A high-efficiency δ 9,11 -The preparation method of canrenone
A canrenone and high-efficiency technology, applied in the field of high-efficiency Δ9,11-canrenone preparation, can solve the problems of increased impurity content, long process route, cumbersome process, etc., and achieves low production cost, high commercial competitiveness, and availability. Actionable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
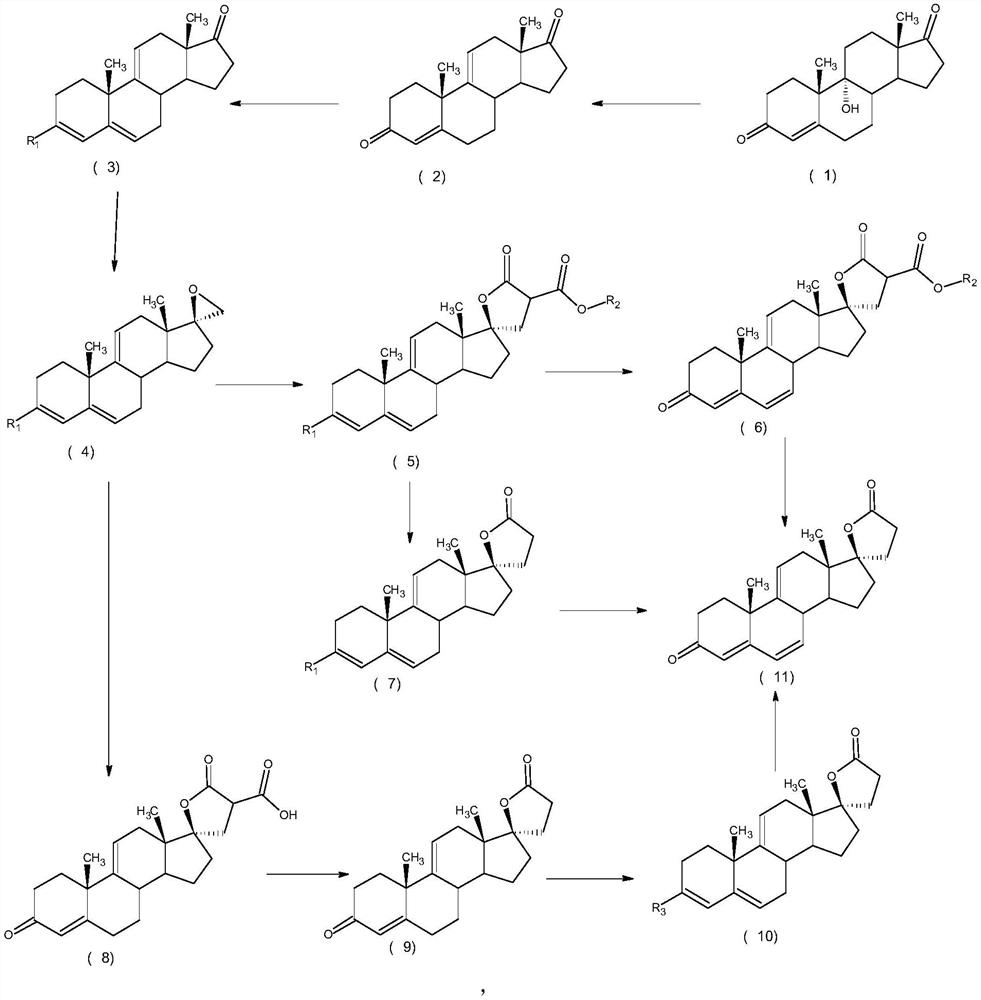
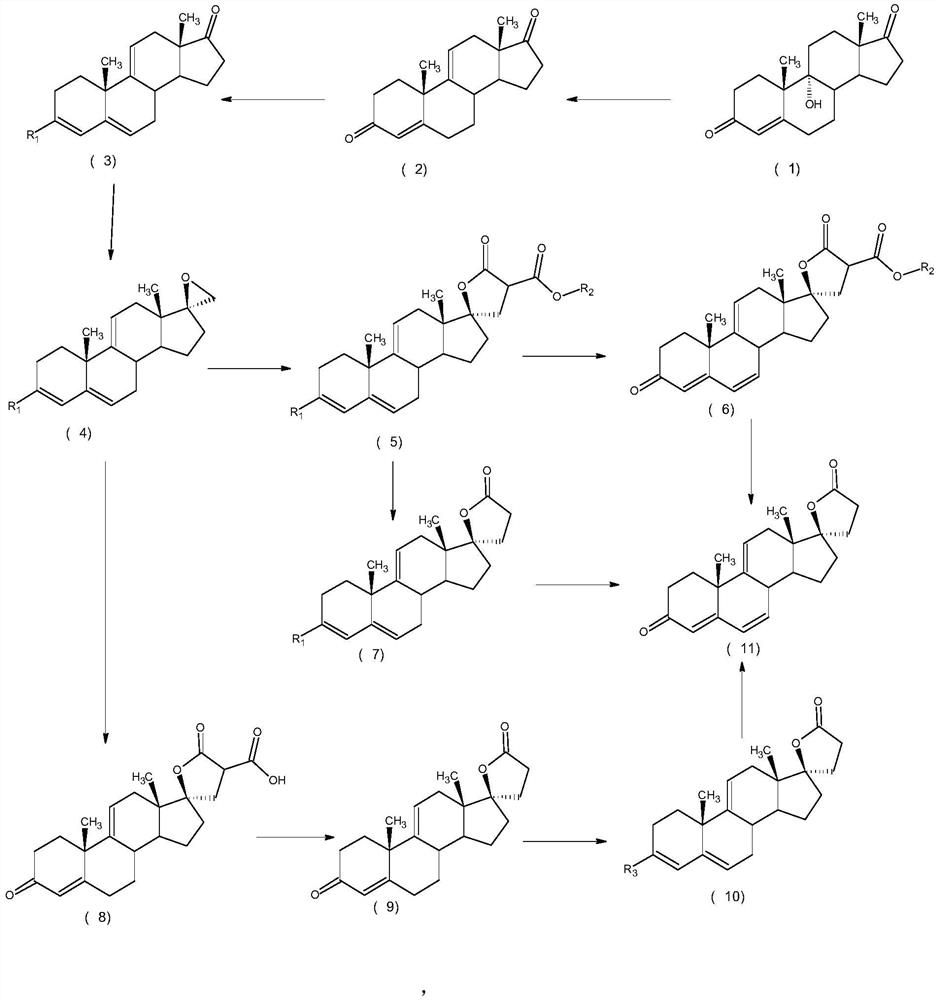
