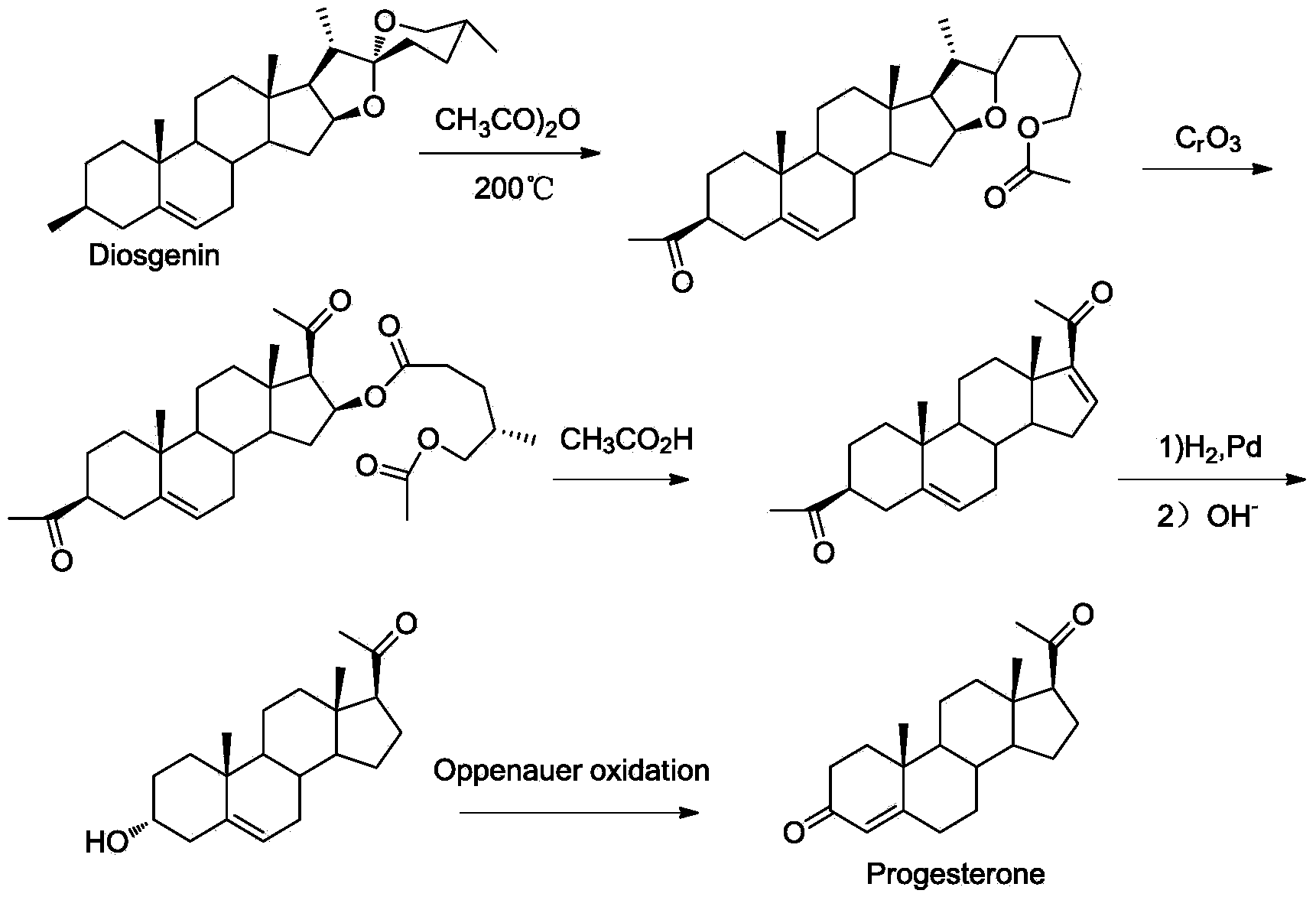
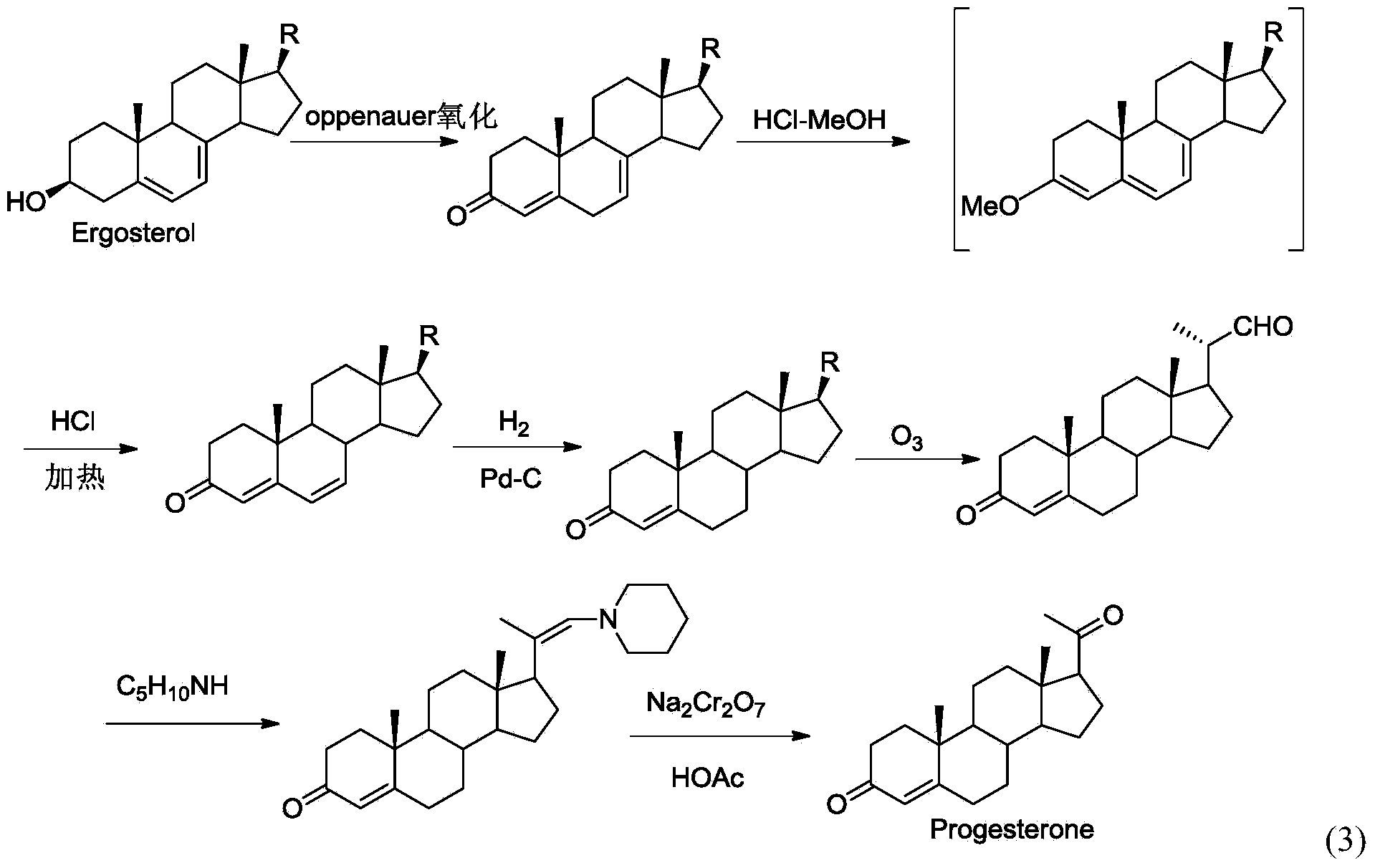
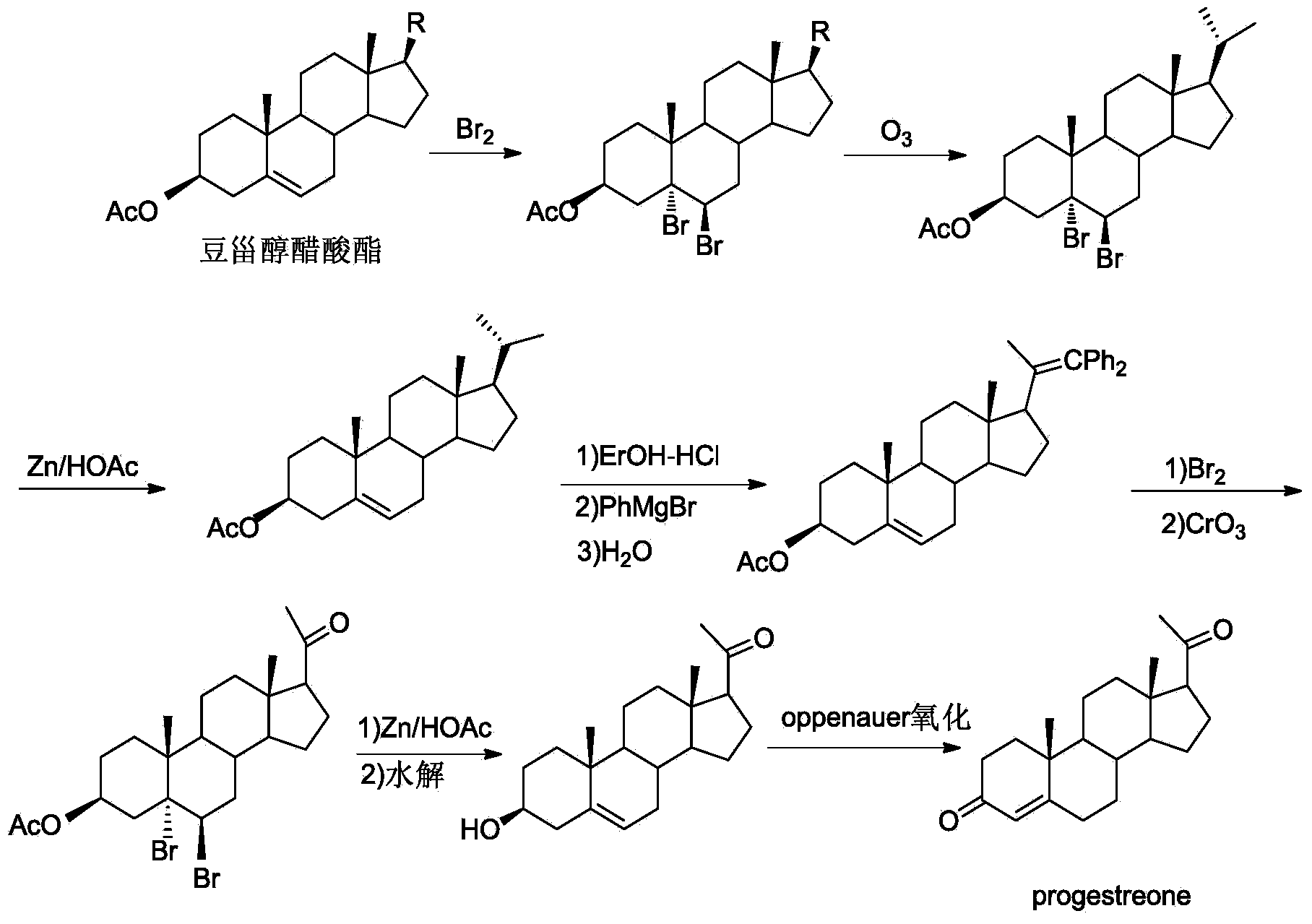
New technique for synthesizing progesterone
A new process, progesterone technology, applied in the direction of steroids, organic chemistry, etc., can solve the problems of low synthesis efficiency and increased raw material cost, and achieve the effects of good product quality, low cost and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Step 1: Take 10.0g of N-chlorosuccinimide and add it to 150ml of toluene solution, stir to dissolve, then take 5.0ml of dimethyl ether sulfur and add it to 50ml of toluene. , added to the toluene solution of the above N-chlorosuccinimide to obtain a mixed solution a, then the mixed solution was cooled to -25 ° C, 8.0 g of the starting material was dissolved in 100 ml of dichloromethane solution, and then within 45min Add dropwise to mixed solution a, keep the temperature of the system at -25 °C, and continue stirring for 2 hours to obtain mixed solution b, then take 3.0 g of triethylamine and dissolve it in 10.0 ml of dichloromethane, and add mixed solution b dropwise within 20 min. , after stirring