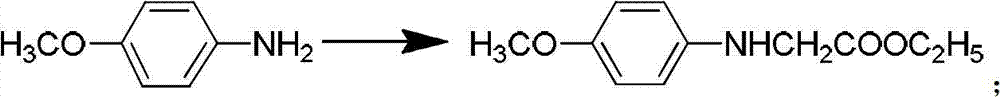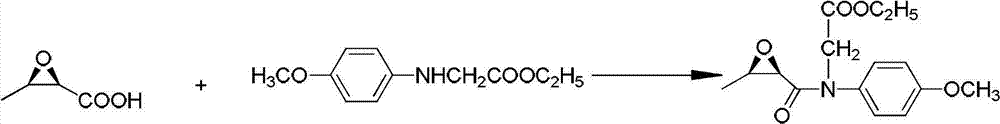Synthetic method for penem and carbapenem antibiotic type key intermediate 4AA
A technology of carbapenems and synthetic methods, which is applied in the field of key intermediates of penems and carbapenem antibiotics, can solve the problems of harsh reaction conditions, low total yield, serious pollution, etc., and achieve easy industrial production , high liquid phase purity and advanced technology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
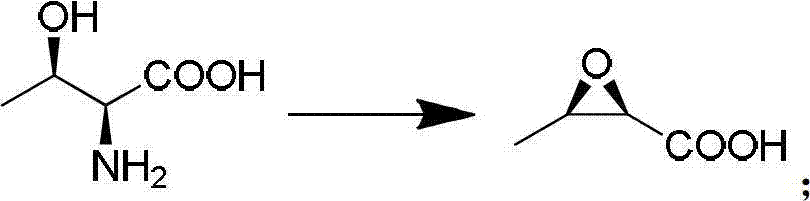
[0080] A method for synthesizing 4AA, a key intermediate of penicillene and carbapenem antibiotics, which consists of the following steps:
[0081] (1) Synthesis of Intermediate I:
[0082] In a 50L reactor, add 10.2kg of industrial hydrochloric acid with a mass concentration of 31% and 5.0L of water. When the internal temperature drops to 0°C, add 3.0kg of L-threonine and 5.0kg of sodium nitrite at -5°C. (The diazotization temperature in step (1)) react for 1 hour. Raise the temperature to 20-25°C and keep it for 1 hour. Turn on the vacuum pump to remove residual NO 2 gas. Cool the inner temperature to 10°C and slowly add 6.5kg of sodium hydroxide and 9.13kg of water to increase the temperature to 25°C and keep it warm for 15 hours. After the heat preservation is completed, cool to -2°C, add concentrated hydrochloric acid dropwise to adjust PH=2.0. Put the material into a 100L extraction kettle, add 50g of tetrabutylammonium bromide and 30.0L of ethyl acetate, stir for 30 minu...
Embodiment 2
[0101] (1) Synthesis of Intermediate I:
[0102] In a 50L reactor, add 10.2kg of industrial hydrochloric acid with a mass concentration of 31% and 5.0L of water. When the internal temperature drops to 0°C, add 3.0kg of L-threonine and 5.0kg of sodium nitrite at 0°C ( The diazotization temperature of step (1) was reacted for 1 hour, and the others were the same as in Example 1. The yield of intermediate I was 65.3%.
[0103] (2) Synthesis of Intermediate II:
[0104] Same as Example 1
[0105] (3) Synthesis of Intermediate III:
[0106] In a 50L reactor, add 1.90kg of Intermediate I, start stirring and pass nitrogen protection, cool the internal temperature to -10°C, start to add 2.2kg of N-methylmorpholine dropwise, control the temperature at -10°C, about 20- After 30 minutes of dripping, slowly add 2.35kg ethyl chloroformate, control the internal temperature at -5°C, and keep it warm for 1 hour. Then add 3.5 kg of Intermediate II, control the internal temperature of -10°C (reaction ...
Embodiment 3
[0118] (1) Synthesis of Intermediate I:
[0119] In a 50L reactor, add 10.2kg of industrial hydrochloric acid with a mass concentration of 31% and 5.0L of water. When the internal temperature drops to 0°C, add 3.0kg of L-threonine and 5.0kg of sodium nitrite at -10°C. (The diazotization temperature in step (1)) was reacted for 1 hour, and the others were the same as in Example 1. The yield of intermediate I was 71.6%.
[0120] (2) Synthesis of Intermediate II:
[0121] The same as in Example 1.
[0122] (3) Synthesis of Intermediate III:
[0123] In a 50L reactor, add 1.90kg of Intermediate I, start stirring and pass nitrogen protection, cool the inner temperature to -10°C, start to add 2.2kg of N-methylmorpholine dropwise, control the temperature at -20°C, about 20- After 30 minutes of dripping, slowly add 2.35kg ethyl chloroformate, control the internal temperature at -5°C, and keep it warm for 1 hour. Then add 3.5 kg of Intermediate II, control the internal temperature of -20°C (r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com