Method for heterogenous expression of PEPR1 protein
A protein and exogenous technology, applied in the field of genetic engineering, can solve problems such as not being able to meet the needs of PEPR1 protein
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
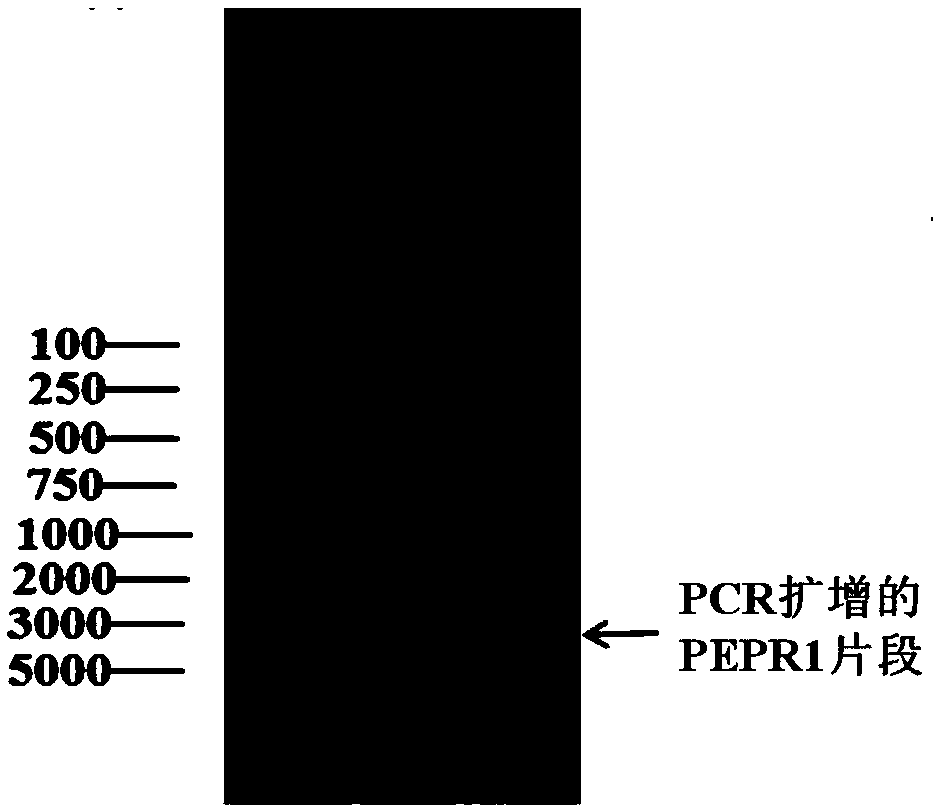
[0046] Example 1 Obtaining of PEPR1 gene exons
[0047] Considering that there is no intron region in the extracellular coding region of PEPR1, the present invention directly amplifies the extracellular domain of PEPR1 from the Arabidopsis genome. In the PCR amplification experiment, high-fidelity PCR enzymes were selected to prevent base mutations during the amplification process, and the primer sequences used were as follows.
[0048] PEPR1_29_BamHI_5: CGCGGATCCTTAAACTCAGATGGGCTAAC
[0049] (SEQ ID NO.4)
[0050] PEPR1_767_6His_SalI_3T: AGGAAGAGTGGCCTTAGCACCCATCATCATCATCATCATTAAGTCGACGTC (SEQ ID NO. 5)
[0051] PCR amplification reaction system: 5 μL 10×pfu buffer, 2.5 μL dNTP (2.5 mM), 0.5 μL each of 0.1 μM upstream and downstream primers, 1 μL pfu polymerase, 0.5 μL template, add double distilled water to 50 μL. The PCR amplification reaction conditions were 95°C, 5min; a total of 30 cycles: 94°C for 30s, 53°C for 30s, and 72°C for 600bp / min to calculate the required ex...
