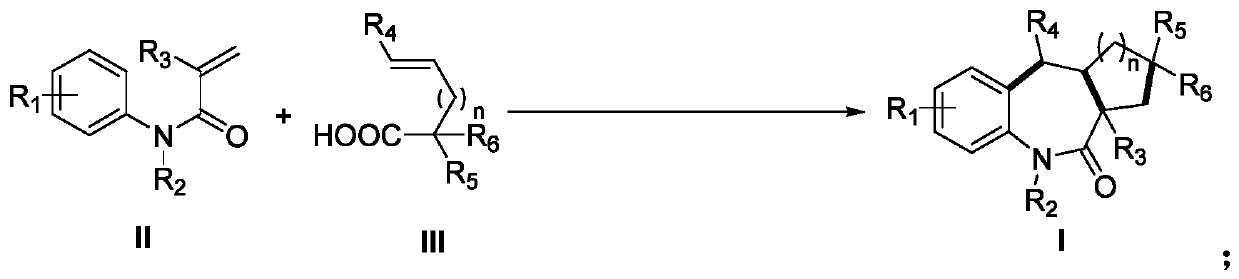
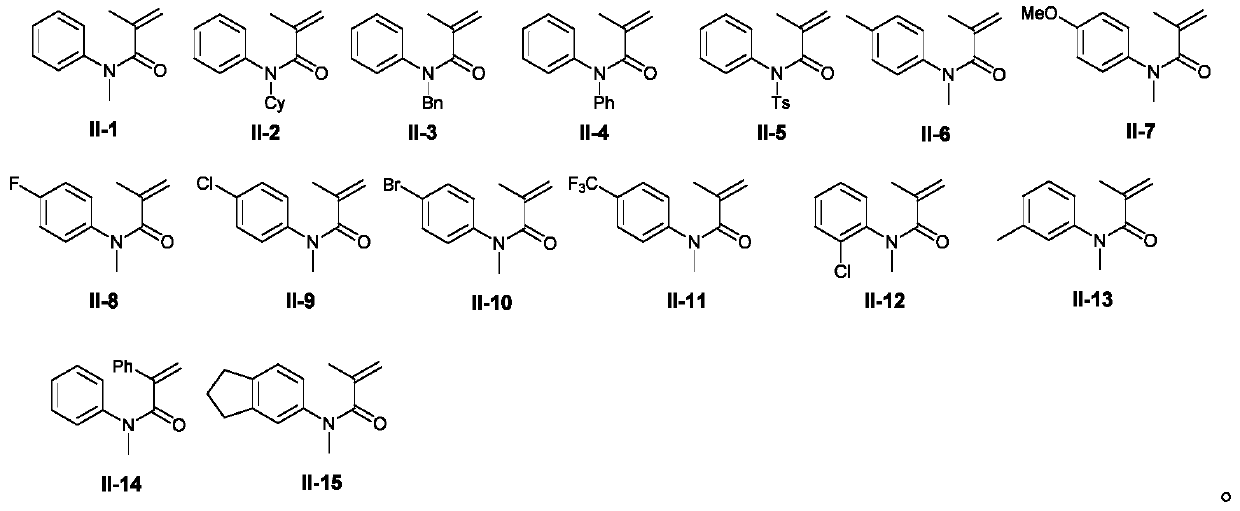
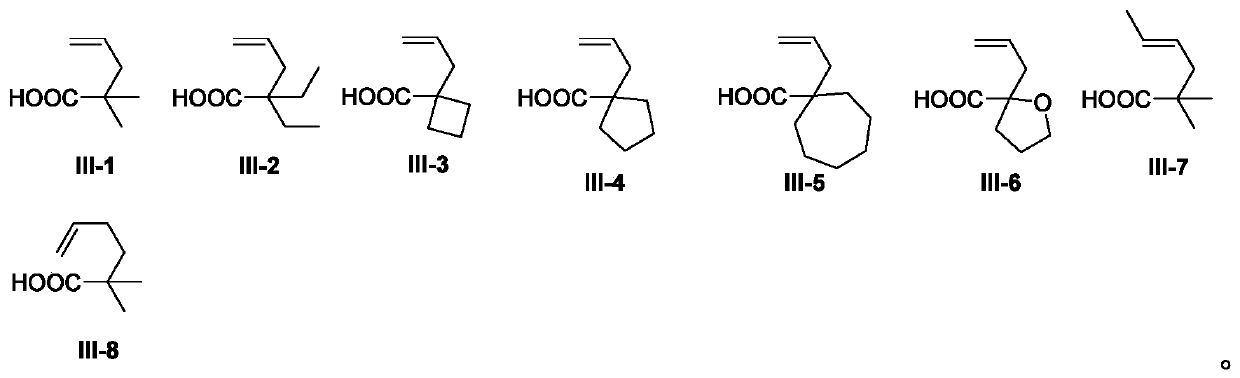
Preparation method of benzoazepine compounds
A technology for benzazepine compounds, which is applied in the field of preparation of benzazepine compounds, can solve problems such as complex starting materials, and achieves the effects of economical greenness, mild reaction conditions and convenient synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-15
[0033] Example 1-15 Reaction condition optimization typical test
[0034]Using N-methyl-N-phenylmethacrylamide II-1 and 2,2-dimethylpent-4-enoic acid III-1 as templates, the effect of different preparation conditions on the yield of target product I-1 was discussed Influence, selected representative embodiment 1-15 wherein, the result is shown in table 1.
[0035]
[0036] Wherein the typical test operation of embodiment 1 is as follows:
[0037] In the Schlenk lock reactor, add N-methyl-N-phenylmethacrylamide II-1 (0.2mmol), 2,2-dimethylpent-4-enoic acid III-1 (2equiv) in sequence , (NH 4 ) 2 S 2 o 8 (2 equiv), and DMSO (2.0 mL). Then the reaction mixture was placed in a 50°C oil bath under an argon atmosphere and stirred for 12 hours. After the reaction was monitored by TLC and / or GC-MS analysis, the reaction solution was extracted with ethyl acetate, and the organic phase was washed with table salt. After washing with water, the organic phases were combined and dr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com