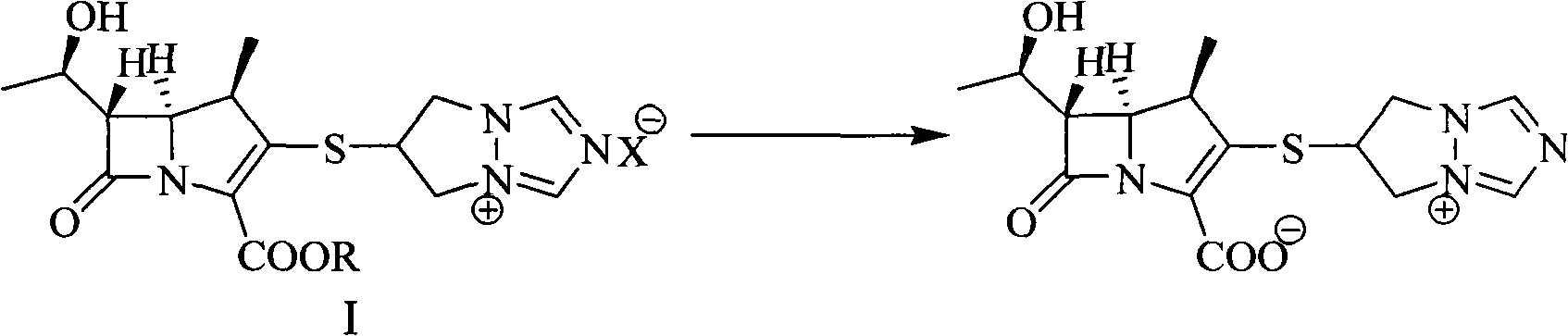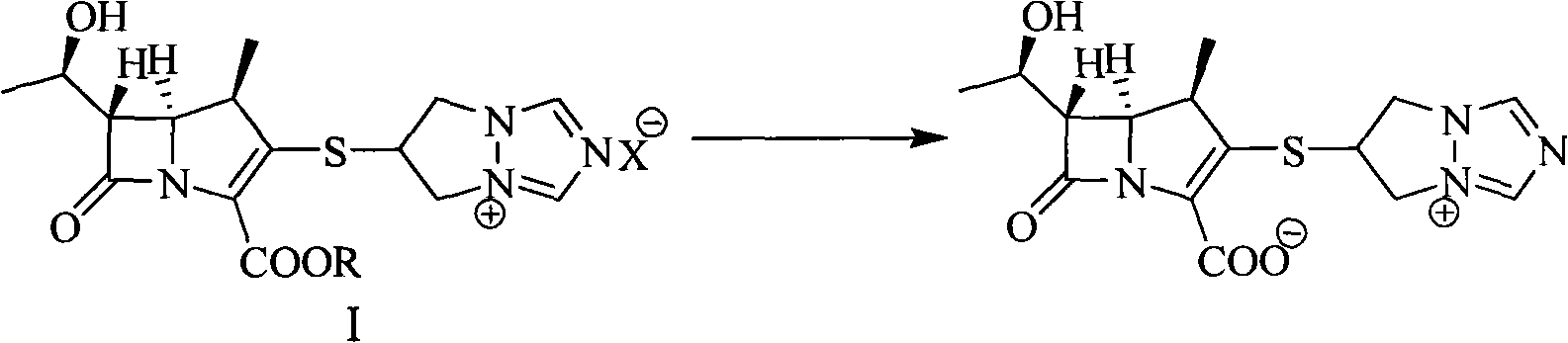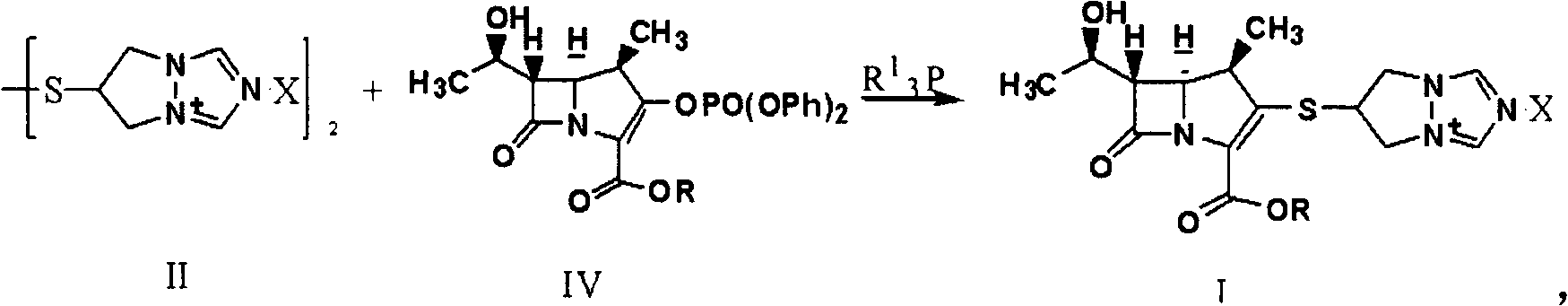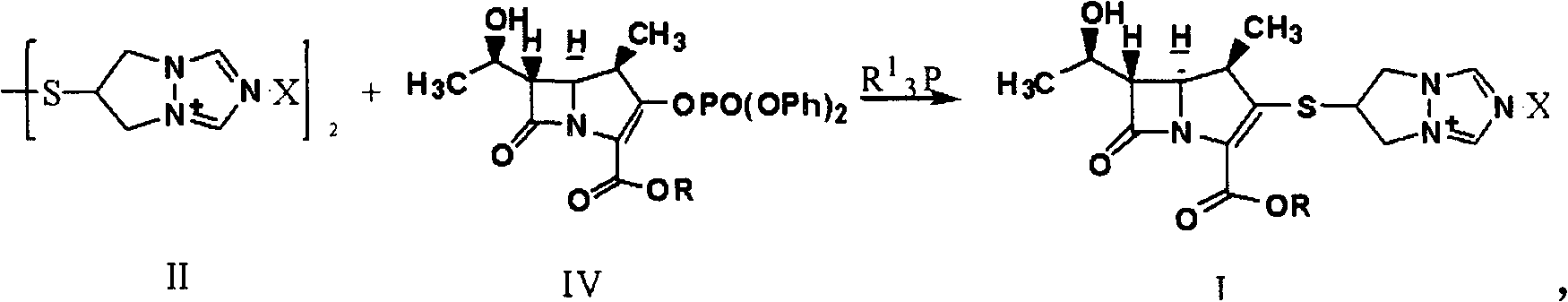Patents
Literature
52 results about "Biapenem" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
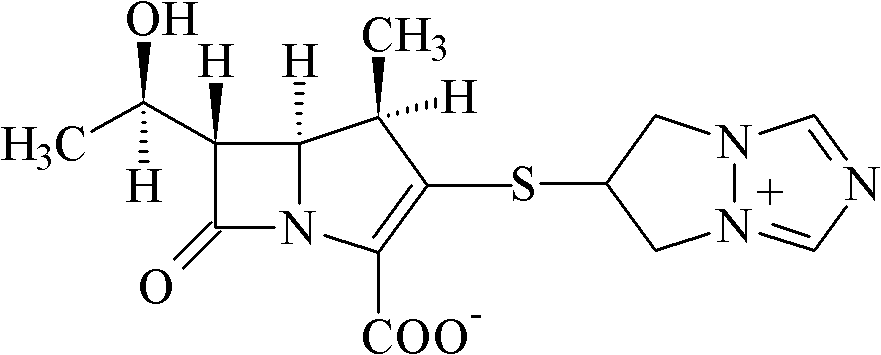
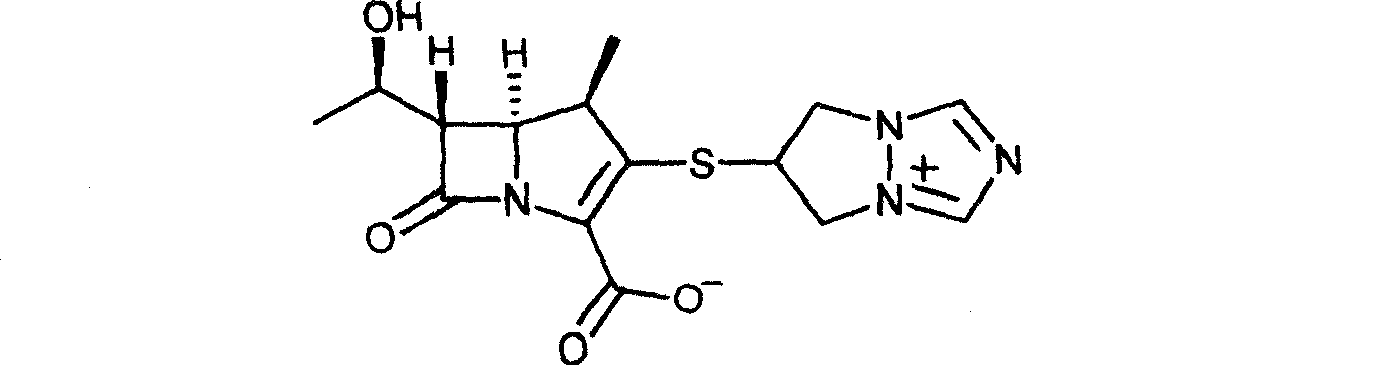
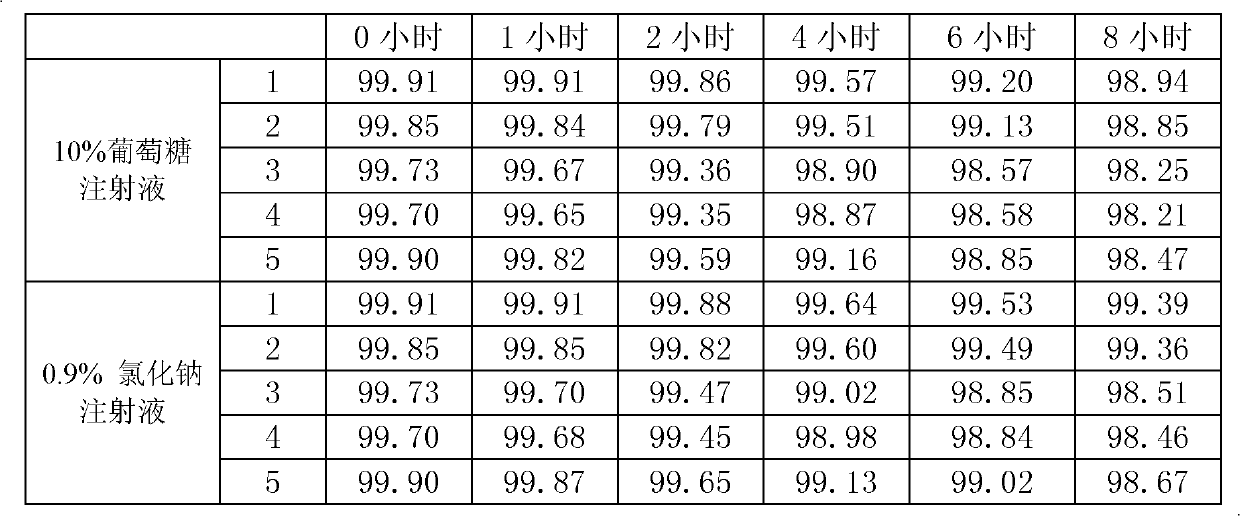
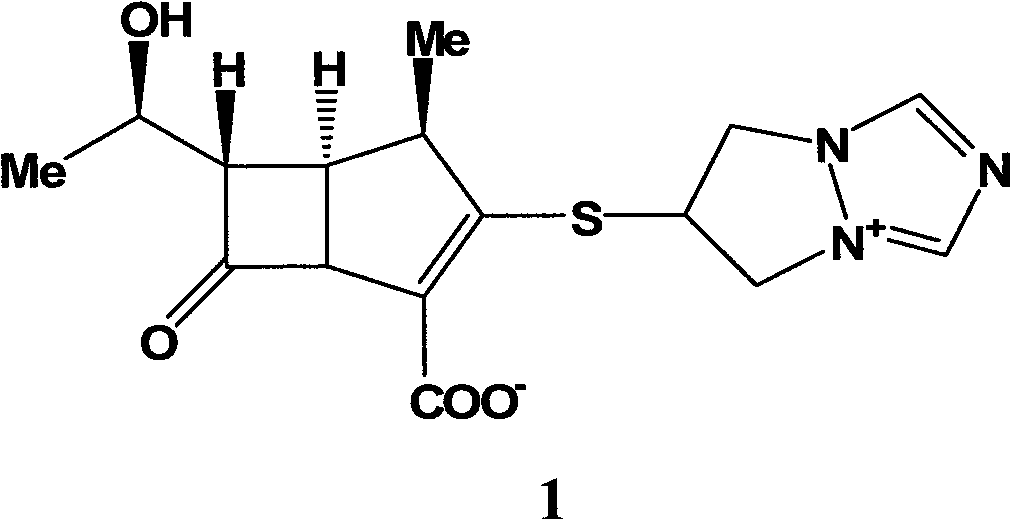
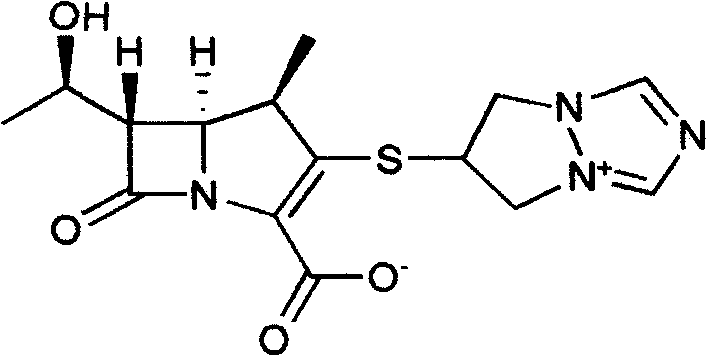
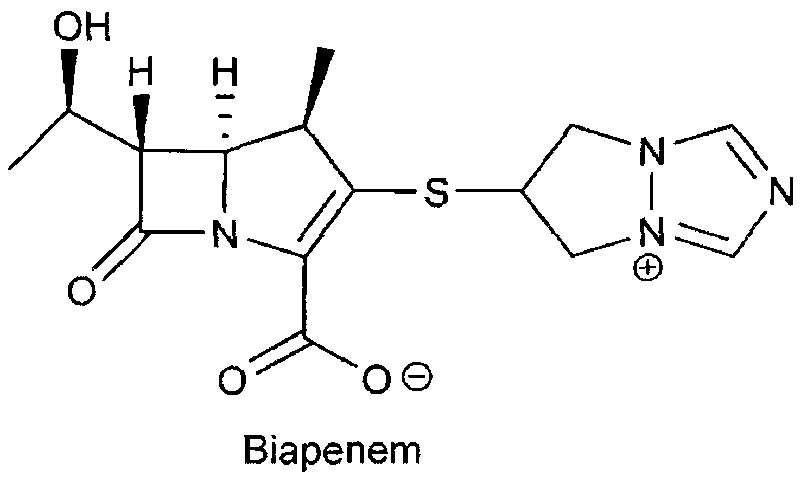
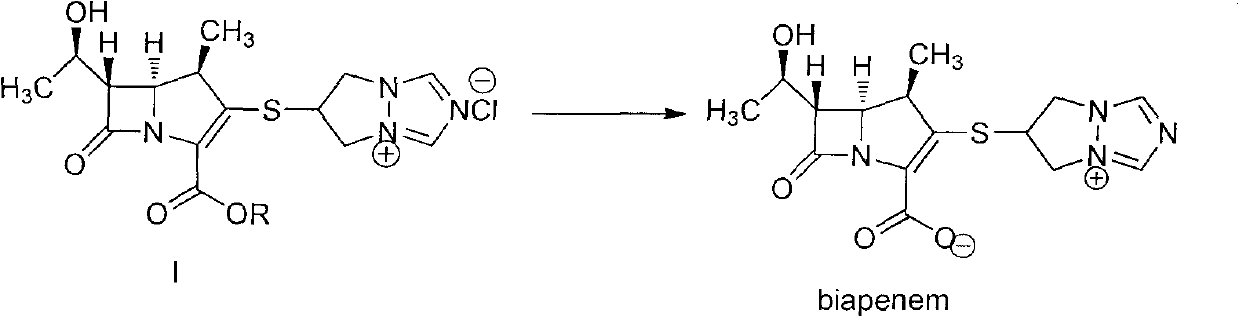
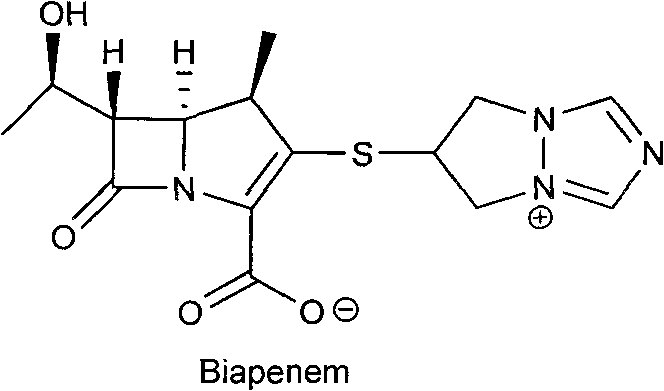
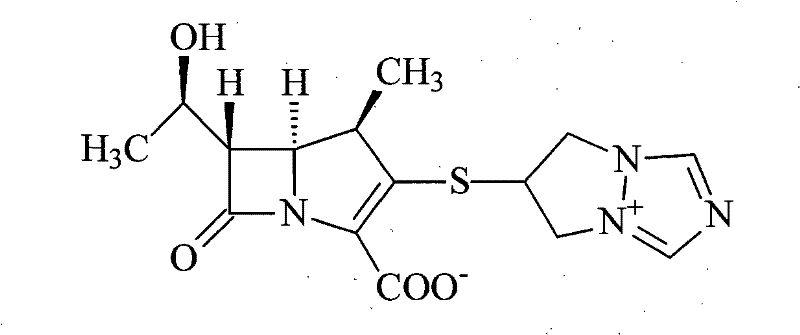
Biapenem (INN) is a carbapenem antibiotic. It has in vitro activity against anaerobes. 1-β-methyl-carbapenem antibiotic. Approved in Japan in 2001.
