Preparation method of dicyanoethyl tertiary amine
A technology of dicyanoethyl tertiary amine mother and dicyanoethyl, applied in the field of preparation of dicyanoethyl tertiary amine, can solve the problems of large steric hindrance effect, low yield, heat release, etc., and achieve fast reaction rate , the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
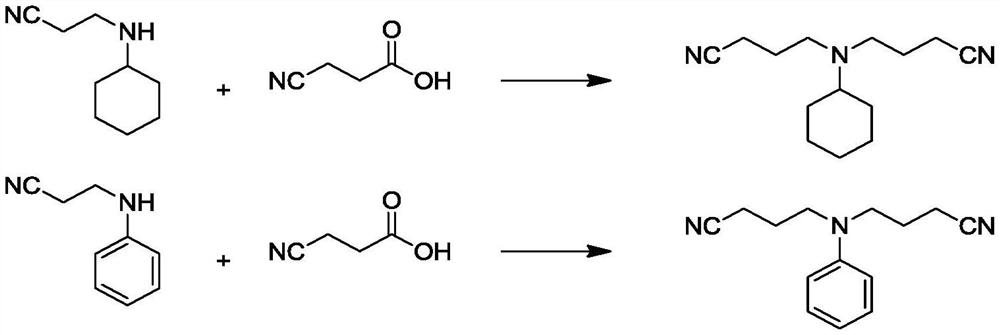
[0050] Preparation catalyst: 24.2 g Fe (NO 3 ) 3 and 2.42 g Cu(NO 3 ) 2Together dissolved in PW water to prepare 161.33g of metal salt solution, and then 242g H-ZSM-5 molecular sieve was added to the three-neck flask containing the metal salt solution, stirred at 100 °C heated reflux state for 3h, through vacuum filtration, and then washed 3 times with ethanol and water, 10h drying at 100 °C, and then roasted at 500 °C for 3h to obtain Catalyst A.
[0051] Addition process: using semi-intermittent process, with 15.2g monocyanatoethyl cyclohexylamine and 3g methanol compound solution and 0.15g homemade catalyst A for laying, with nitrogen purged 3 times after heating to the reaction temperature of 100 ° C, and then 407.69g concentration of 5wt% of the aqueous solution of 3-cyanopropionic acid at a rate of 2g / min gradually added into the monocyanatoethyl cyclohexylamine alcohol solution stirred, after all the feeding is over, continue to react for 2h, cooling, Cryogenic collection...
Embodiment 2
[0053] Preparation catalyst: 24.2 g Fe (NO 3 ) 3 and 4.48g Bi(NO 3 ) 3 Together dissolved in PW water to prepare 69.14g of metal salt solution, and then 363g H-β molecular sieve was added to the three-neck flask containing metal salt solution, stirred at 150 °C heated reflux state for 5h, filtered by vacuum, and then washed 3 times with ethanol and water, dried at 120 °C for 10h, and then roasted at 600 °C for 5h to obtain catalyst B.
[0054]Addition process: using semi-intermittent process, 15.2g monocyanatoethyl cyclohexylamine and 5g ethanol compound solution and 0.76g homemade catalyst B for laying the bottom, with nitrogen purged 3 times after heating to the reaction temperature of 120 ° C, and then the 161.78g concentration of 15wt% of the aqueous solution of 3-cyanopropionic acid at a rate of 3g / min gradually added dropwise into the alcohol solution of monocyanatoethyl cyclohexylamine for stirring, after all the feeding is completed, continue to react for 3h, cooling, Cr...
Embodiment 3
[0056] Preparation catalyst: 16.2 g FeCl 3 and 1.62g CuCl 2 Dissolved in PW water to prepare 64.8g of metal salt solution, and then 194.4g H-ZSM-5 molecular sieve was added to the three-neck flask containing metal salt solution, stirred at 120 °C heated reflux state for 4h, filtered by vacuum, and then washed 3 times with ethanol and water, dried at 110 °C for 10h, and then roasted at 550 °C for 4h to obtain catalyst C.
[0057] Addition process: using a semi-batch process, with 14.6g monocyanatoaniline and 3.65g of methanol to form a solution and 0.58g of homemade catalyst C for laying the bottom, with nitrogen purged 3 times after heating to the reaction temperature of 110 ° C, and then the 266.94g concentration of 8wt% of the aqueous solution of 3-cyanopropionic acid at a rate of 2.5g / min gradually added into the alcohol solution of monocyanatoethylaniline for stirring, after all the feeding is completed, continue to react for 4h, cooling, Cryogenic collection of dicyanoethyl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com