for 5 min, the cooling bath was removed, 150 ml of ether was added, 1% HCl was added to neutralize excess triethylamine, the organic layer was extracted with water, the organic layer was dried with anhydrous sodium sulfate, concentrated, and then mixed with ethyl acetate-n B...
Embodiment 2
[0042] Step 1: Add 25.0g of N-chlorosuccinimide to 400ml of ethylene glycol dimethyl ether solution, stir to dissolve, then take 15.0ml of dimethyl ether sulfur and add it to 100ml of ethylene glycol dimethyl ether solution, mix After homogeneous, add the above N-chlorosuccinimide in the ethylene glycol dimethyl ether solution under the condition of nitrogen flow at -5°C to obtain mixed solution a, and then cool the mixed solution to -30°C, 20.0 g of the starting material was dissolved in 200 ml of chloroform solution, then added dropwise to mixed solution a within 1 h, and stirred continuously for 4 h at -30 °C to obtain mixed solution b, and then 15.0 g of triethylamine was dissolved in 80 ml of Trichloromethane was added dropwise to mixed solution b within 45min, the cooling bath was removed after stirring for 5min, 300ml of ether was added, excess triethylamine was neutralized with 1% HCL, the organic layer was extracted with water, and the organic layer was extracted with ...
Embodiment 3
[0045] Step 1: Take 65.0g of N-chlorosuccinimide and add it to 500ml of ethyl acetate solution, stir to dissolve, then take 40.0ml of dimethyl ether sulfur and add it to 250ml of ethyl acetate. Under the condition of nitrogen, add the ethyl acetate solution of the above N-chlorosuccinimide to obtain the mixed solution a, then cool the mixed solution to -25 ℃, dissolve 50.0 g of the starting material in 500 ml of 1,2- The dichloroethane solution was added dropwise to the mixed solution a within 1.5 h, and the mixture was stirred continuously for 2.5 h at -25 °C to obtain the mixed solution b. Then 30.0 g of triethylamine was dissolved in 150 ml of 1,2-dichloromethane. Ethane was added dropwise to mixed solution b within 1 h, the cooling bath was removed after stirring for 5 min, 500 ml of ethyl acetate was added, excess triethylamine was neutralized with 1% HCl, the organic layer was extracted with water, and the organic layer was extracted with anhydrous sulfuric acid It was d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com