[0034] Example 1Δ 9,11 -The preparation method of canrenone, concrete steps are as follows:
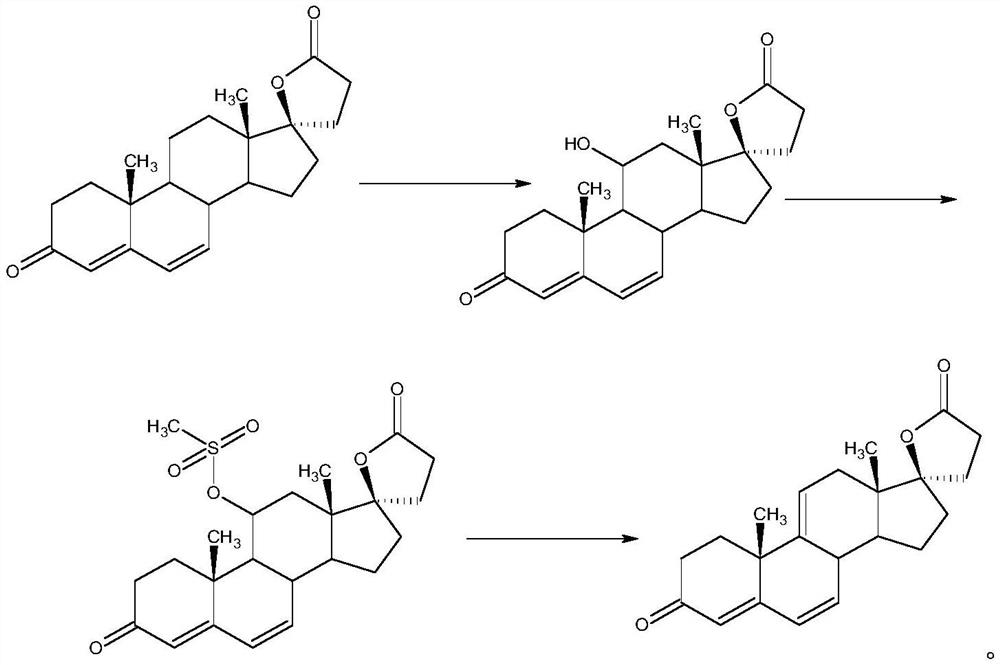
[0035] 1) Put 100ml of sulfuric acid solution with a mass concentration of 20% into a three-necked bottle, put in 10g of 9α-hydroxy-4-androstene-3,17dione, and keep warm at 60°C for reaction. Methane extraction, washing with water, concentration, water analysis, filtration, and drying to obtain Δ 9,11 -4-androstene-3,17dione 9.1 g.