Embodiment 2
[0053] Example 2 Exogenous expression vector pFastBac TM Construction of Hem
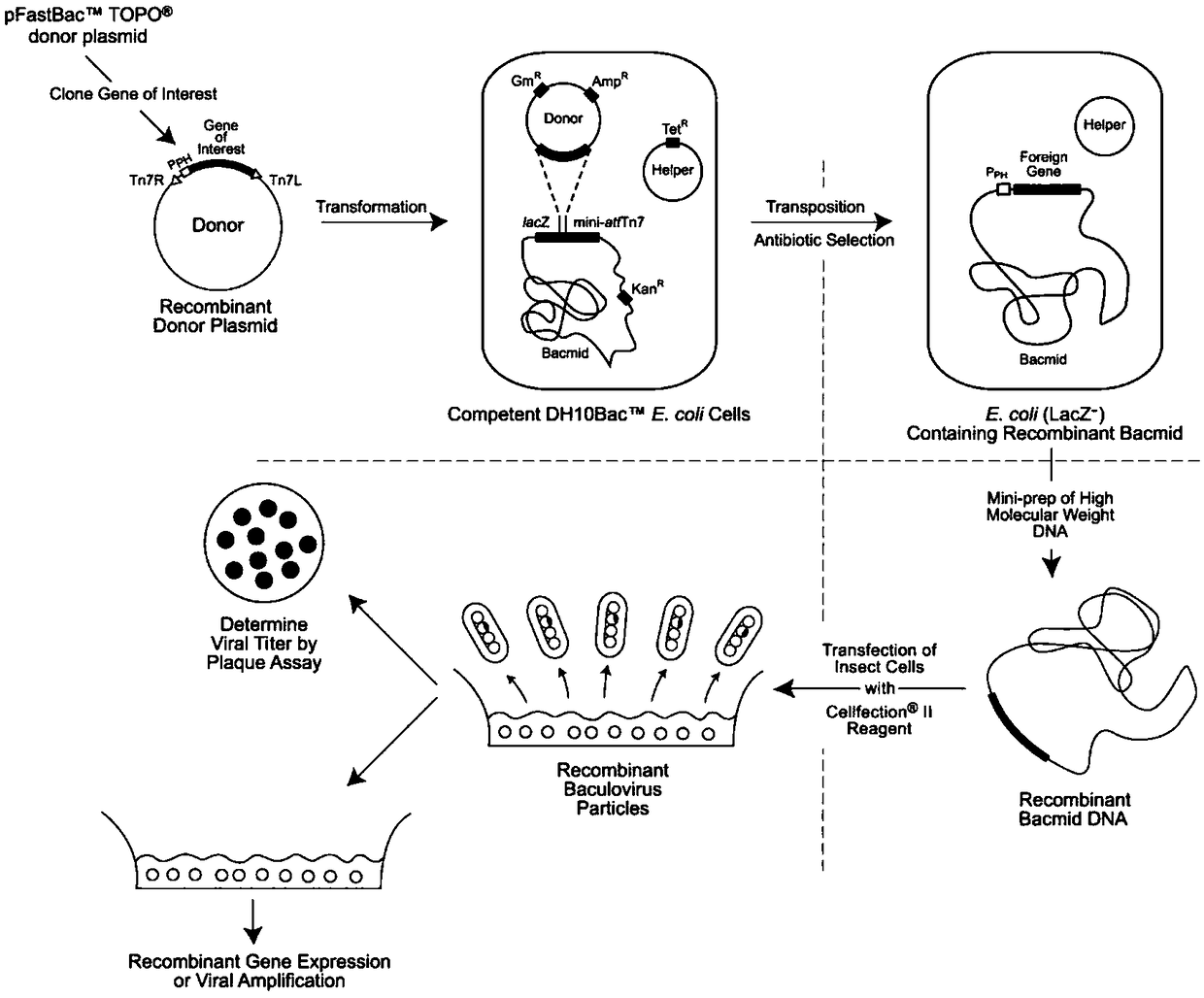
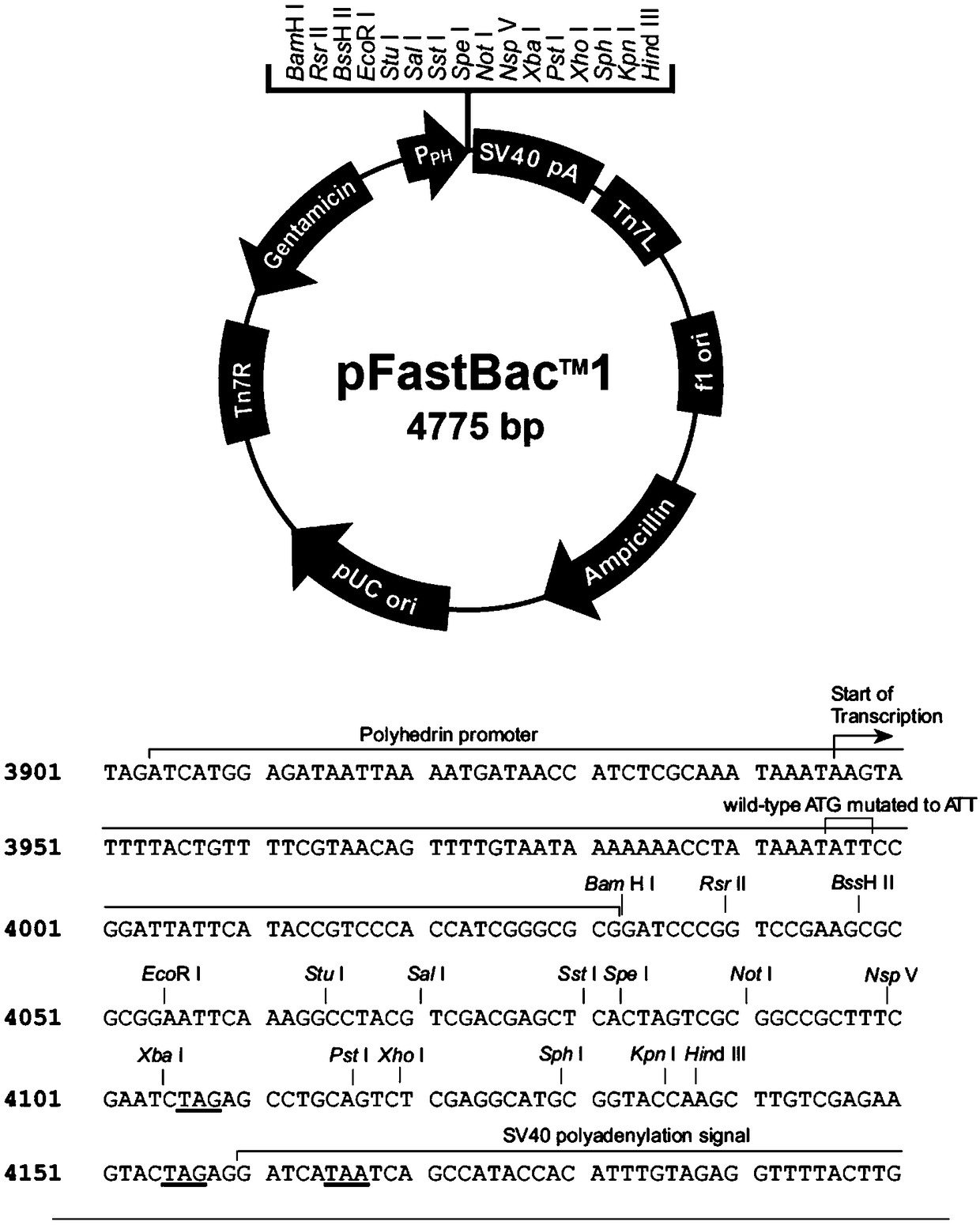
[0054] Signal peptides play an important role in the process of protein expression and processing. During exogenous expression of plant proteins such as PEPR1 in insect cells, it is unknown whether the signal peptides of plant proteins can still function normally when transferred to insect cells. own function, so this embodiment uses the baculovirus expression vector pFastBac in the insect cell expression system TM 1( image 3 ) was transformed, and the newly transformed carrier was pFastBac TM -Hem( Figure 4A and Figure 4B ). i.e. in pFastBac TM 1 A baculovirus-derived Hemolinpeptide signal peptide is added to the front end of the vector connected to the N-terminus of the target gene, the nucleotide sequence of which is shown in SEQ ID NO.3.
[0055] The present invention unexpectedly finds that the use of the Hem signal peptide is better than the use of the N-terminal signal peptide of PE...
Embodiment 3
[0057] Example 3 PEPR1 gene exons and exogenous expression vector pFastBac TM Hem ligation and preparation of recombinant bacmid
[0058] The choice of restriction endonucleases is based on the fact that the cut sequences of the two restriction endonucleases selected on both sides of the gene cannot appear in the target gene sequence, otherwise, in the later enzyme digestion experiment, the required The gene sequence connected into the vector will be cut by restriction endonucleases, so that a successful recombinant plasmid cannot be obtained. Based on the above principles, in this embodiment, two pairs of restriction endonucleases BamH1 and Xho1, Bgl2 and Sal1 were selected when screening enzyme cutting sites. BamH1 and Bgl2 are homologous to each other, and Xho1 and Sal1 are homologous to each other.
[0059] These two groups of homologous enzymes digest the target gene obtained in Example 1, and one of each group of homologous enzymes is selected, and used in pairs. Ther...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com