Levetiracetam tablet and preparation method thereof
A technology for tablets and colloidal silicon dioxide, which is applied in the directions of pill delivery, pharmaceutical formulations, and medical preparations with inactive ingredients, etc. The effect of saving process operation time, low production cost and simple process flow
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] A preparation method of levetiracetam tablet, described preparation method comprises the following steps:
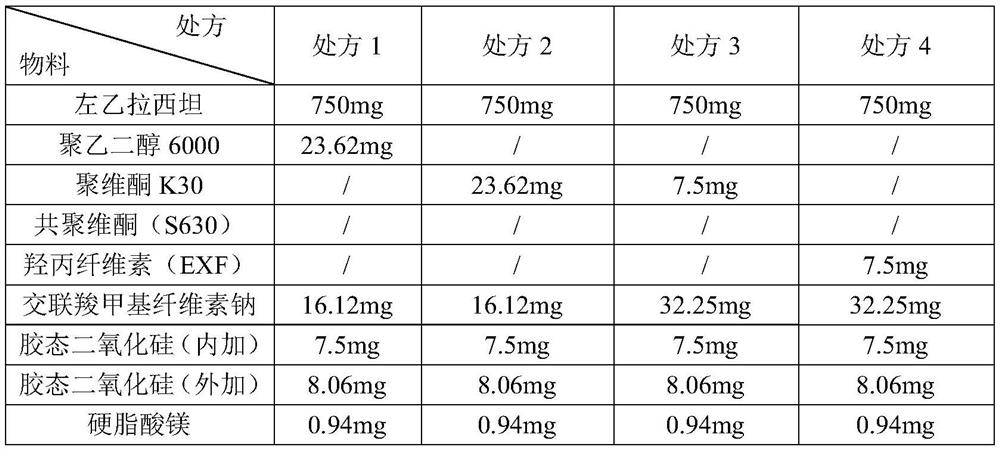
[0038] (1) Weighing and preparing materials: carry out weighing and preparing materials according to the prescription ratio in the table below; Table 1
[0039]
[0040] (2) Preparation method
[0041] Table 2
[0042]
[0043]
Embodiment 2
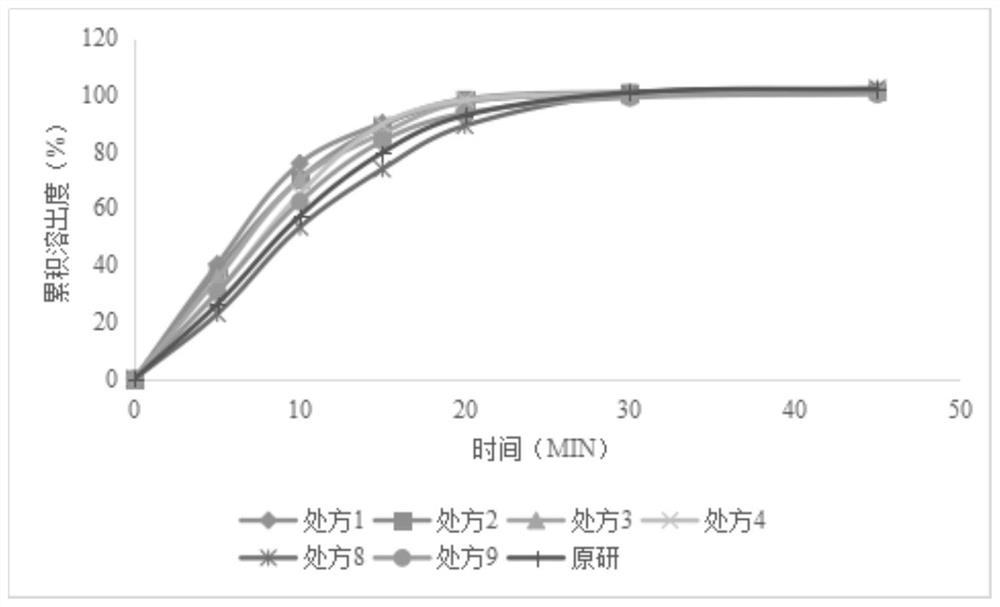
[0045] Get the levetiracetam tablet that different prescriptions prepare among the embodiment 1, measure friability, investigate its composition
[0046] Formability; The experimental results are as follows: According to the measurement results, it can be known that the tablet is prepared by hydroxypropyl cellulose whose model is EXF, the friability of the plain tablet is small, and the formability is better.
[0047] table 3
[0048] Prescription 1 Prescription 2 Prescription 3 Prescription 4 Friability (%) 0.50 (with missing corner) 0.52 (with missing corner) 0.45 (with slight chipping) 0.33 (no chipped corner) Hardness (kg) 12-14 12-15 14-17 14-17
[0049] Note: During the testing of hardness and friability in this example, 10 preparations of the same batch were taken for each test, and the hardness data in Table 3 is the hardness distribution range of the 10 preparations.
Embodiment 3
[0051] Table 4
[0052]
[0053] Preparation
[0054] table 5
[0055]
[0056]
[0057] It can be seen from the above examples that the addition method of colloidal silicon dioxide has a great influence on the forming of the tablet. When colloidal silicon dioxide is added in the form of internal addition and external addition at the same time, the compressibility of the material is significantly improved, and the plain The hardness of the tablet increases obviously, and the friability decreases obviously.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com