[0036] 2) Put Δ 9,11 -4-Androstene-3,17dione was put into 4.5ml of methanol, 3.5ml of trimethyl orthoformate and 0.09g of pyridine hydrochloride were added, and the temperature was kept at 20°C for reaction. After the reaction was completed, it was filtered and dried to obtain 3-methanol Oxy-androst-3,5,9(11)-trien-17-one 9.2 g.
[0037] 3) Put 1.2g sodium methoxide and 12.9g trimethylsulfonium iodide into the reaction bottle, 9.2g 3-methoxy-androst-3,5,9(11)-triene-17-one, put 92ml Dimethyl sulfoxide, keep warm at 0°C for reaction, after the reactio...
Embodiment 2
[0041] Example 2Δ 9,11 -The preparation method of canrenone, concrete steps are as follows:
[0042] 1) Put 20ml of sulfuric acid solution with a mass concentration of 80% into the three-necked bottle, cool down to 10°C, put in 10g of 9α-hydroxy-4-androstene-3,17dione, keep warm at 10°C for reaction, after the reaction is completed, water analysis , extracted with 200ml dichloromethane, washed with water, concentrated, water analyzed, filtered and dried to obtain Δ 9,11 -4-androstene-3,17-dione 9.2 g.
[0043] 2) The 9.2gΔ 9,11 -4-androstene-3,17dione was put into 92ml tetrahydrofuran, added 16.2ml pyrrolidine, 0.46g pyridinium hydrobromide, and kept at 50°C for reaction. After the reaction was completed, it was filtered and dried to obtain 3-pyrrolyl-androstene Ste-3,5,9(11)-trien-17-one 9.48 g.
[0044] 3) Put 5.36g of potassium ethylate and 10.05g of trimethylsulfonium bromide into the reaction bottle, 9.48g of 3-pyrrolyl-androst-3,5,9(11)-trien-17-one, and put into 19m...
Embodiment 3
[0048] Example 3Δ 9,11 -The preparation method of canrenone, concrete steps are as follows:
[0049] 1) Put 50ml of sulfuric acid solution with a mass concentration of 50% into a three-neck flask, put in 10g of 9α-hydroxy-4-androstene-3,17dione, and keep warm at 40°C for reaction. After the reaction is completed, analyze with water and extract with 100ml of toluene , washed with water, concentrated, hydrolyzed, filtered, and dried to obtain Δ 9,11 -4-androstene-3,17dione 9.0 g.
[0050] 2) Add 9.0gΔ 9,11 -4-Androstene-3,17-dione was put into 27ml of tetrahydrofuran, 26.4ml of triethyl orthoformate and 0.9g of p-toluenesulfonic acid were added, and the temperature was kept at 40°C for reaction. After the reaction was completed, it was filtered and dried to obtain 3-ethyl Oxy-androst-3,5,9(11)-trien-17-one 9.0 g.
[0051] 3) Put 10.2g potassium tert-butoxide and 15.3g trimethylsulfonium chloride into the reaction flask, 9g 3-ethoxy-androst-3,5,9(11)-triene-17-one, put 27ml ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com