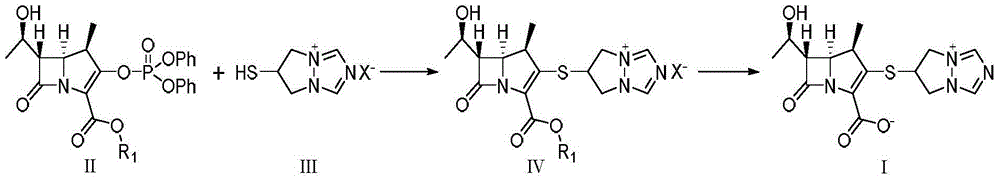
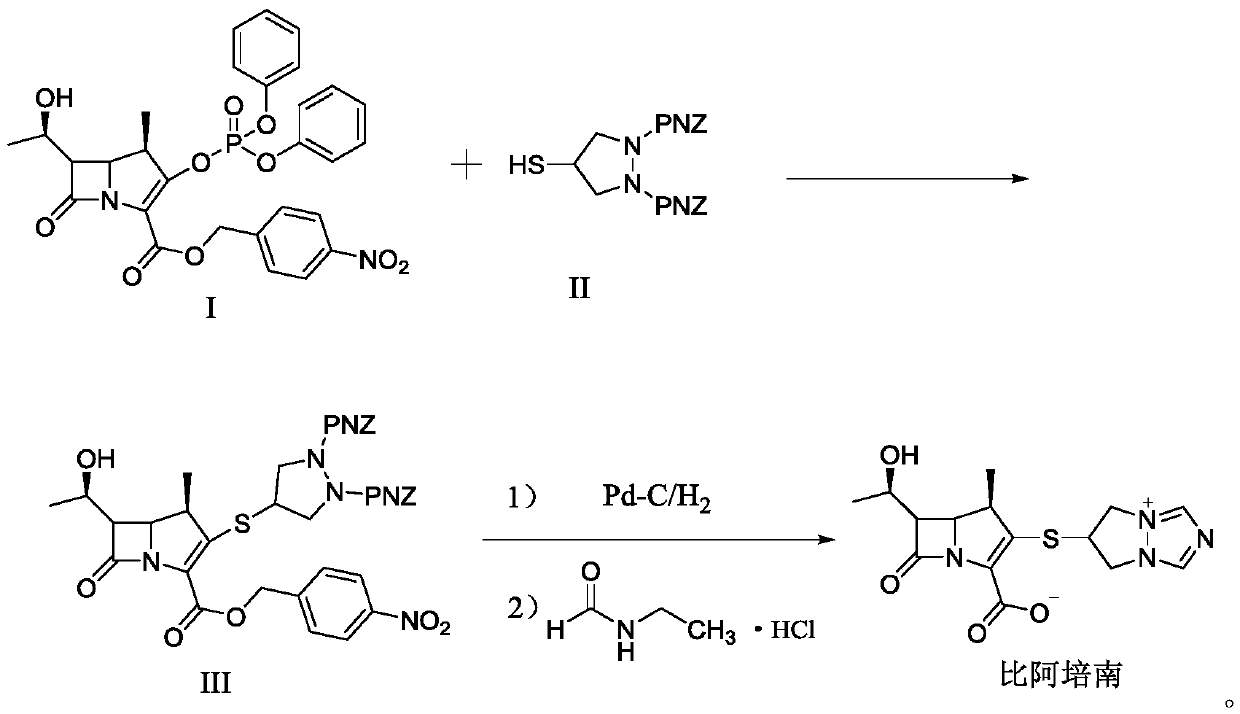
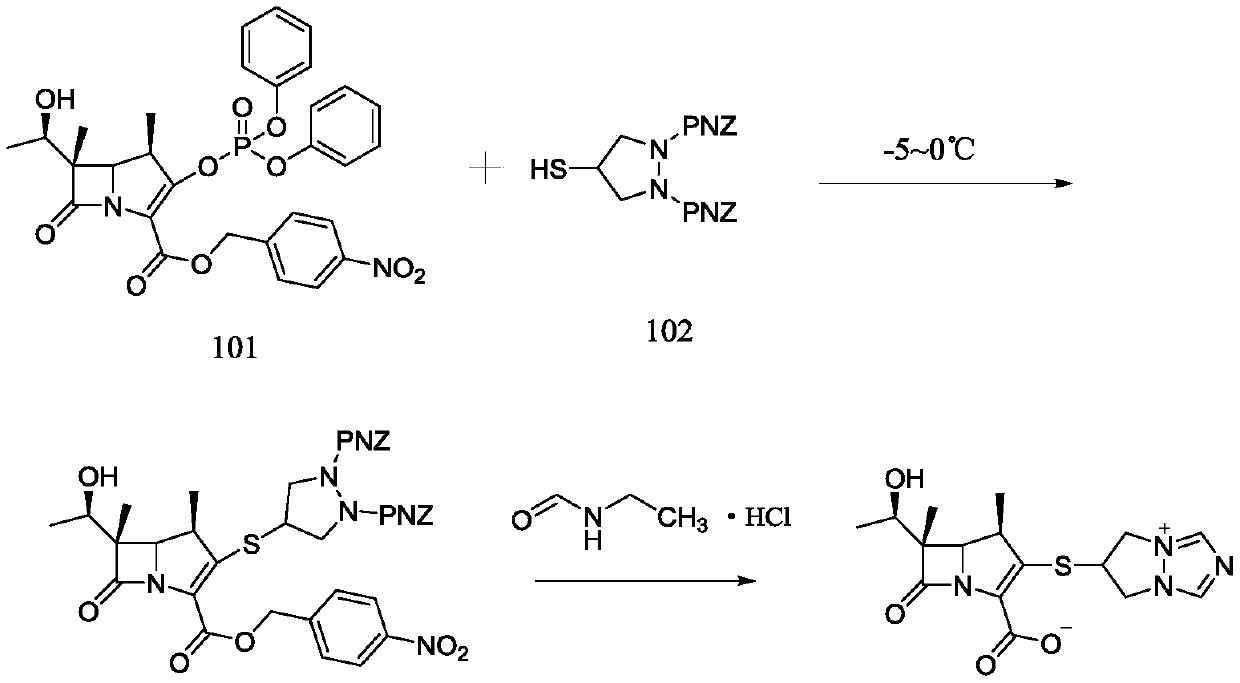
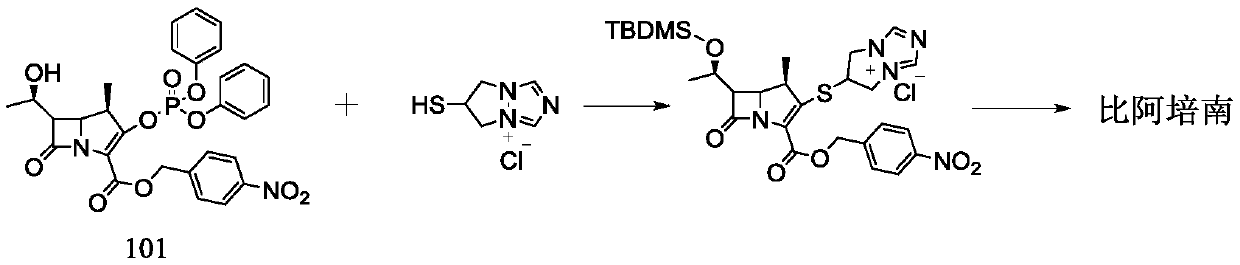
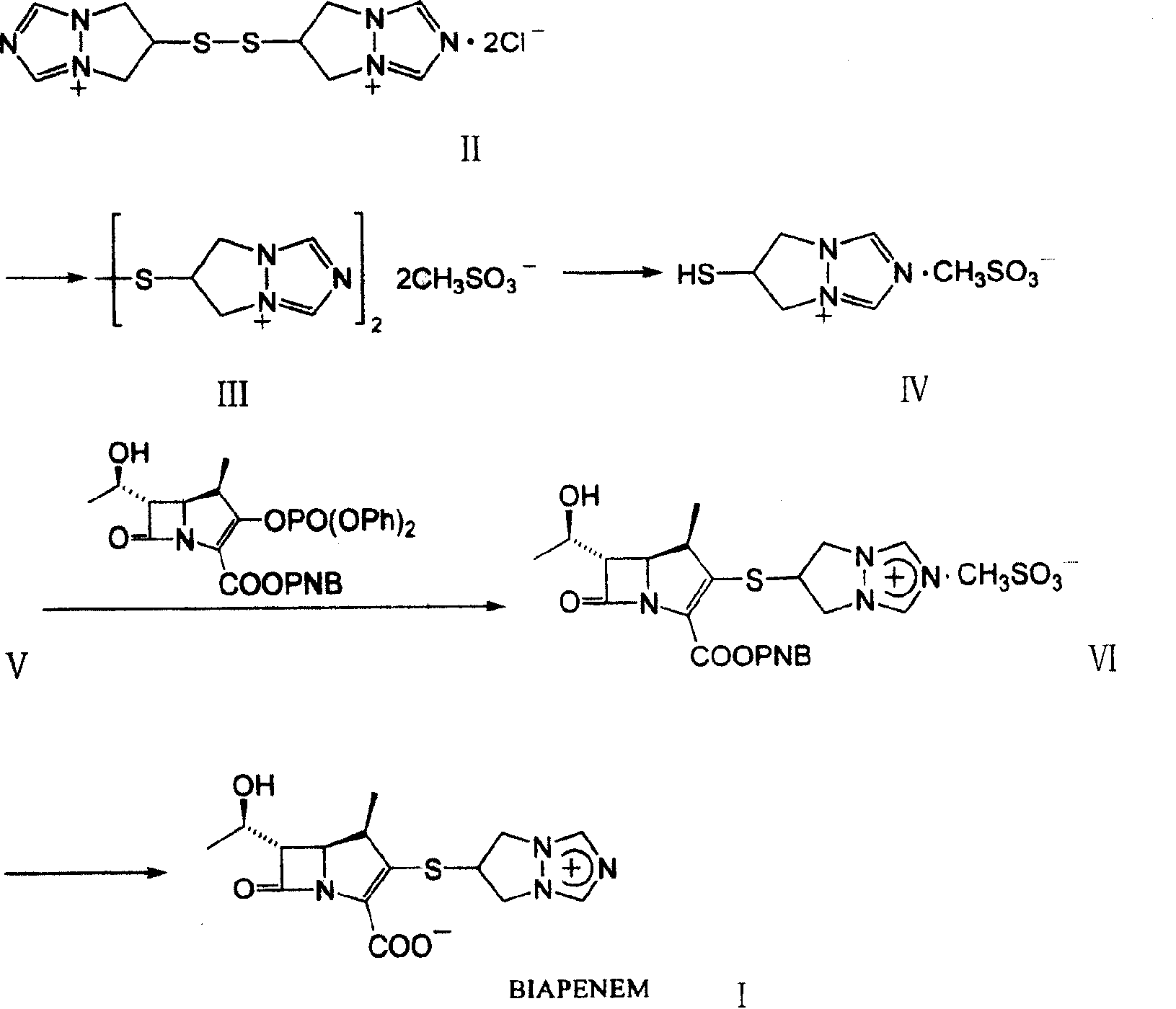
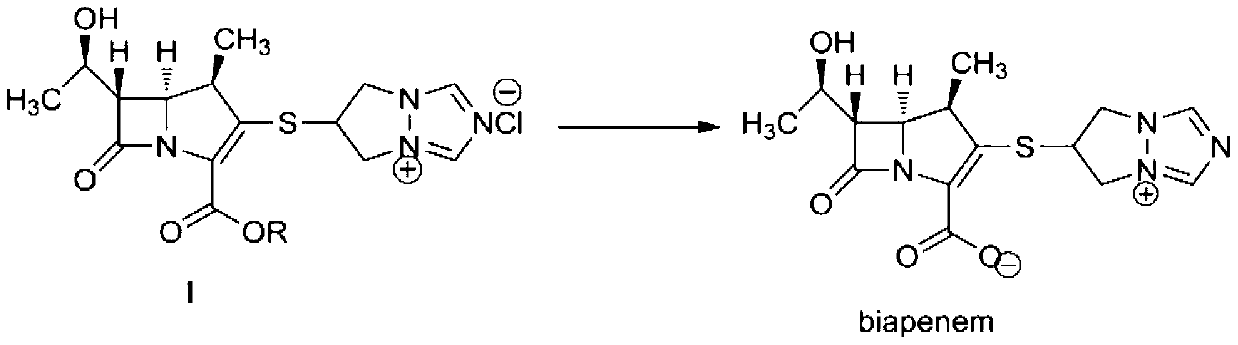
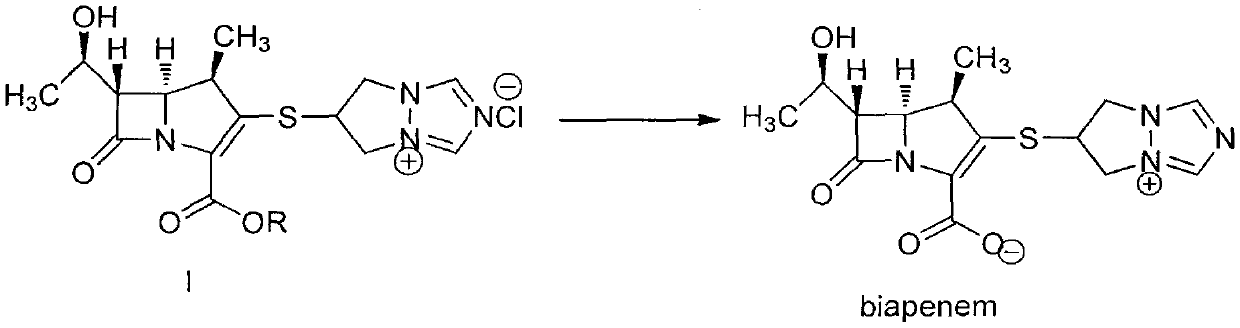
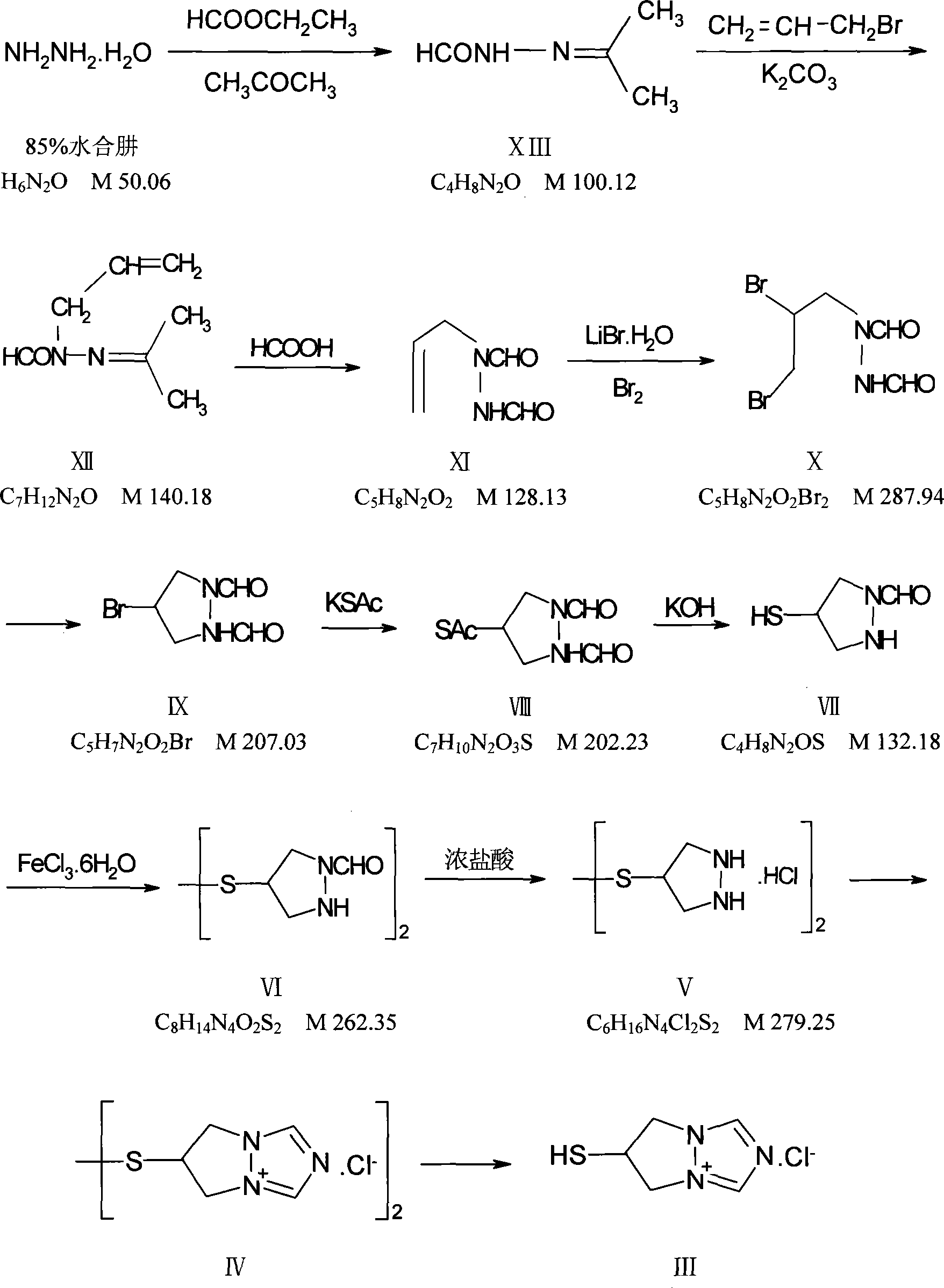
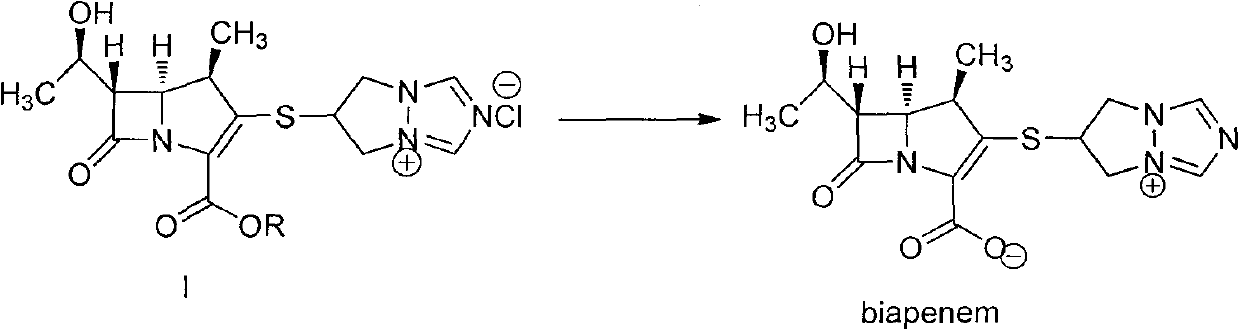
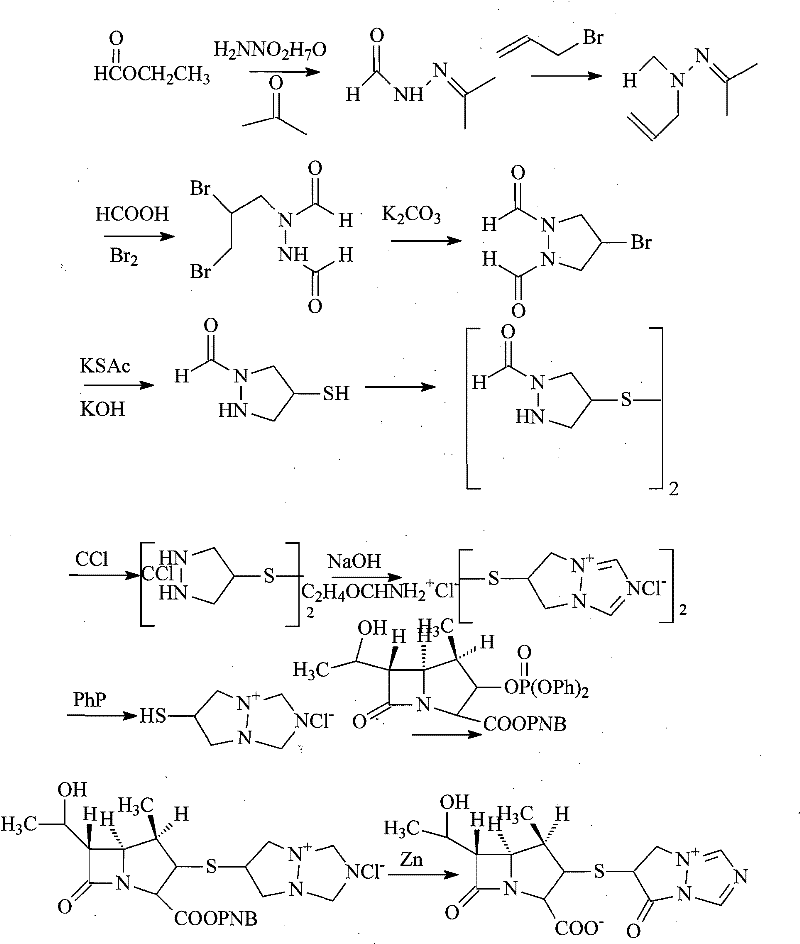
Synthesis method for biapenem
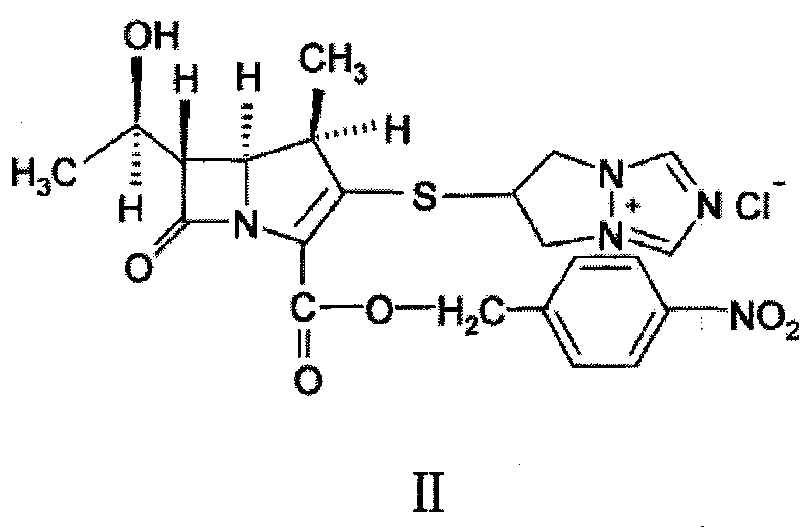
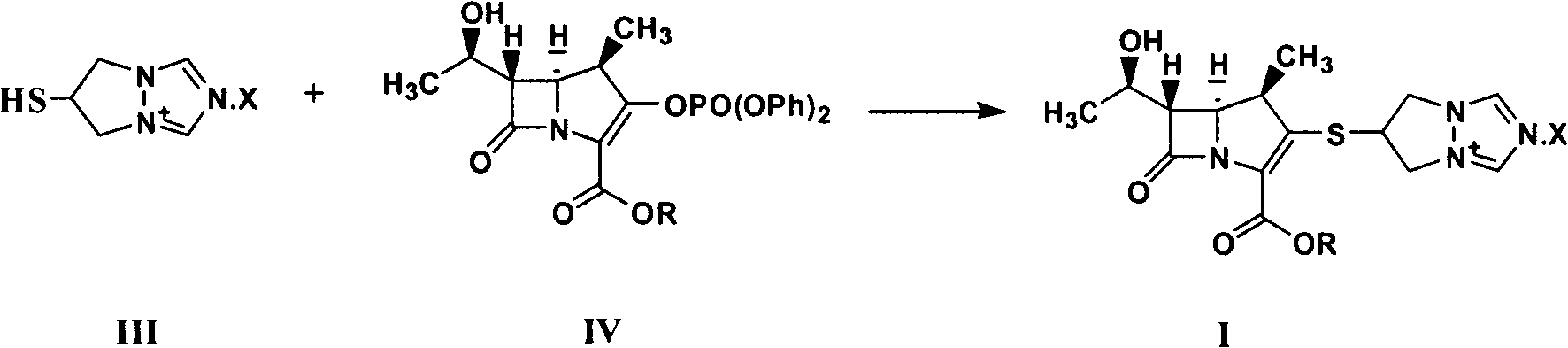
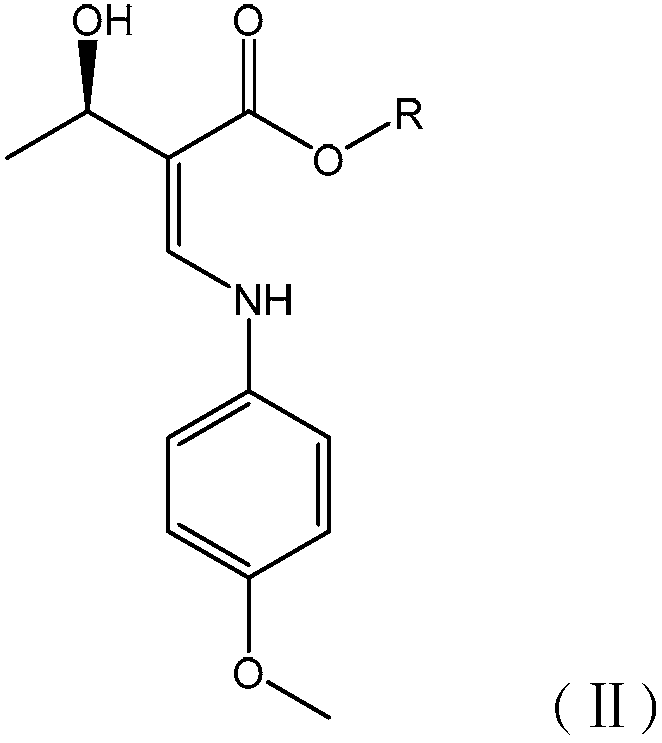
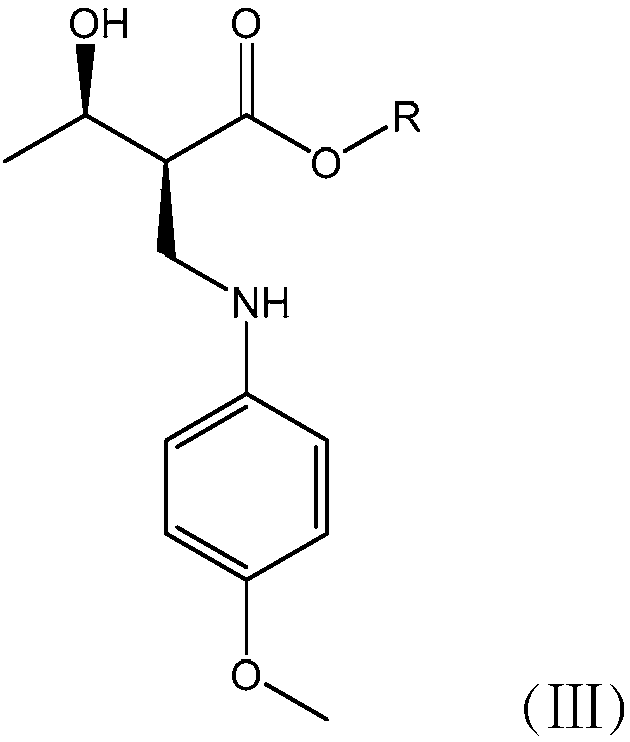
