the same as in Example 1.
[0329] 1.2.2 Design and synthesis of primers for the target gene
[0330] Primers were designed to amplify the human papillomavirus type 18 main capsid protein L1 gene (SEQ ID NO: 5) by PCR. The amplification primers of the designed human papillomavirus type 18 main capsid protein L1 gene are:
[0331] HPV18L1 upstream:
[0332] 5′-CGCC GGATCC AACATGTGCCTGTATACACGGGTCCTG-3...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com