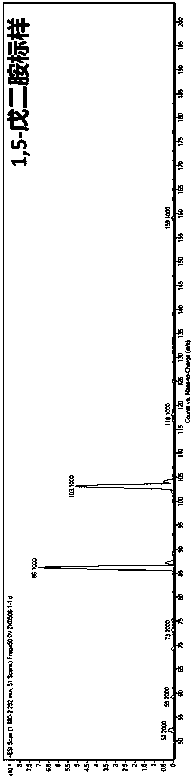
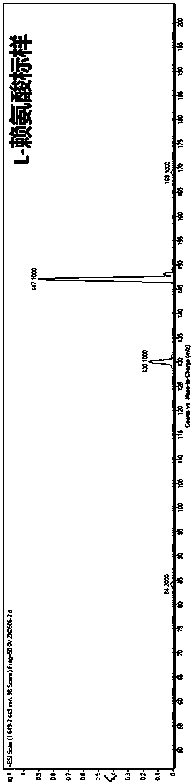
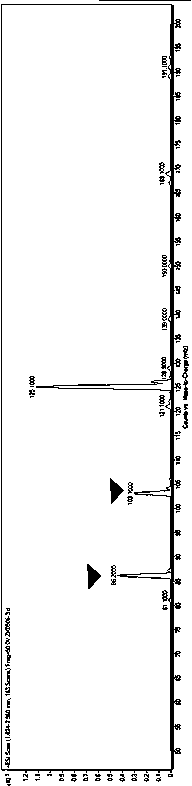
Method for production of 1,5-pentanediamine by chemical decarboxylation of L-lysine and separation and extraction method
A technology of amino acid chemistry and extraction method, applied in the field of chemical intermediate preparation, can solve problems such as few reports, and achieve the effects of low cost, mild reaction conditions and environmental friendliness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] A kind of L-lysine chemical decarboxylation produces the method for 1,5-pentanediamine, and its steps are:
[0041] 1) Dissolve 15.9mmoL of L-lysine dihydrochloride (L-Lysine dihydrochloride) in 90mL of citric acid-disodium hydrogen phosphate buffer solution (pH 5);
[0042] 2) Add 20mL of N-bromosuccinimide (NBS) in dimethylformamide (DMF) solution dropwise to the solution in step 1) at 25°C, and react for 30 minutes;
[0043]3) Add 96.5moL of nickel chloride hexahydrate to the reaction solution in step 2), and gradually add 158mmoL of sodium borohydride powder in a small amount, and react for 1h in an ice-water bath.
[0044] Above-mentioned a kind of L-lysine chemical decarboxylation produces 1, the separation and extraction method of 5-pentanediamine, and its steps are:
[0045] a) Filter the obtained solution containing 1,5-pentanediamine with diatomaceous earth as a filter aid, and dilute the filtrate to 500 mL;
[0046] b) Add the diluted filtrate to a column o...
Embodiment 2
[0053] A kind of L-lysine chemical decarboxylation produces the method for 1,5-pentanediamine, and its steps are:
[0054] 1) Dissolve 15.9mmoL of L-lysine dihydrochloride (L-Lysine dihydrochloride) in 90mL of citric acid-disodium hydrogen phosphate buffer solution (pH 5);
[0055] 2) Add 20mL of N-bromosuccinimide (NBS) in dimethylformamide (DMF) solution dropwise to the solution in step 1) at 25°C, and react for 30 minutes;
[0056] 3) Add 96.5moL of cobalt chloride hexahydrate to the reaction solution in step 2), and gradually add 158mmoL of sodium borohydride powder in small amounts, and react for 1h in an ice-water bath.
[0057] Above-mentioned a kind of L-lysine chemical decarboxylation produces 1, the separation and extraction method of 5-pentanediamine, and its steps are:
[0058] a) Filter the obtained solution containing 1,5-pentanediamine using diatomaceous earth as a filter aid, and dilute the filtrate to 500 mL;
[0059] b) Add the diluted filtrate to a column ...
Embodiment 3
[0066] A kind of L-lysine chemical decarboxylation produces the method for 1,5-pentanediamine, and its steps are:
[0067] 1) Dissolve 15.9mmoL of L-lysine dihydrochloride (L-Lysine dihydrochloride) in 90mL of citric acid-disodium hydrogen phosphate buffer solution (pH 5);
[0068] 2) Add 20mL of N-bromosuccinimide (NBS) in dimethylformamide (DMF) solution dropwise to the solution in step 1) at 25°C, and react for 30 minutes;
[0069] 3) Add 96.5moL of ferric chloride to the reaction solution in step 2), and gradually add 158mmoL of sodium borohydride powder in small amounts, and react for 1h in an ice-water bath.
[0070] Above-mentioned a kind of L-lysine chemical decarboxylation produces 1, the separation and extraction method of 5-pentanediamine, and its steps are:
[0071] a) Filter the obtained solution containing 1,5-pentanediamine using diatomaceous earth as a filter aid, and dilute the filtrate to 500 mL;
[0072] b) Add the diluted filtrate to a column of strongly ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com