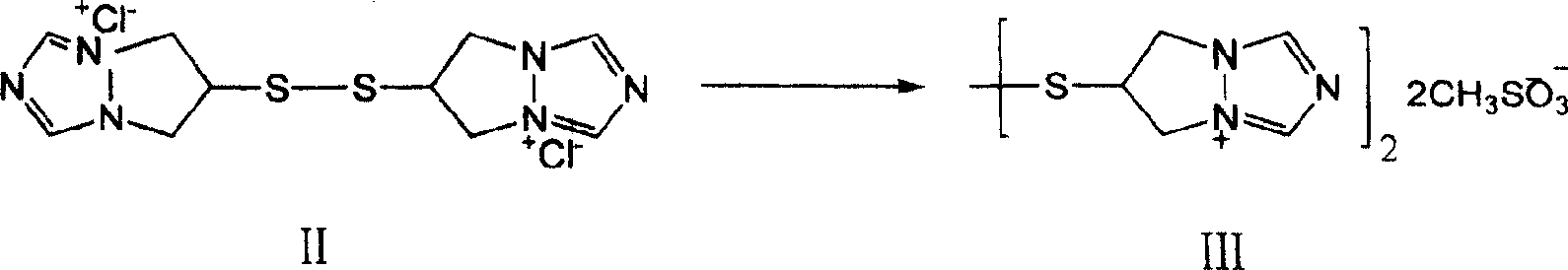
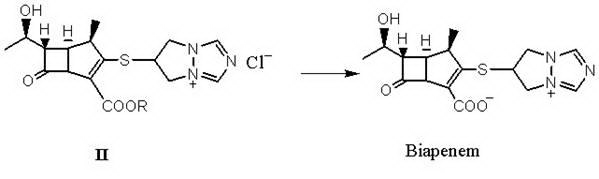
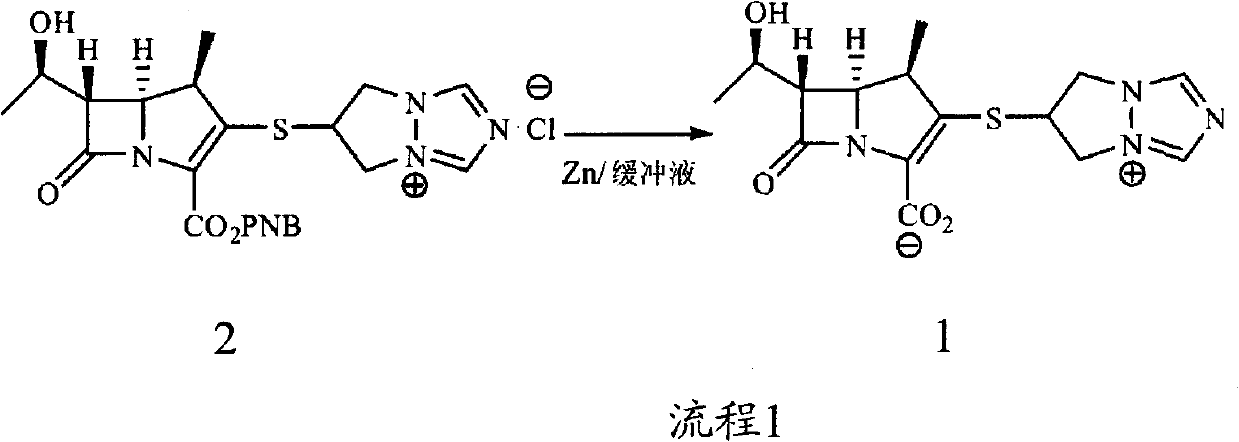
The invention is a synthesis method of the biapenem, with the MAP ((1R, 5R, 6R) 2-(drophenyl phosphate)-6-[(R)-1- hydroxyethyl]-1-methyl-1-1 - carbapenems-2-en-3-formic acid benzyl ester of p-nitrophenol)) and the 6, 7-dihydro-6-mercapto-5 H-pyrazol [1,2 - a] [1,2 , 4]-triazole chloride ( the intermediate III) as the starting materials; after the condensation, hydrolysis and the fine preparation reaction and synthesis, the biapenem is made.
