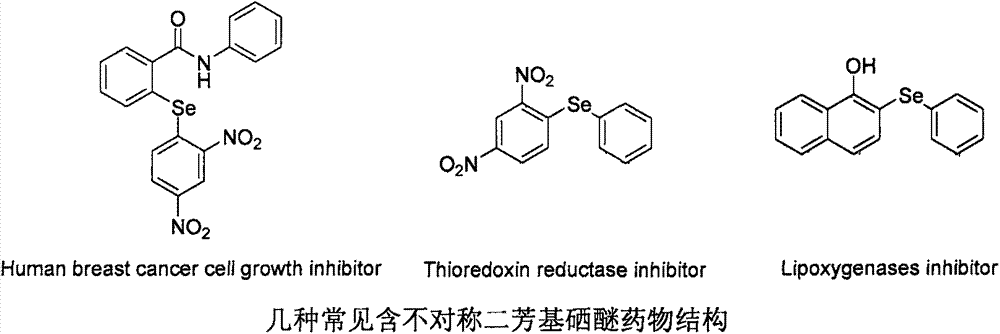
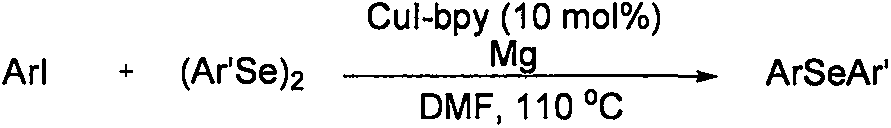Method for synthesizing asymmetric diaryl selenide compound
A technology of diaryl selenide and diaryl diselenide, which is applied in the field of organic compound synthesis, can solve the problems of poor functional group tolerance, complicated operation of asymmetric diaryl selenide compounds and the like, and achieves simple operation and simple post-processing. , the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080] Synthesis of (4-methoxy-2-nitrophenyl)(phenyl)selenoethers
[0081]
[0082] At room temperature, 2-nitro-4-methoxybenzoic acid (0.4mmol, 1equiv), diphenyldiselenide (0.8mmol, 2equiv), Cu(OAc) 2 (0.06mmol), 1,10-phenanthroline (0.06mmol), potassium carbonate (1.2mmol, 3equiv) and 2mL toluene join in the reaction tube, fill with oxygen then, and replace three times, under oxygen reaction environment, Stir at 150°C reaction temperature for 24h. After the end of the reaction was monitored by thin-layer chromatography, the reaction mixture was cooled, then ethyl acetate was added to filter, then the solvent was spun off, and the product was separated by column chromatography (eluent: petroleum ether: ether=98: 2), and the product was Yellow liquid, yield 95%, product weight 117mg.
[0083] The data of the proton nuclear magnetic resonance spectrum of gained product are as follows:
[0084] 1 H NMR (500MHz, CDCl 3 ): δ7.80(d, J=2.8Hz, 1H), 7.69-7.67(m, 2H), 7.50-7.47...
Embodiment 2
[0091] Synthesis of (5-methyl-2-nitrophenyl)phenyl selenide
[0092]
[0093] At room temperature, 5-methyl 2-nitrobenzoic acid (0.4mmol, 1equiv), diphenyldiselenide (0.8mmol, 2equiv), Cu(OAc) 2 (0.06mmol), 1,10-phenanthroline (0.06mmol), potassium carbonate (1.2mmol, 3equiv) and 2mL toluene join in the reaction tube, fill with oxygen then, and replace three times, under oxygen reaction environment, Stir at 150°C reaction temperature for 24h. After the end of the reaction was monitored by thin-layer chromatography, the reaction mixture was cooled, then ethyl acetate was added to filter, then the solvent was spun off, and the product was separated by column chromatography (eluent: petroleum ether: ether=98: 2), and the product was Yellow liquid, yield 90%, product weight 105mg.
[0094] The data of the proton nuclear magnetic resonance spectrum of gained product are as follows:
[0095] 1 H NMR (500MHz, CDCl 3 ): δ8.20(d, J=8.4Hz, 1H), 7.71-7.69(m, 2H), 7.51(t, J=7.2Hz,...
Embodiment 3
[0102] Synthesis of (2-nitro-4-trifluoromethylphenyl)phenylselenide
[0103]
[0104] At room temperature, 2-nitro-4-trifluoromethylbenzoic acid (0.4mmol, 1equiv), diphenyldiselenide (0.8mmol, 2equiv), Cu(OAc) 2 (0.06mmol), 1,10-phenanthroline (0.06mmol), potassium carbonate (1.2mmol, 3equiv) and 2mL toluene join in the reaction tube, fill with oxygen then, and replace three times, under oxygen reaction environment, Stir at 150°C reaction temperature for 24h. After the end of the reaction was monitored by thin-layer chromatography, the reaction mixture was cooled, then ethyl acetate was added to filter, then the solvent was spun off, and the product was separated by column chromatography (eluent: petroleum ether: ether=98: 2), and the product was Yellow liquid, yield 85%, product weight 118mg.
[0105] The data of the proton nuclear magnetic resonance spectrum of gained product are as follows:
[0106] 1 H NMR (500MHz, CDCl 3 ): δ8.56(s, 1H), 7.71-7.69(m, 2H), 7.57-7.5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com