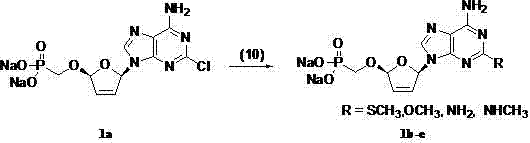
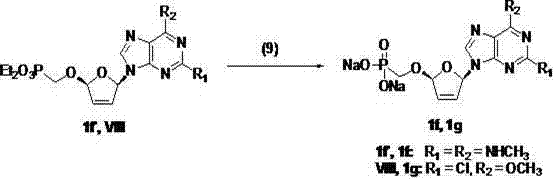
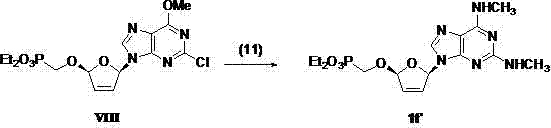(2R,5R)-5-phosphoryl methoxy-2-(2-substituted adenine-9-yl)-2,5-dihydrofuran nucleoside analog as well as preparation method and application thereof
A dihydrofuran nucleoside and phosphoryl methoxy technology, applied in the field of nucleotide chemistry and medicinal chemistry, can solve the problems of complex nucleoside synthesis process and no reports
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Example 1: Preparation of compound II
[0062] Dissolve 3,5-di-O-p-methylbenzoyl-2-deoxy-D-riboside (I, 7.7 g, 20 mmol) in 15 ml of glacial acetic acid, and pass through dry HCl gas at 0°C , after the solid was no longer precipitated, it was suction filtered, and the filter cake was washed with acetone to obtain 7.0 g (90%) of compound II as a white solid.
[0063] Experimental data:
[0064] white solid, 1 H NMR (400 MHz, CDCl 3 ) δ 8.02 (d, J = 8.2 Hz, 1H, Ar-H), 7.92 (d, J = 8.2 Hz, 1H, Ar-H), 7.28 (dd, J = 11.7, 7.0 Hz, 4H, Ar-H), 6.50 (d, J = 5.0 Hz, 1H, H-1'), 5.59 (dd, J = 7.0, 2.6 Hz, 1H, H-3'), 4.88 (dd, J = 6.8, 3.2 Hz, 1H, H-4'), 4.71 (dd, J = 12.1, 3.1 Hz, 1H, H-5'a), 4.62 (dd, J = 12.1, 4.2 Hz, 1H, H-5'b), 2.89 (ddd, J = 15.1, 7.4, 5.2 Hz, 1H, H-2'a), 2.77 (d, J = 15.1 Hz, 1H, H-2'b), 2.45 (s, 1H, -CH 3 ), 2.44 (s, 1H, -CH 3 ). 13 C NMR (101 MHz, CDCl 3 ) δ 166.4 (C=O), 166.1 (C=O), 144.3, 144.1, 129.9, 129.7, 129.3, 129.2, 126.8...
Embodiment 2
[0065] Example 2 : Preparation of Compound III
[0066] Dissolve 1.15 g (6 mmol) of 2,6-dichloropurine in 12 ml of anhydrous tetrahydrofuran. After it is completely dissolved, add it to 10 ml of acetonitrile solution containing 240 mg of sodium hydride under nitrogen protection. After no more gas was generated, 1.95 g (5 mmol) of compound II was added in portions at 25°C. The reaction progress was monitored by thin-layer chromatography. After the reaction was completed, it was filtered with suction, the filter cake was washed with ethyl acetate, the filtrate was concentrated under reduced pressure, and separated by silica gel column chromatography to obtain 2.1 g of compound III (yield 78%).
[0067] Experimental data:
[0068] White solid, 1 H NMR (400 MHz, CDCl 3 ): δ 8.32 (s, 1H, H-8), 7.99 (d, J = 8.1 Hz, 2H, Ar-H), 7.84 (d, J = 8.1 Hz, 2H, Ar-H), 7.31 (d, J = 8.1 Hz, 2H, Ar-H), 7.22 (d, J = 8.1 Hz, 2H), 6.57 (t, J = 6.9 Hz, 1H, H-1'), 5.81 (m, 1H, H-3'), 4...
Embodiment 3
[0069] Example 3: Preparation of compound Ⅳ
[0070] Suspend 1.2g (2.2 mmol) of compound III in 50ml of anhydrous methanol, add 375mg (7 mmol) of sodium methoxide, and stir the reaction at 25°C. The reaction progress was monitored by thin-layer chromatography. After the reaction, glacial acetic acid was added to adjust the pH to 7. The reaction solution was concentrated and separated by silica gel column chromatography to obtain 500 mg of compound IV (yield 75%).
[0071] Experimental data:
[0072] Goo. 1 H NMR (400 MHz, CDCl 3 ) δ 8.14 (s, 1H, H-8), 6.38 (dd, J = 8.3, 5.9 Hz, 1H, H-1'), 4.80 (d, J = 5.1 Hz, 1H, H-3'), 4.20 (s, 4H, H-4', -OCH 3 ), 3.99 (dd, J = 12.7, 1.5 Hz, 1H, H-5'a), 3.84 (dd, J = 12.6, 1.8 Hz, 1H, H-5'b), 2.91 (ddd, J = 13.7, 8.4, 5.6 Hz, 1H, H-2'a), 2.43 (m, 1H, H-2'b). 13 C NMR (101 MHz, CDCl 3) δ 161.6 (C-2), 153.0 (C-4), 151.8 (C-6), 142.2 (C-8), 121.6 (C-5), 89.0 (C-4'), 86.9 (C-1 '), 72.4 (C-3'), 62.9 (C-5'), 55.3 (-OCH 3 ), 40.9 (C-2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com