Owner:严洁
Preparation method of biapenem
ActiveCN102212077AReduce dosageEasy to operateOrganic chemistryBulk chemical productionOrganic acidAlcohol
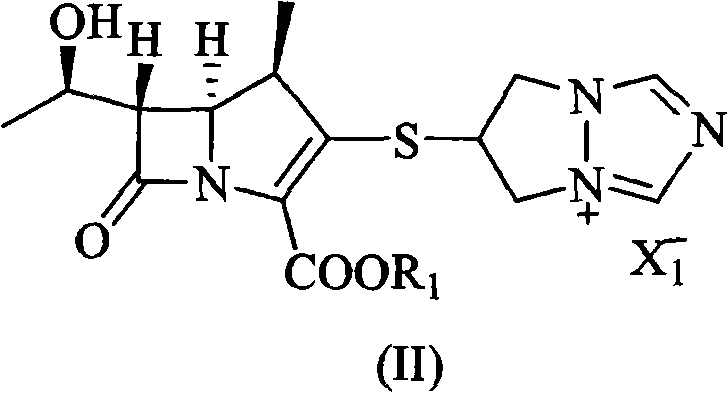
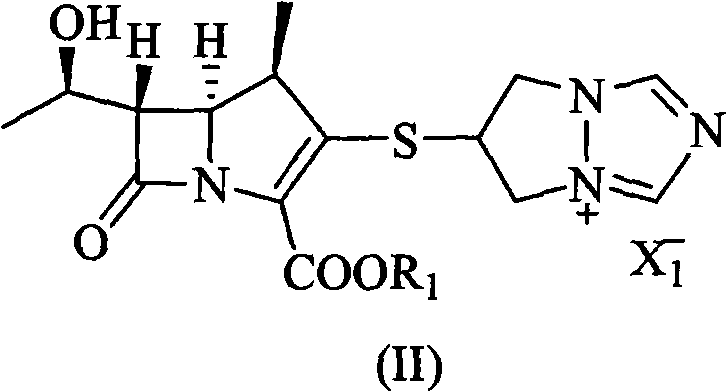
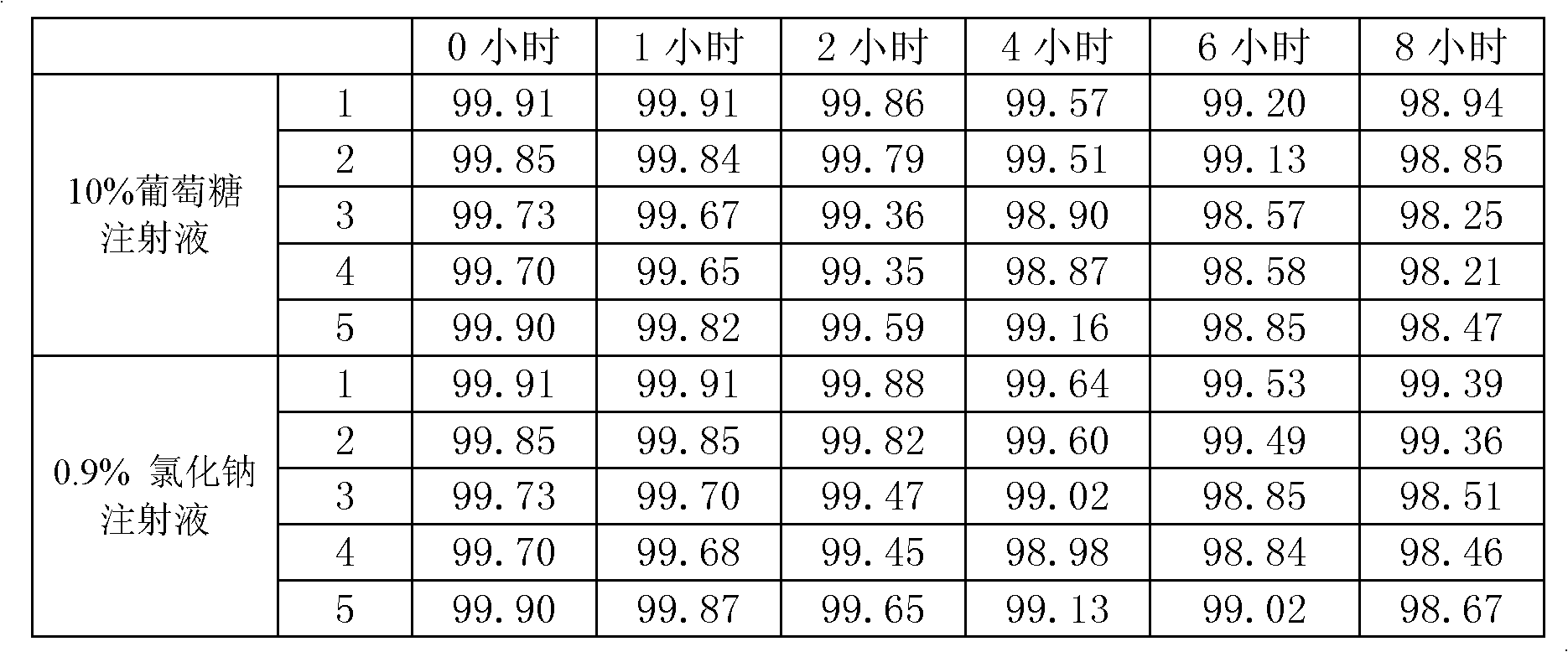
The invention discloses a preparation method of biapenem. The method comprises the following steps of: undergoing a catalytic hydrogenation reaction on a compound which is shown as a formula I and serves as a raw material and H2 in a mixed solvent of water and an organic solvent; adding an organic base immediately for regulating the pH value after the reaction; adding an organic solvent for precipitating biapenem crystals; and recrystallizing the crystals in water, organic acid and ethanol or ketone to obtain a refined biapenem product. The preparation method has the advantages of easiness for operating, no need of adjusting the pH value with a buffer salt during hydrogenation, no need of resin purification after hydrogenation, no need of special equipment, greatly-lowered water consumption, high yield and high product purity, and is suitable for industrial production.
Owner:SHANGHAI INST OF PHARMA IND CO LTD +1
New crystal form of biapenem and its synthesis method
InactiveCN102268024AHygroscopic differenceStability differenceAntibacterial agentsOrganic active ingredientsPharmaceutical drugCarboxylic acid
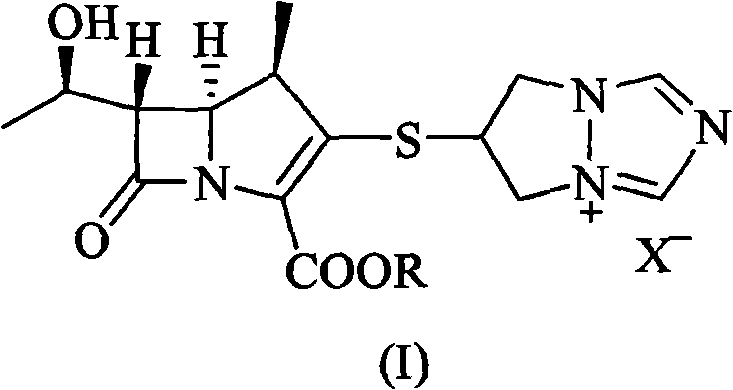
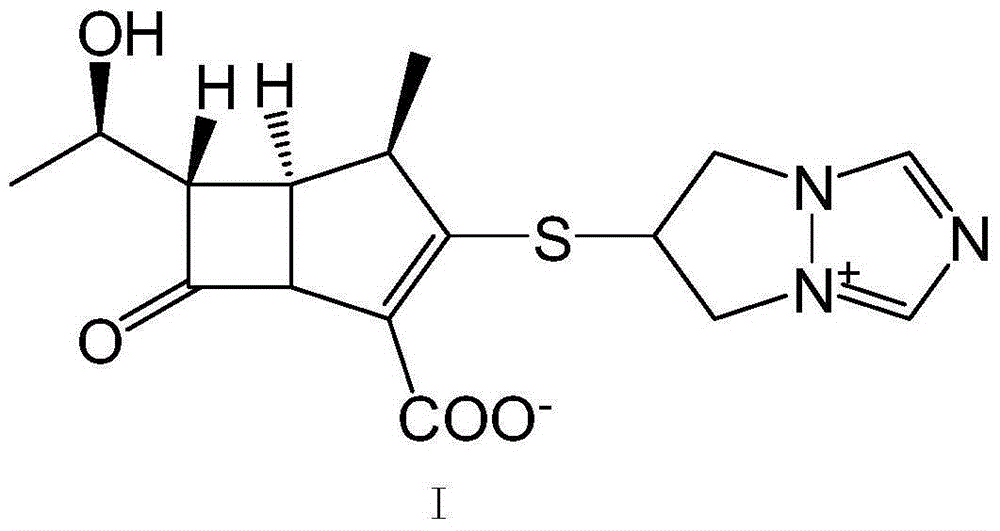
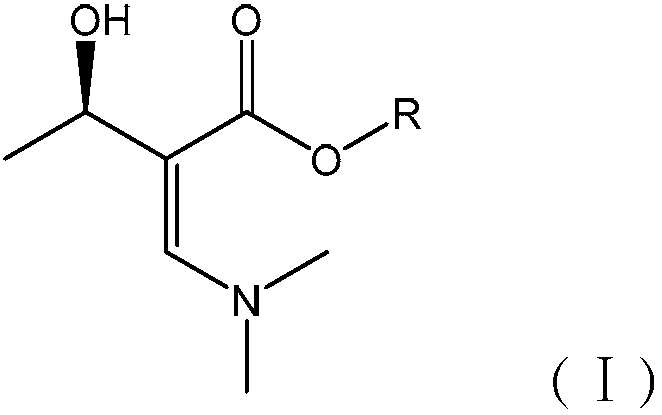
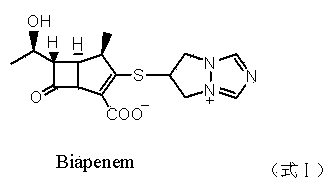
The invention relates to crystal form I of (1R,5S,6S)-2-[(6,7-dihydro-5H-pyrazolo[1,2-alpha]-[1,2,4]triazole-hexabase)]sulfur-6R-1-hydroxyethyl]-1-methyl-carbapenem-3-carboxylate (biapenem), a preparation method of crystal form I, and a pharmaceutical composition containing the crystal form I of biapenem and one or more pharmaceutically acceptable carriers, excipients or diluents. Formula (I) is as described in the specification.
Owner:HARBIN PHARMA GRP CO LTD GENERAL PHARMA FACTORY
A kind of biapenem compound and its preparation method
The invention relates to a biapenem compound and a preparation method thereof, which comprises the following steps: 1) dissolving crude biapenem in water, then adding activated carbon, keeping warm, fully stirring, filtering the deactivated carbon, and collecting the filtrate; 2) dissolving The filtrate obtained in the previous step is separated and purified by column chromatography, and the eluent is collected; 3) the temperature is increased to concentrate the above-mentioned eluent solution, and then adding ethanol and acetone with a volume ratio of 1: 0.5~2 mixed solvent solvent, and control temperature for crystallization, the precipitated crystals are centrifuged, washed, and dried to obtain a refined biapenem compound; 4) Optionally, the crystallization mother liquor is returned to step 3), that is, added to the eluting solution obtained in step 2) together with a mixed solvent. By adopting the method of the invention, the purity of crude biapenem can be greatly improved, the quality of preparation products can be improved, and toxic and side effects can be reduced.
