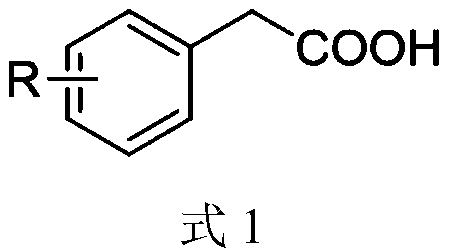
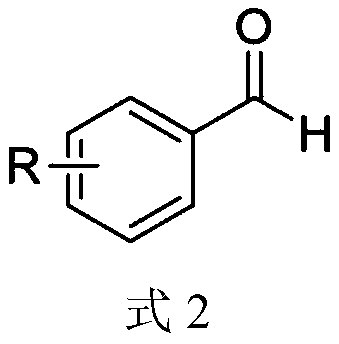
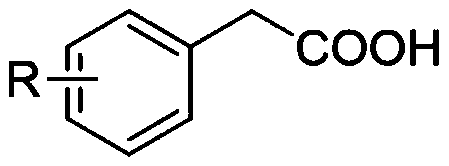
Synthesis method of aryl aldehyde compound
A synthesis method and compound technology, which is applied in the field of synthesis of aryl aldehyde compounds, can solve the problems of waste treatment, inconvenient production and application, etc., and achieve the effects of low cost, mild reaction conditions and high reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] The preparation method of aryl aldehyde compounds, the steps are as follows:
[0047] Add photocatalyst 4CzIPN (0.1mol%), DBU (10mol%), solvent acetonitrile 1.5mL, 4-methoxyphenylacetic acid (0.2mmol) into the 25mL reaction tube, stir in the air at room temperature 25℃, under blue light irradiation After 4 hours of reaction, the final product was separated by silica gel column chromatography, and the yield of the final product was 90% based on the molar amount of aryl acetic acid as 100%.
[0048] The specific results are as follows:
[0049]
[0050] 1 H NMR (400MHz, Chloroform-d) δ 9.87 (s, 1H), 7.83 (d, J = 8.8 Hz, 2H), 6.99 (d, J = 8.8 Hz, 2H), 3.87 (s, 3H). 13 C NMR (101 MHz, Chloroform-d) δ 190.80, 164.60, 131.96, 129.93, 114.31, 55.56.
Embodiment 2
[0052] The preparation method of aryl aldehyde compounds, the steps are as follows:
[0053] Add photocatalyst 4CzIPN (0.1mol%), DBU (10mol%), solvent acetonitrile 1.5mL, 3-methoxyphenylacetic acid (0.2mmol) into the 25mL reaction tube, stir in the air at room temperature 25℃, under blue light irradiation After 8 hours of reaction, the final product was separated by silica gel column chromatography. The yield of the final product was 85% based on the molar amount of aryl acetic acid as 100%.
[0054] The specific results are as follows:
[0055]
[0056] 1 H NMR (400MHz, Chloroform-d) δ9.98 (s, 1H), 7.51 - 7.42 (m, 2H), 7.43 - 7.37 (m, 1H), 7.18 (m, 1H), 3.86 (s, 3H). 13 C NMR (101 MHz, Chloroform-d) δ 192.15, 160.16, 137.82, 130.04, 123.55, 121.53, 112.06, 55.48.
Embodiment 3
[0058] The preparation method of aryl aldehyde compounds, the steps are as follows:
[0059] Add photocatalyst 4CzIPN (0.1mol%), DBU (10mol%), solvent acetonitrile 1.5mL, 2-methoxyphenylacetic acid (0.2mmol) into the 25mL reaction tube, stir in the air at room temperature 25℃, under blue light irradiation After 8 hours of reaction, the final product was separated by silica gel column chromatography, and the yield of the final product was 86% based on the molar amount of aryl acetic acid as 100%.
[0060] The specific results are as follows:
[0061]
[0062] 1 H NMR (400MHz, Chloroform-d) δ 10.46 (s, 1H), 7.81 (dd, J = 7.9, 1.8 Hz, 1H), 7.54 (m, 1H), 7.08–6.95 (m, 2H), 3.91 ( s, 3H). 13 C NMR (101MHz, Chloroform-d) δ189.78, 161.83, 135.98, 128.44, 124.79, 120.62, 111.66, 55.61.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com