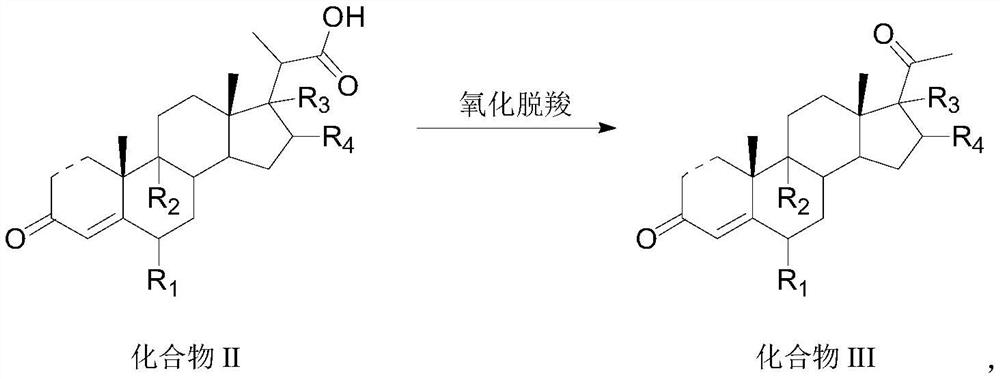
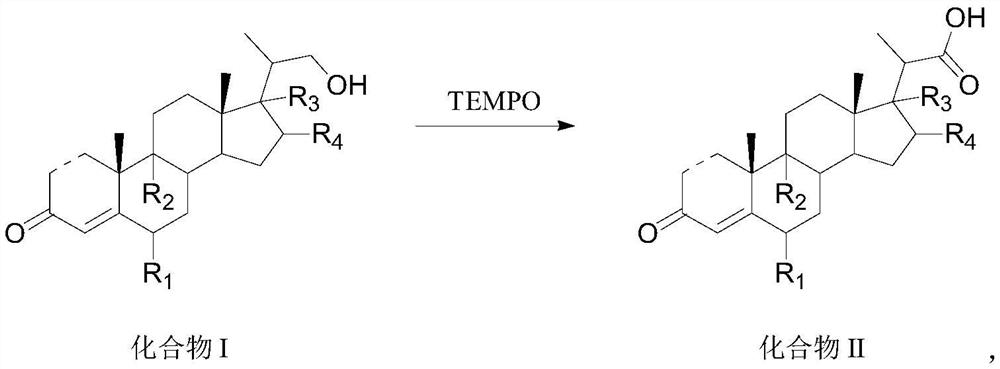
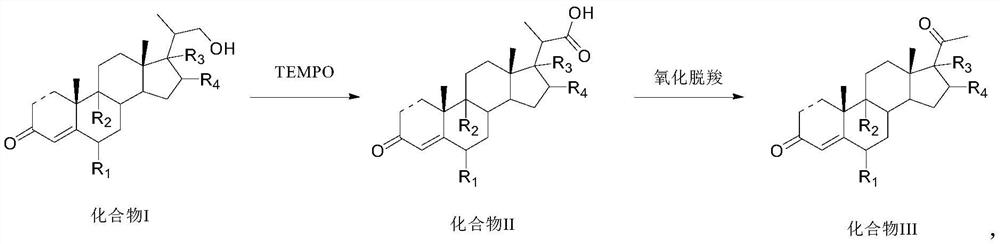
Preparation method and application of steroid intermediate
A technology for intermediates and steroids, which is applied in the field of preparation of steroid intermediates, can solve the problems of high cost of oxidizing reagent Dess Martin, difficult to realize industrialized production, and many reaction by-products, etc., and achieves improved production applicability and easy control. , the effect of increasing the reaction temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
[0084]
Embodiment 1-1-1
[0086] 15.0 g of Compound I-a and 75 mL of dichloromethane were added to the reaction flask, and stirred at room temperature to dissolve. Add 0.5g of potassium bromide, 1.0g of sodium bicarbonate, 0.04g of TEMPO and 7.5mL of water, stir to cool down to -5-0°C, start adding 16.9g of calcium hypochlorite in portions, control the temperature at 0°C, and complete the addition in about 30 minutes , insulated and stirred for 1 h, and no compound I-a was detected by TLC. Add 36 mL of an aqueous solution of sodium thiosulfate under an ice bath, return to room temperature and stir for 30 min, let the layers stand, wash with water, separate the organic layer, dry, filter, and concentrate under reduced pressure to obtain 14.6 g of compound II-a with a molar yield of 93.5%. HPLC purity 99.8%.
Embodiment 1-1-2
[0088] 15.0 g of Compound I-a and 75 mL of acetonitrile were added to the reaction flask, and stirred at room temperature to dissolve. Add 0.9g of sodium bromide, 0.9g of potassium bicarbonate, 0.07g of TEMPO and 7.5mL of water, stir and cool down to -10~-5°C, start adding 5.1g of sodium hypochlorite in portions, control the temperature at 5°C, finish adding for about 30 minutes, and keep warm The reaction was stirred for 1 h, and no compound I-a was detected by TLC. Add 36 mL of an aqueous solution of sodium thiosulfate under an ice bath, return to room temperature and stir for 30 min, let the layers stand, wash with water, separate the organic layer, dry, filter, and concentrate under reduced pressure to obtain 14.1 g of compound II-a with a molar yield of 90.3%. HPLC purity 95.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com