Owner:HAINAN MEILAN SMITH KLINE PHARMA
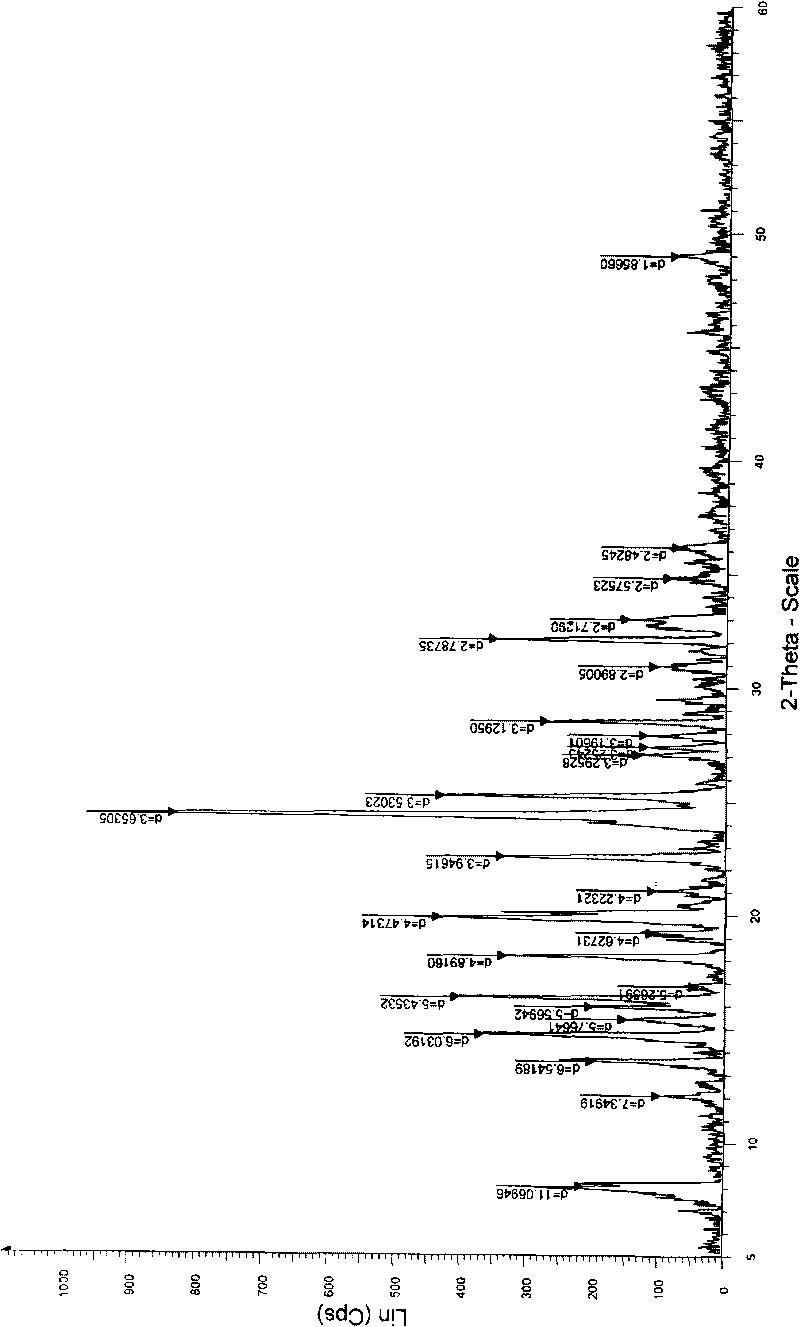
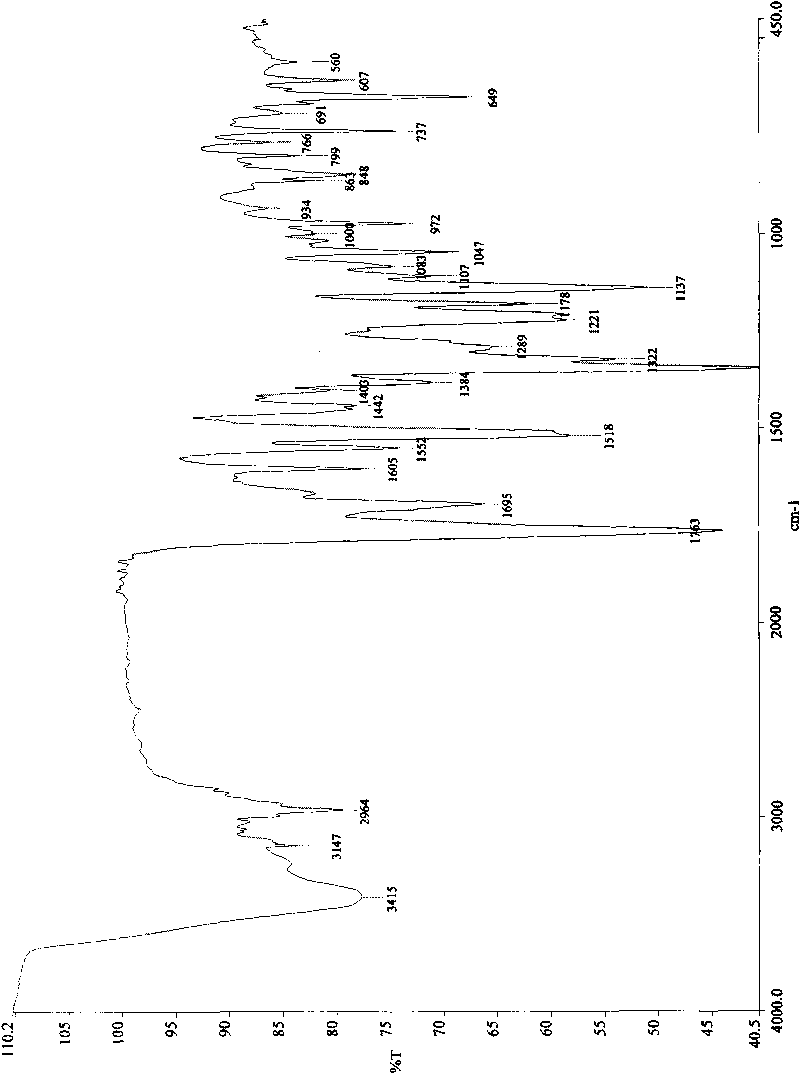
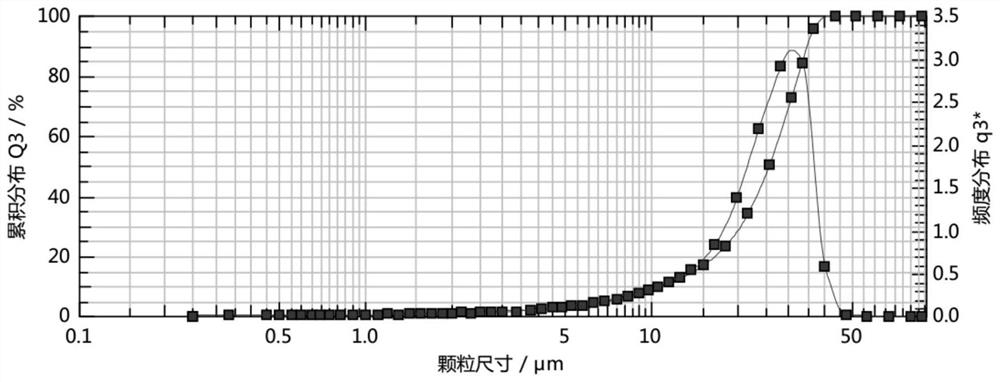
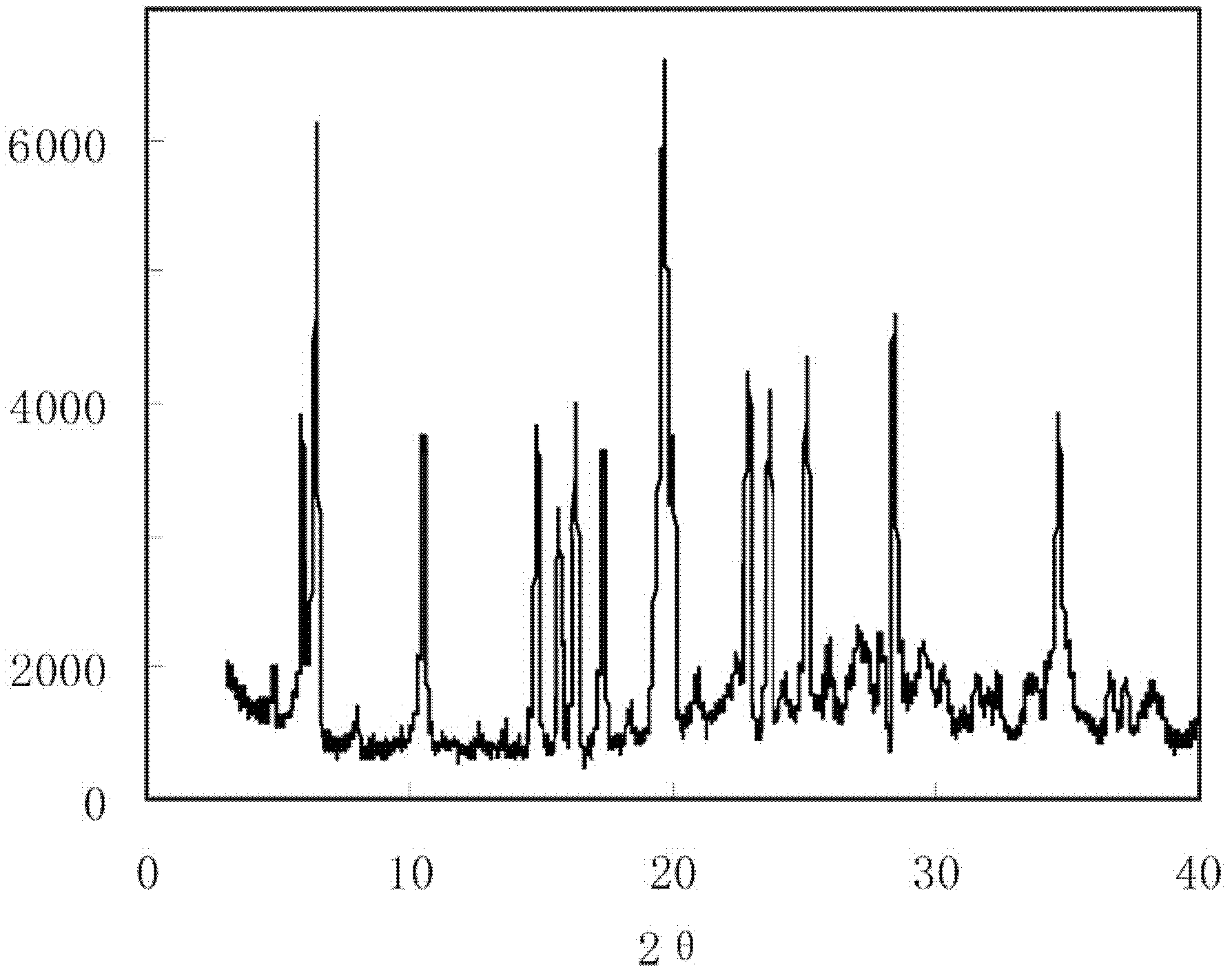
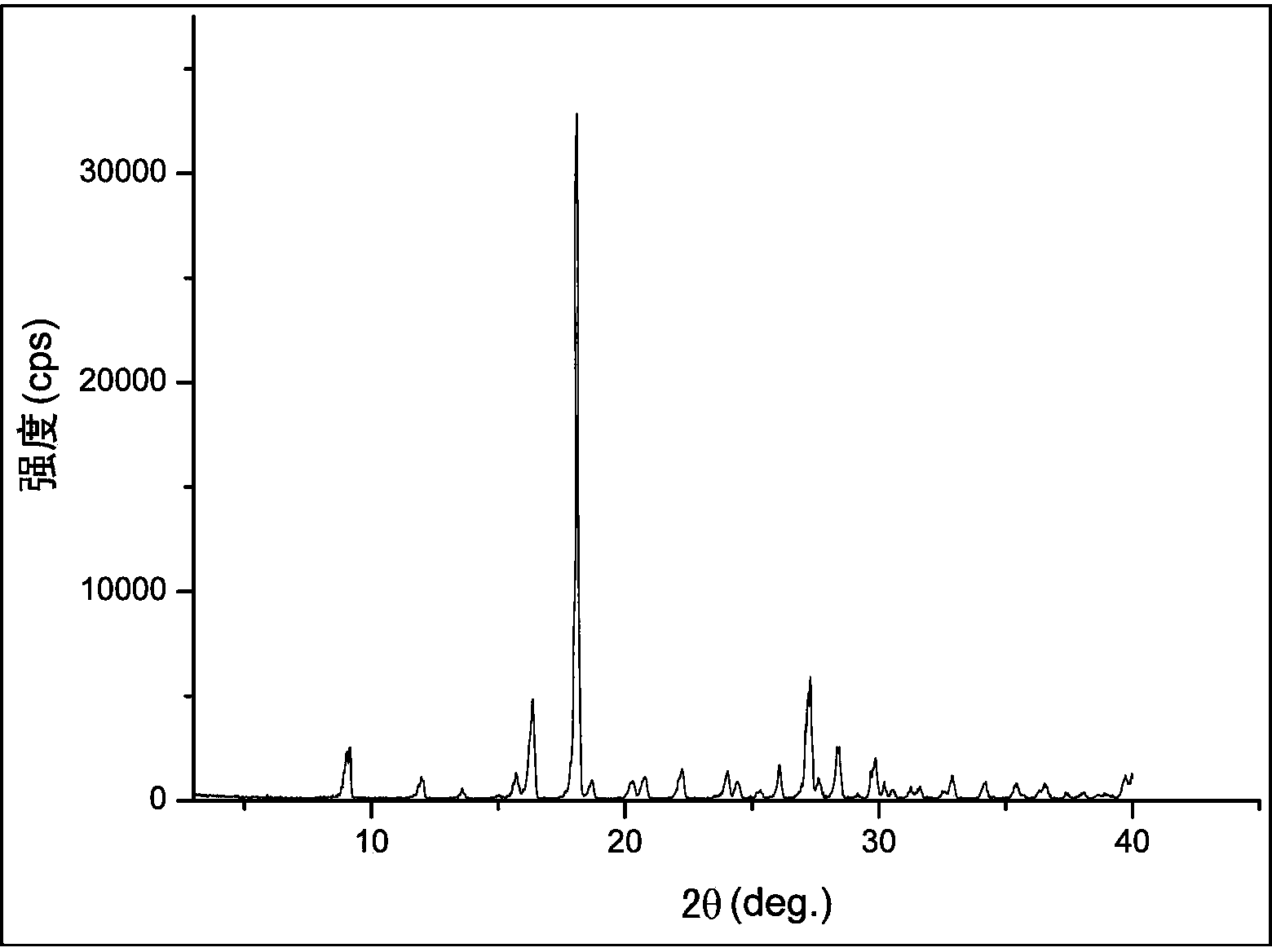
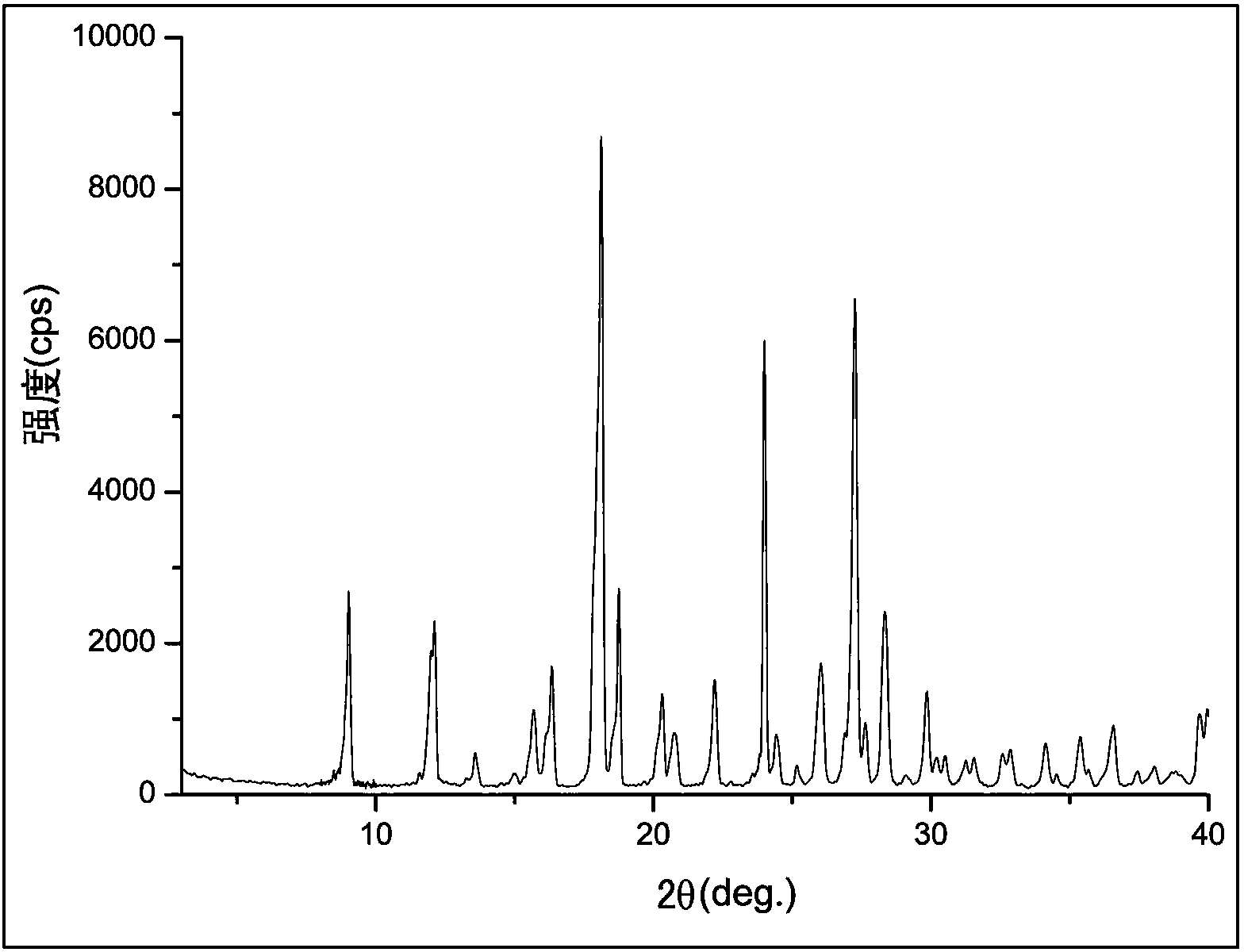
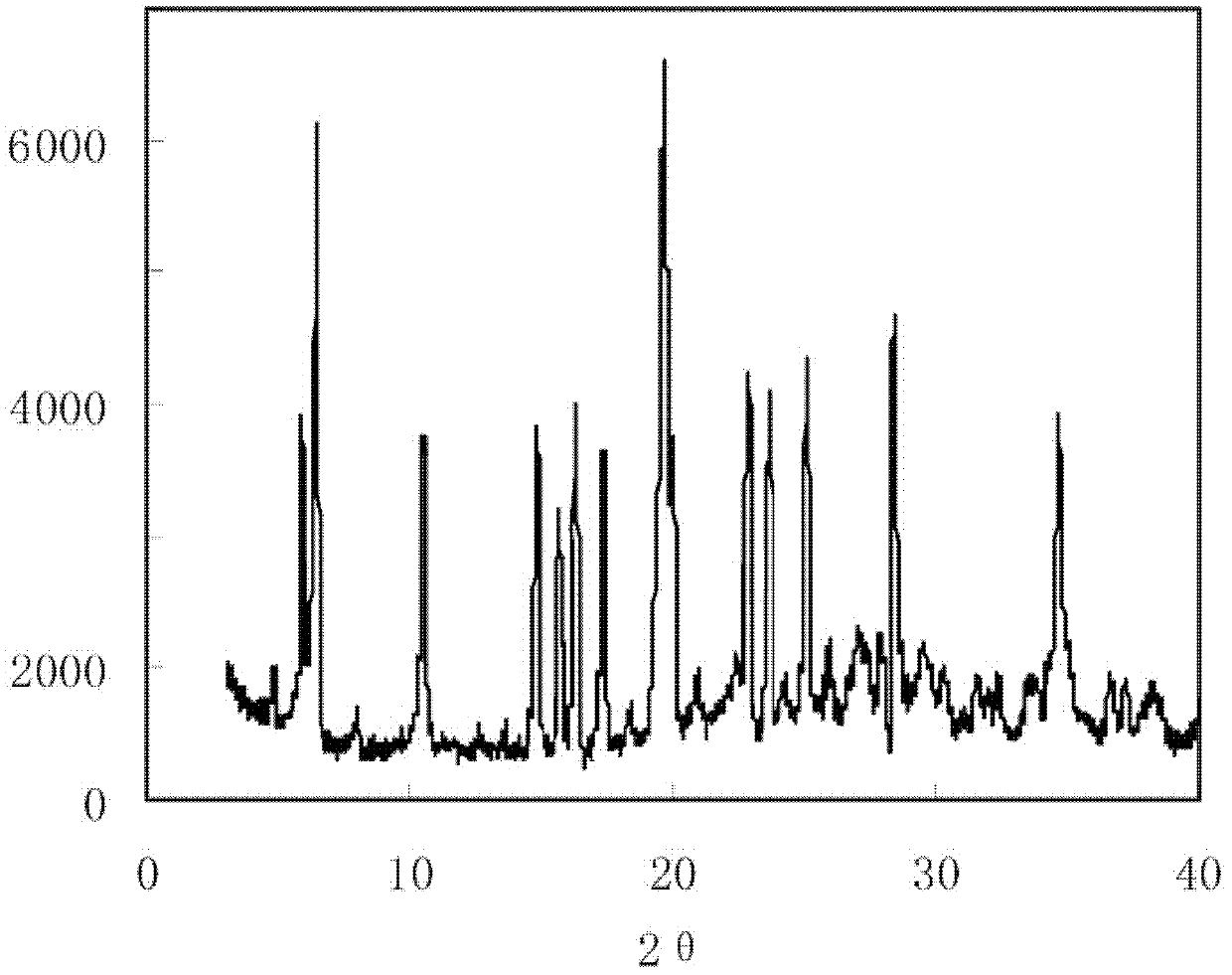
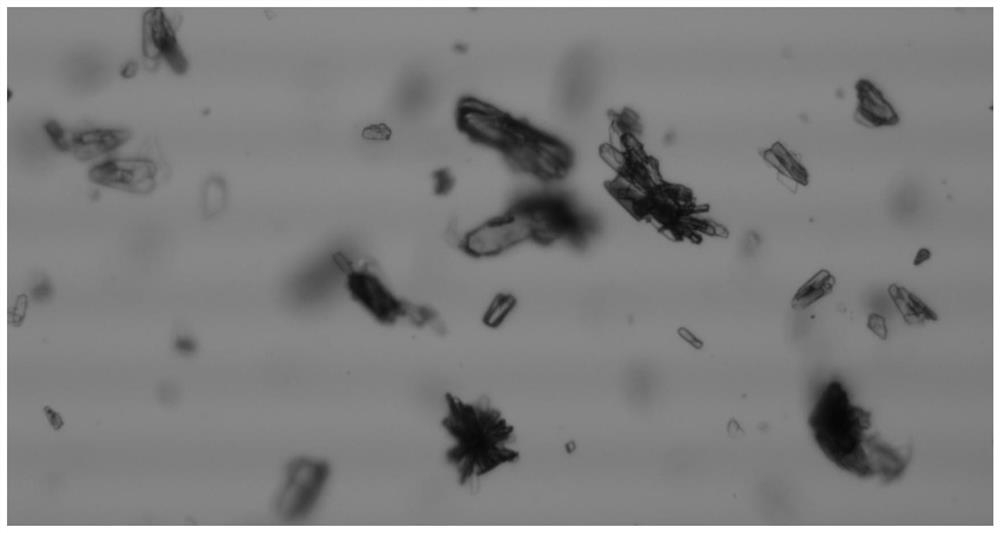
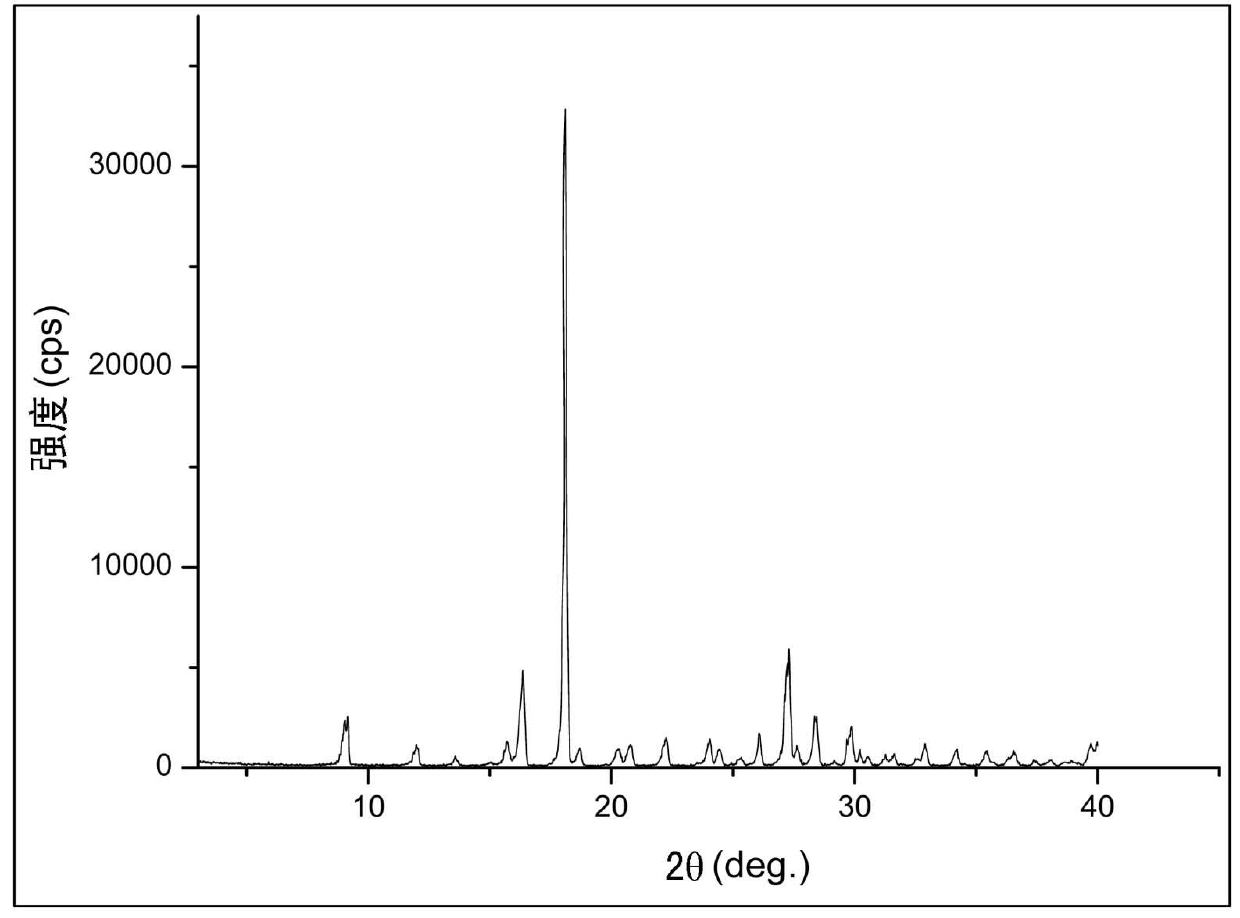
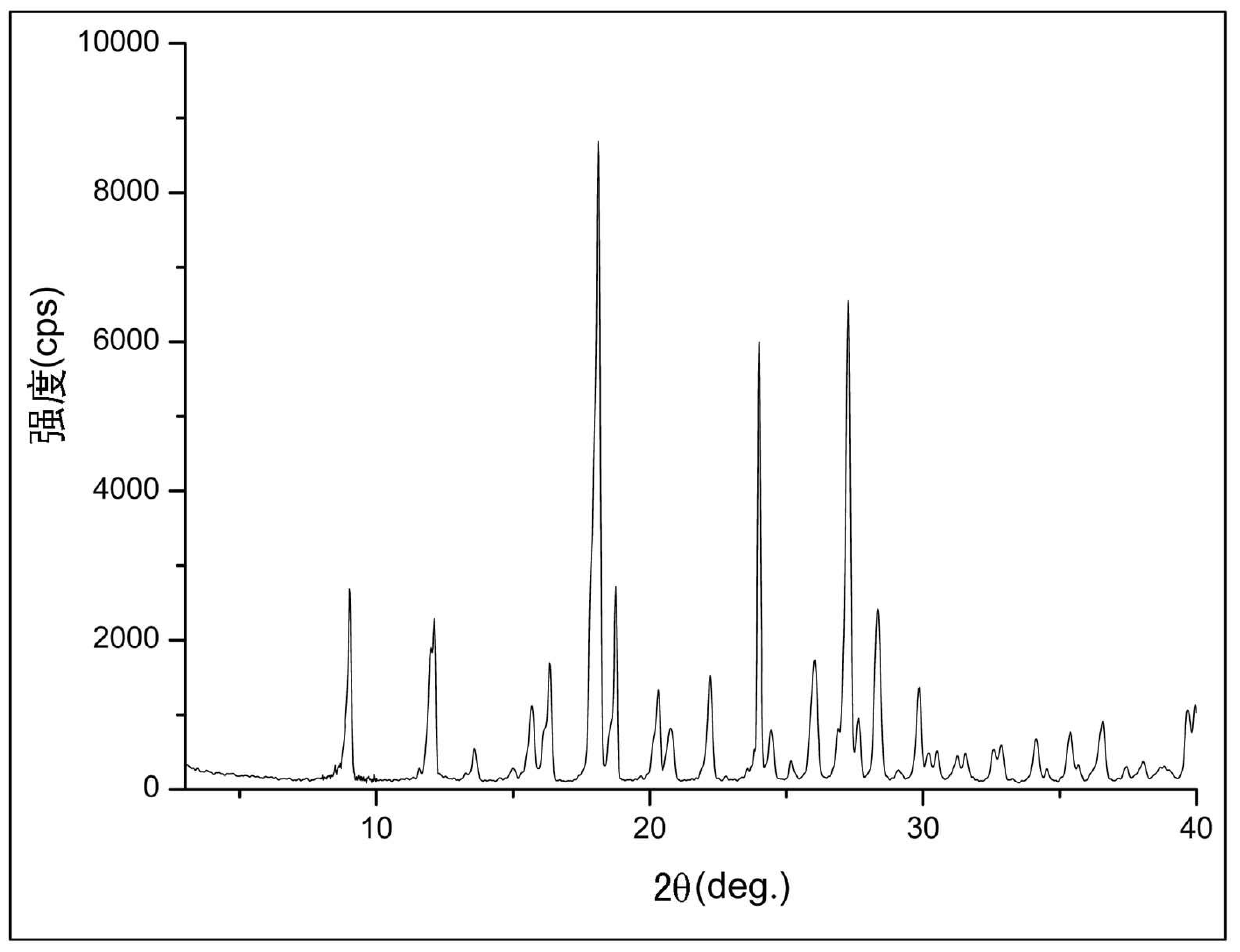
Crystal form of 6, 7-dihydro-6-mercapto-5H-pyrazolo[1,2-alpha][1,2,4] triazoliumchloride and preparation method thereof
ActiveCN101735220AEasy to operateImprove efficiencyOrganic chemistryColumn chromatographyDiffraction spectrum
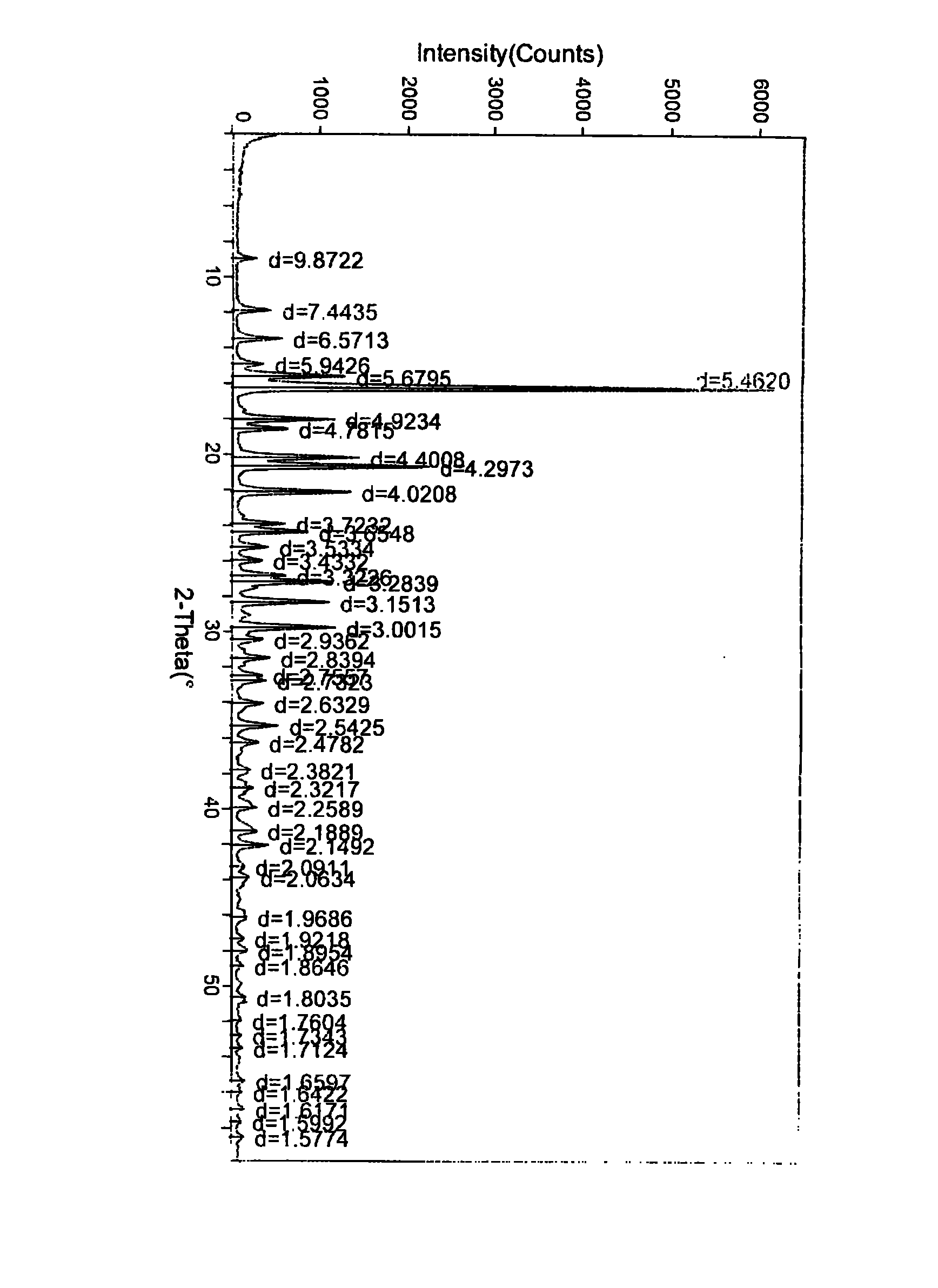
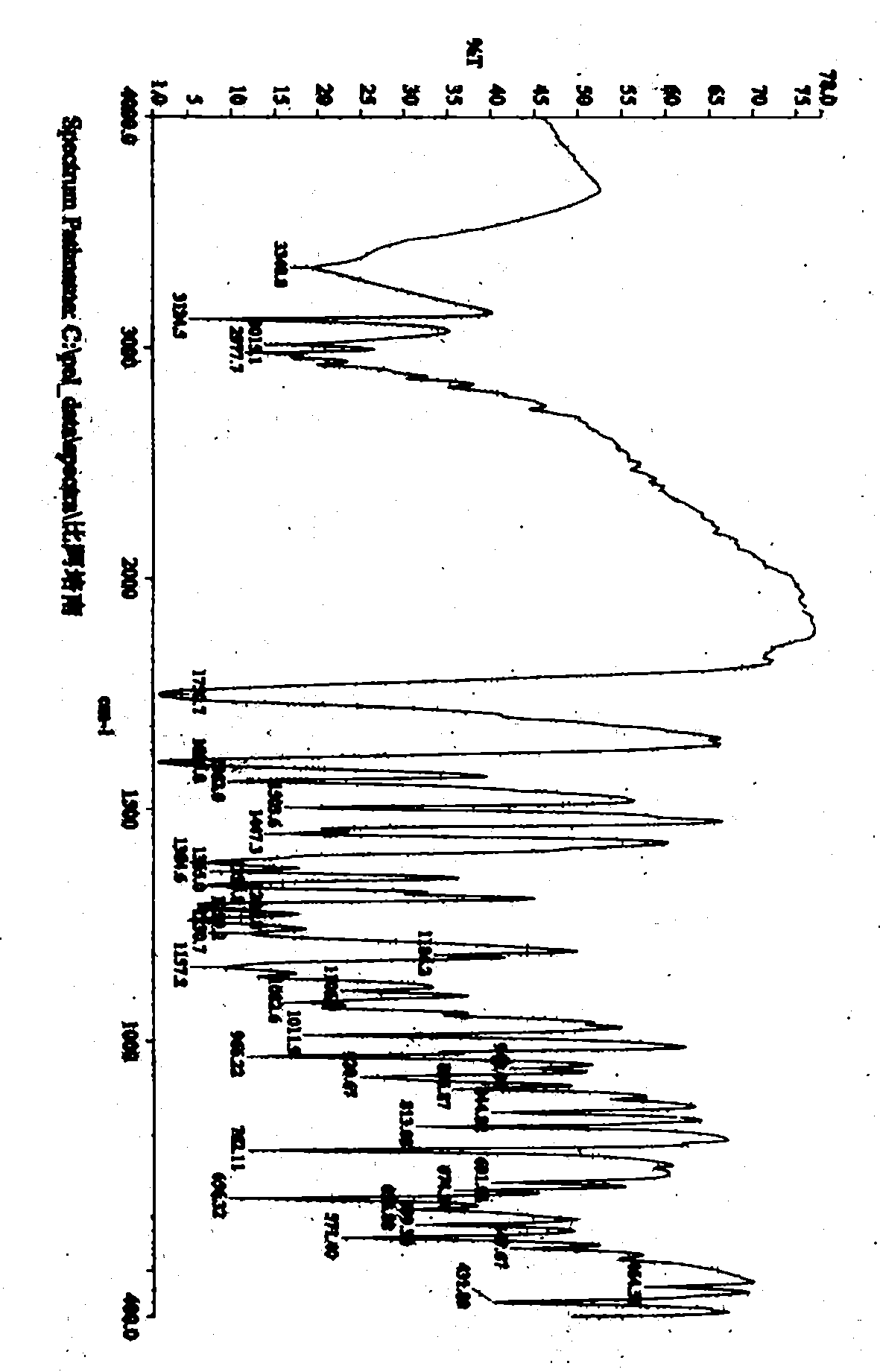
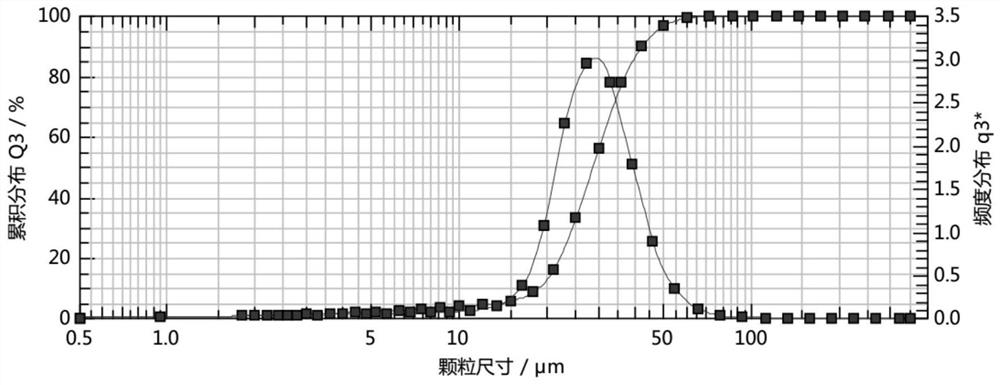
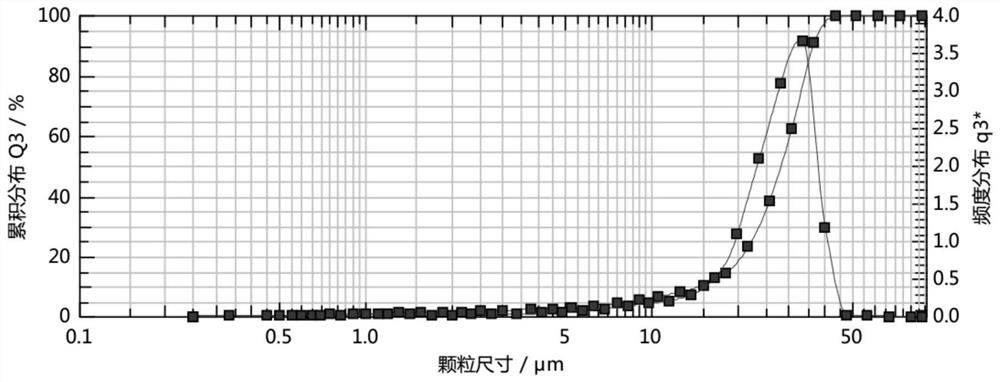
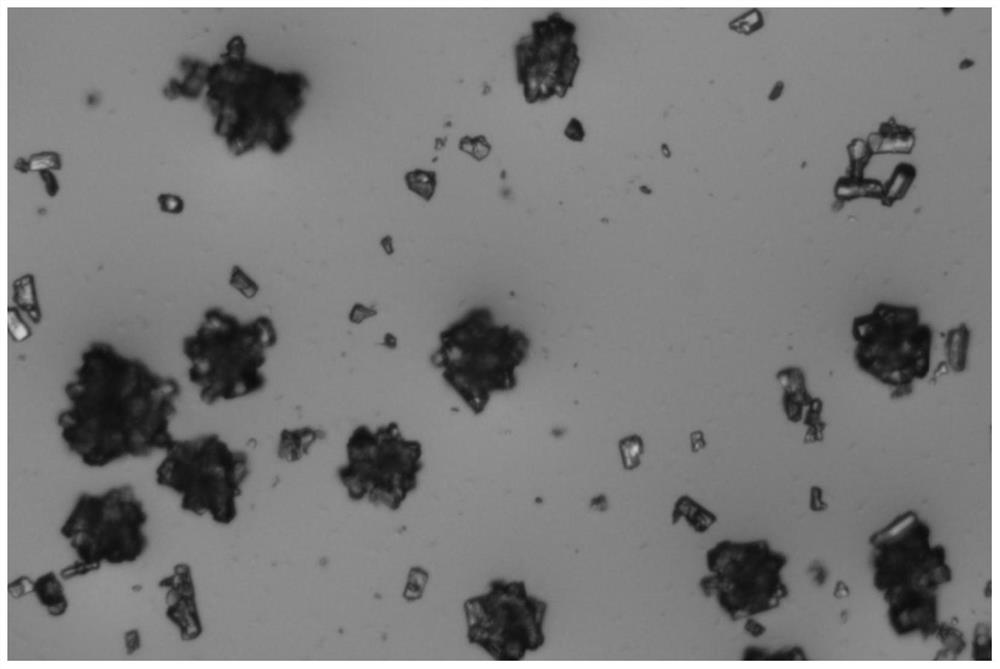
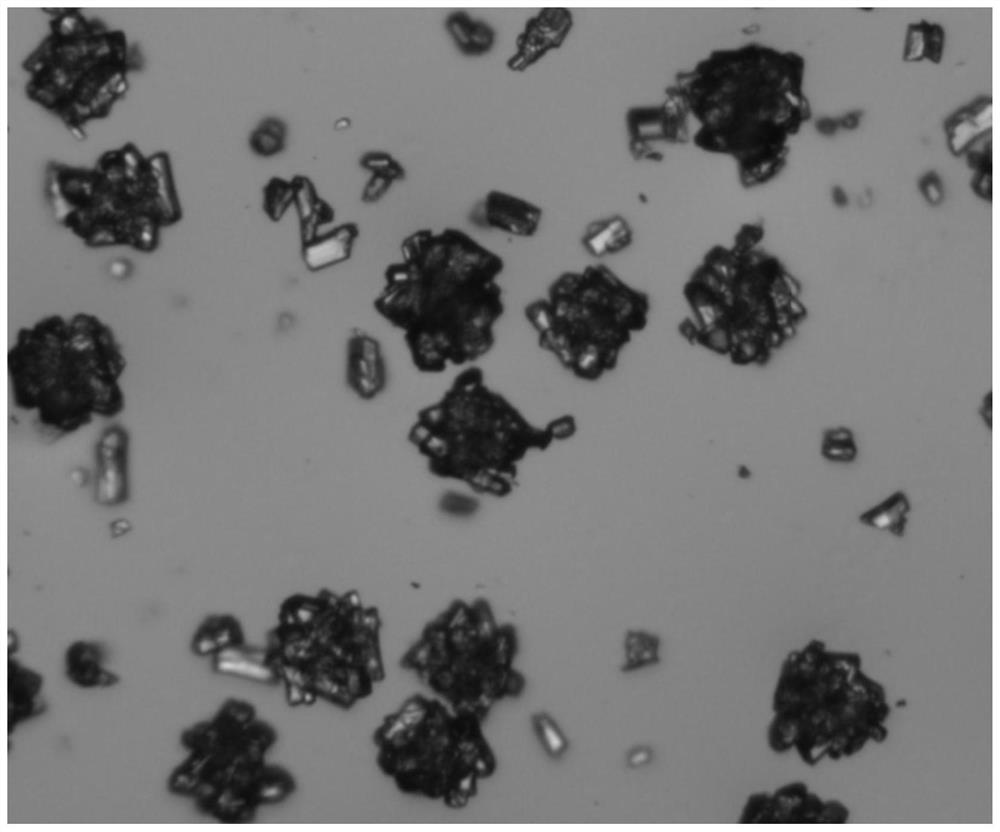
The invention relates to a crystal form of 6, 7-dihydro-6-mercapto-5H-pyrazolo [1,2-alpha][1,2,4] triazoliumchloride and a preparation method thereof. The 6, 7-dihydro-6-mercapto-5H-pyrazolo [1,2-alpha][1,2,4] triazoliumchloride is a key intermediate for synthesizing the carbapenem antibiotic biapenem. The crystal form of 6,7-dihydro-6-mercapto-5H-pyrazolo[1,2-alpha][1,2,4] triazoliumchloride is characterized in that the X-ray diffraction spectrum of powder of the crystal form is represented by an angle of 2theta, and has peaks at 27.487+ / -0.2 and 26.707+ / -0.2, the intensity of the peak is 100% when 2theta is 27.487+ / -0.2, and the ratio of intensity of the peak I / I0 is no less than 60% when 2theta is 26.707+ / -0.2. The 6, 7-dihydro-6-mercapto-5H-pyrazolo[1,2-alpha][1,2,4] triazoliumchloride is prepared by the recrystallization method which abandons the column chromatography and has high efficiency and low cost and is more suitable for industrialized production.
Owner:石药集团中诺药业(石家庄)有限公司 +1
Method for preparing biapenem
ActiveCN101768174AIncrease production capacityThe amount of reaction solvent is smallAntibacterial agentsOrganic chemistrySolubilityOrganic solvent
The invention discloses a method for preparing biapenem, which is applicable to industrialization. The method comprises the following steps: performing a hydrogenation reaction on aprotic polar organic solvent serving as the solvent for a catalytic hydrogenation reaction and H2 in the presence of a catalyst; removing the protective group of a compound in a formula (II); and collecting the biapenem in reaction mixture. By using the method, the usage amount of reaction solvent is small, and the production capability of a pressure reaction still is greatly improved. Moreover, the reaction condition is mild; the operation is simple; and the biapenem can directly be precipitated from water and the organic solvent by using the solubility of the product without buffer, purification of ion exchange resin, concentration of mass aqueous solution or low temperature freezing crystallization, so that the separation and purification operation processes of the product are simplified, and the reaction yield is improved; and the obtained product has high purity, and the method is applicable to industrial and large-scale production.
Owner:SICHUAN KELUN PHARMA CO LTD
Biapenem crystalline solid and preparation method thereof
The invention discloses a biapenem crystalline solid and a preparation method thereof. X ray diffraction spectrogram of the biapenem crystalline powder has a peak at the position where an angle of 2theta is 16.228+ / -0.2. The biapenem crystalline solid is prepared by means of crystallization, three kinds of reagents are used, operation is simple, cost is low, crystalline particles are uniform, solid-liquid separation and dying are convenient and the preparation method is suitable for industrial production. The biapenem crystalline solid prepared with the preparation method has the advantages that purity of crystalline solid is high, and the crystalline solid has good stability in bright, hot and humid environments.
Owner:SICHUAN KELUN PHARMA RES INST CO LTD
Method for preparing biapenem with high purity
ActiveCN101805359AEasy to operateHigh purityOrganic chemistryBulk chemical productionOrganic solventHydrogenation reaction
The invention relates to a method for preparing biapenem with high purity, which takes low-cost Raney N1 as catalyst, and comprises the steps of: leading the catalyst and H2 to have hydrogenation reaction in buffer solution or the mixed solution of the buffer solution and organic solvent, removing protecting group, and then collecting biapenem from the reaction product. The method has mild reaction condition, simple operation, no need of resin purification, high purity of the obtained product and low production cost, and is suitable for industrialized production.
Owner:ZHEJIANG HUAHAI PHARMA CO LTD
Preparation method for biapenem condensation compound and crystalline solid thereof
ActiveCN101747352ALarge crystal grainsLarge particlesAntibacterial agentsOrganic chemistryAlcoholSolvent
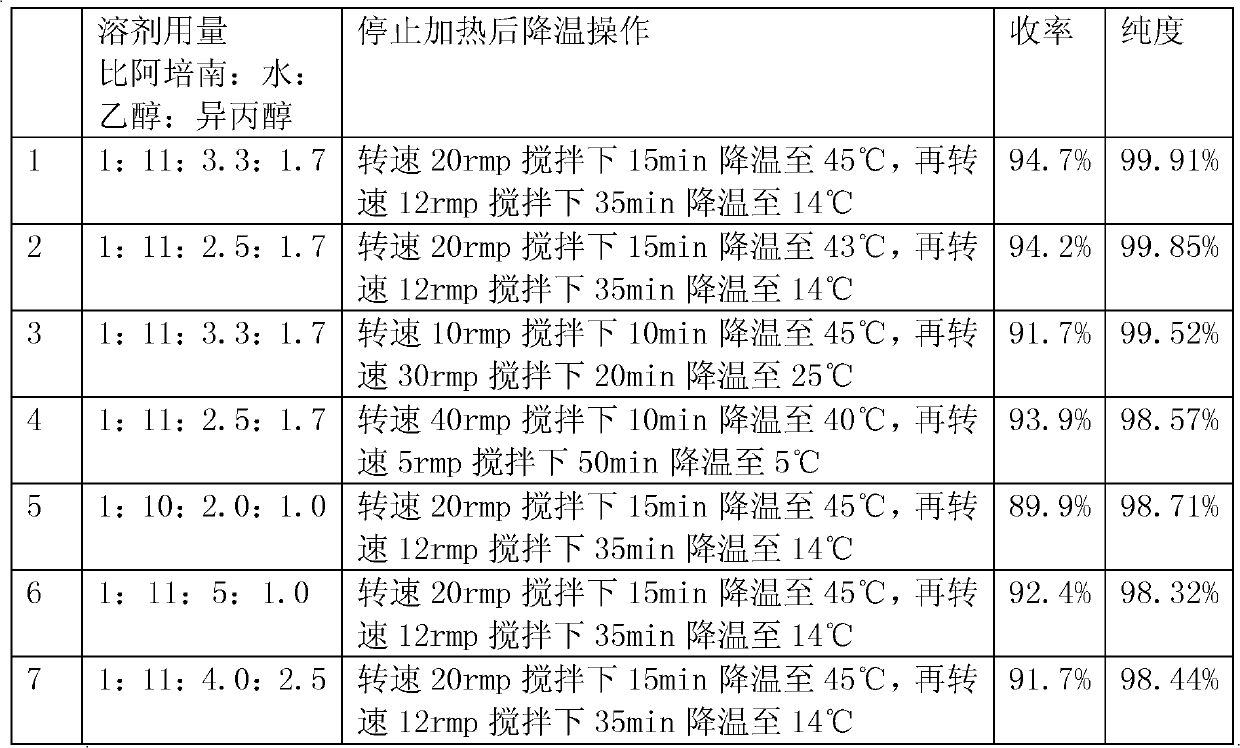
The invention relates to a preparation method for a biapenem condensation compound shown in a formula II and a crystalline solid thereof. Impurities in reaction raw materials can be effectively removed by adding a lower alcohol solvent in a reaction system so as to obtain the crystalline solid of the biapenem condensation compound with high purity. Because particles of the obtained crystalline solid of the biapenem condensation compound are large, the crystalline solid is beneficial to solid-liquid separation and drying, and is more suitable for industrial production.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD +1
Preparation process of biapenem
InactiveCN103570750ASimple processLow cost of industrializationOrganic chemistryChlorideCarboxylic acid
The invention discloses a preparation process of biapenem. The preparation process of the biapenem comprises the following steps: preparing bis(6,7-dihydro-5H-pyrazolyl-[1,2-alpha][1,2,4]-triazolyl ylide-6-yl) disulfide dichloride; preparing dihydro-6-mercapto-5H-pyrazolyl[1,2-alpha][1,2,4]-triazolium chloride; preparing (1R,5S,6S)-2-[(6,7-dihydro-5H-pyrazolo[1,2-alpha][1,2,4]triazolyl-6-yl)sulfenyl-6-[(R)-1-hydroxyethyl]-1-methyl-carbapenem-2-ene-3-p-nitrobenzyl carboxylate chloride; preparing the biapenem; refining the biapenem. The preparation process is simple; no special equipment is required; a solvent is recyclable, so that the industrial production cost is reduced; the product yield is high.
Owner:ANHUI YOUCARE KAIYUE PHARMA
Synthesis method of biapenem ester
ActiveCN101891756ASimple and fast operationEasy to manufactureOrganic chemistryOrganic solventSynthesis methods
The invention relates to a synthesis method of biapenem ester, which comprises the steps of: 1) under the condition of air isolation, adding organic solvent, compound (II), compound (IV), R13P and alkali into a reactor, controlling the reaction temperature, and reacting for more than 15min; 2) carrying out HPLC monitoring, and using acid to adjust a system to acidity after reaction; and 3) crystallizing, cooling the reaction liquid, adding crystallization solvent for stirring and crystallization, filtering and obtaining the target compound (compound I). The invention has simple and convenient operation, can directly crystallize without needing purification in the reaction, and avoids using biapenem branched chain which is compound (III) and is easy in moisture absorption and difficult to prepare and store.
Owner:SHENZHEN HAIBIN PHARMA
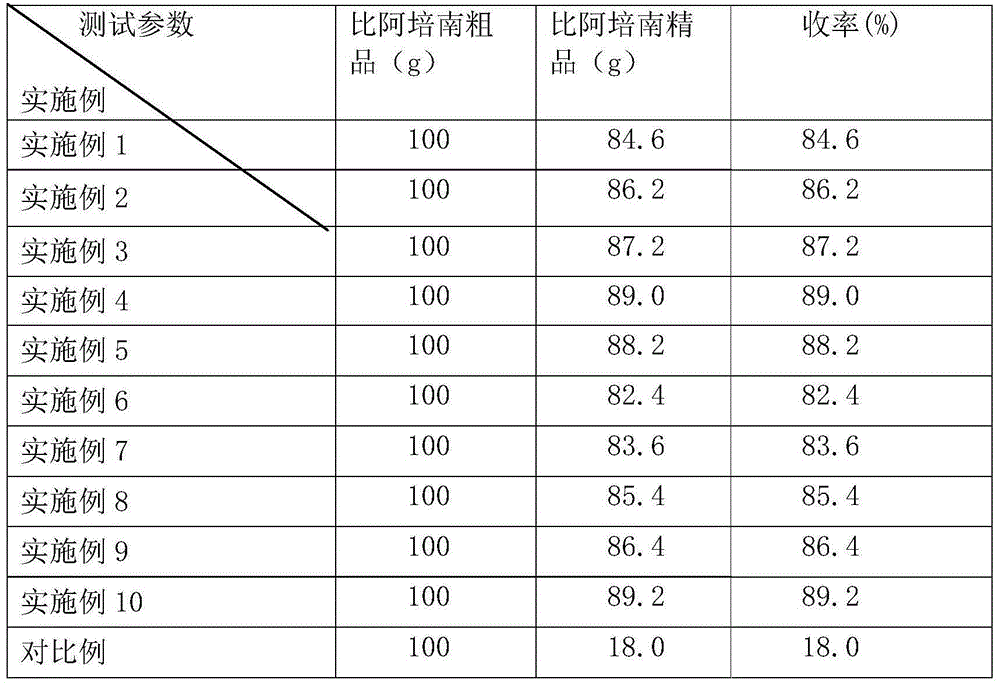
Refining method of biapenem crude product
The invention discloses a refining method of a biapenem crude product. The method comprises the following steps: (1) under room temperature, dissolving the biapenem crude product into a methanoic acid water solution, wherein the percentage mass content of methanoic acid in the methanoic acid water solution is 30 to 80 percent; (2) adding activated carbon into the solution, stirring and destaining for 15 to 30 minutes; (3) filtering filter liquor through a filter membrane of 0.22 micrometers, then transferring the filter liquor into a reaction flask, dropwise adding 800 to 1000 mL of anti-solvent, and stirring for reacting for two hours for crystallization after finishing adding; (4) filtering solid matters in the solution, and drying to obtain a biapenem refined product. According to the method provided by the invention, the compound property is stable, the method is simple, biapenem is easy to produce and can be prepared effectively, the yield of the biapenem is improved, and the use amount of solvents are reduced.
Owner:NANJING SIMCERE DONGYUAN PHARM CO LTD
Preparation method of high-purity biapenem
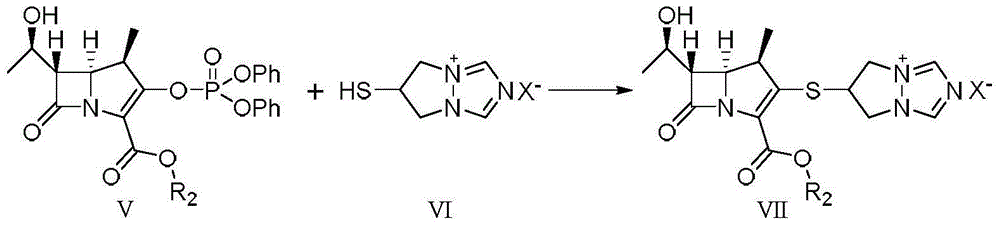
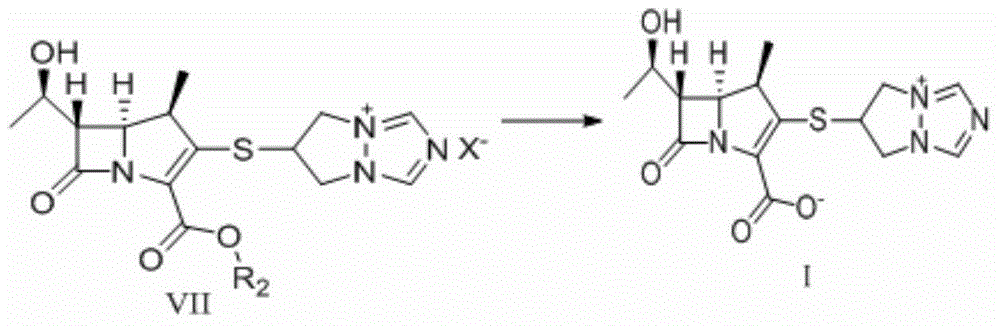
InactiveCN104829633AOvercome stabilityOvercome costsOrganic chemistryBulk chemical productionN dimethylformamideCarboxylic acid
The invention relates to a preparation method of high-purity biapenem, which includes the steps of: (A) carrying out a condensation reaction with a compound represented as the formula V and a compound represented as the formula VI in an acetonitrile solvent in the presence of a less amount of N,N-dimethylformamide and an organic alkali to obtain a compound represented as the formula VII; (B) carrying out a catalytic hydrogenation to the reaction product in the step (A) in a mixed solution composed of an alcohol solvent and a non-proton polarity organic solvent in the presence of a catalyst and an organic alkali to remove a carboxylic acid protective group in the compound represented as the formula VII; and (C) filtering a reaction mixed solution to obtain a filter cake, adding the filter cake in water with stirring to obtain a filtrate, mixing the filtrates, adding an organic solvent under stirring, performing precipitation crystallization, and filtering and drying a product to obtain the biapenem.
Owner:JIANGSU TASLY DIYI PHARMA CO LTD
Method for synthesizing biapenem medicine intermediates
ActiveCN108069998ARoute raw materials are cheap and easy to getSimple stepsGroup 4/14 element organic compoundsBiapenemMedicinal chemistry
The invention discloses a method for synthesizing biapenem medicine intermediates. The method has the advantages that the biapenem medicine intermediates 4-AA are prepared from (R)-3-polyhydroxybutyrate which is a raw material, and the raw material in the routes is inexpensive, is easily available and can be substantially purchased; the method includes simple steps, the various steps are high in yield, and reaction is simple; chiral reagents and chiral resolution are omitted, accordingly, the method is low in cost and high in yield, and reaction conditions are easily available.
Owner:ZHEJIANG GONGSHANG UNIVERSITY +2
Preparation method of biapenem bulk drug
ActiveCN111875622AHigh purityThe status of impurities is clearAntibacterial agentsOrganic chemistryActivated carbonBiapenem
The invention provides a biapenem bulk drug preparation method, which comprises: 1) dissolving a biapenem crude product in water at a certain dissolving temperature T1 to prepare a biapenem crude product aqueous solution; (2) controlling the temperature of the biapenem crude product aqueous solution obtained in the step (1) to be T1 or T2, adding activated carbon with stirring for decolorization,filtering the liquid, and cooling the filtrate to T3 for later use; 3) dropwise adding the filtrate obtained in the step 2) into a mixed solvent of acetone and ethanol, which is cooled to T4 in advance, and crystallizing the filtrate; 4) growing crystal; and 5) separating, washing and drying the crystals separated out in the step 4) to obtain the biapenem bulk drug.
Owner:SHENZHEN HAIBIN PHARMA +2
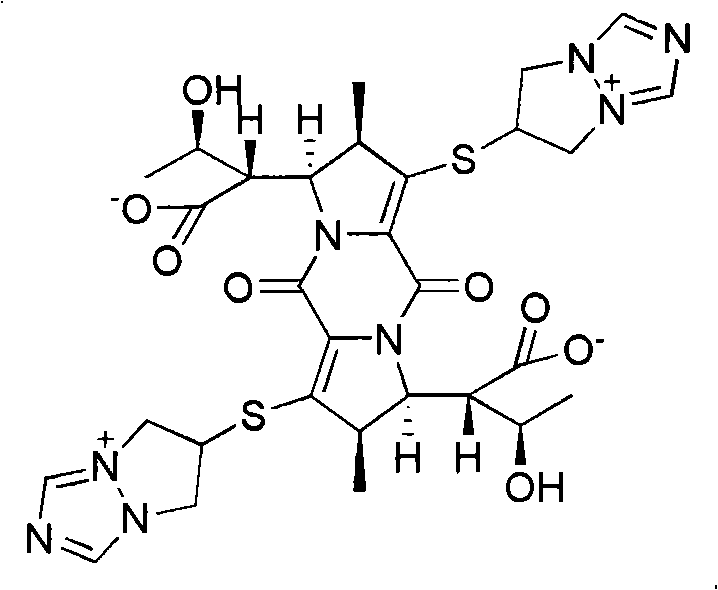
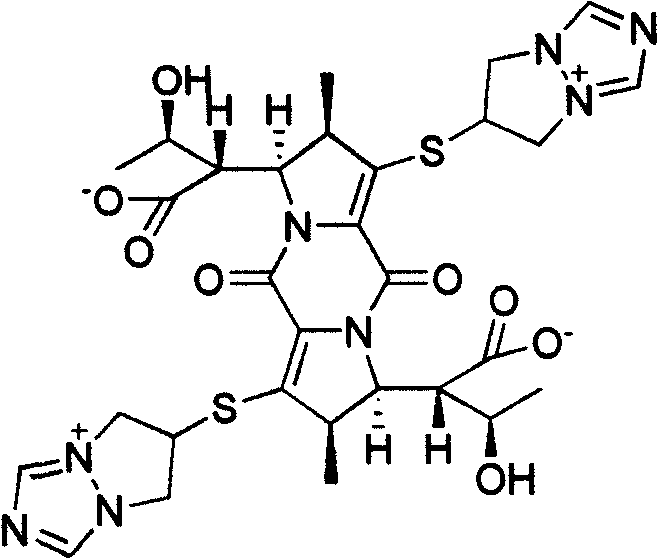
Biapenem crystalline compound and composition powder-needle thereof
ActiveCN102584862AImprove stabilityImprove bioavailabilityAntibacterial agentsOrganic active ingredientsSodium benzoateBiapenem
The invention relates to a biapenem crystalline compound, which is measured by using a powder X-ray diffraction measurement method. An X-ray powder diffraction pattern indicated by a diffraction angle of 2 theta + / -0.2 degree shows feature diffraction peak at the positions of 5.9 degrees, 6.5 degrees, 10.5 degrees, 14.8 degrees, 15.6 degrees, 16.3 degrees, 17.4 degrees, 19.8 degrees, 22.9 degrees, 23.7 degrees, 25.1 degrees, 28.4 degrees and 34.7 degrees. The invention further relates to a biapenem composition powder-needle comprising the biapenem crystalline compound. Components of the composition powder-needle are 95 to 100 parts of the biapenem crystalline compound and 0.1 to 1 part of sodium benzoate.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD +2
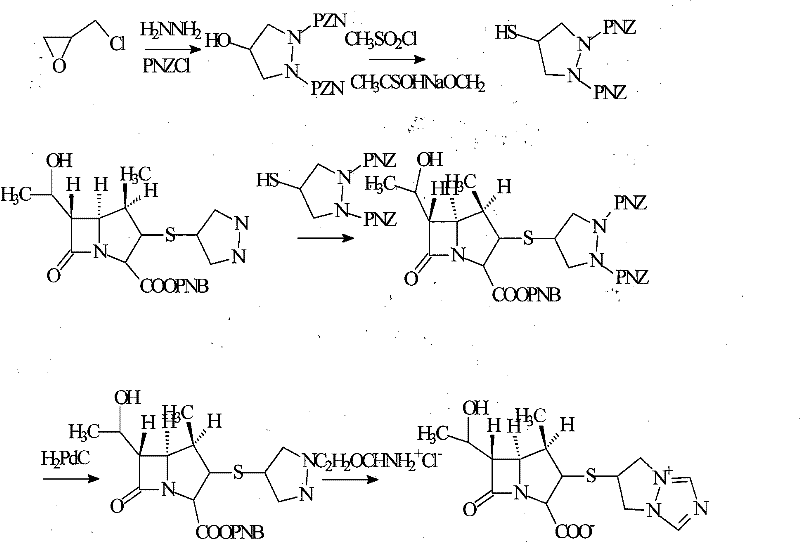
Preparation method of biapenem
The invention belongs to the technical field of medicines, and discloses a preparation method of biapenem. The method comprises the following steps: p-nitrobenzyl (1R,5R,6S)-6-[(1R)-1-hydroxyethyl]-2-[(diphenylphosphono)oxy]-1-methylcarboxyl pen-2-em-3-carboxylate and 4-sulfydryl-N,N-bis(p-nitrobenzyloxycarbonyl)pyrazolidine are used as raw materials, substitution is performed, hydrogenation is performed and cyclization is performed to synthesize the biapenem. The method provided by the invention overcomes the defects of a long reaction route, easy degradation of raw materials in the reactionprocess, a low yield and the like in the prior art, and is more suitable for industrial production.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD +2
Improved Biapenem preparation method
Owner:NANJING SIMCERE DONGYUAN PHARM CO LTD +1
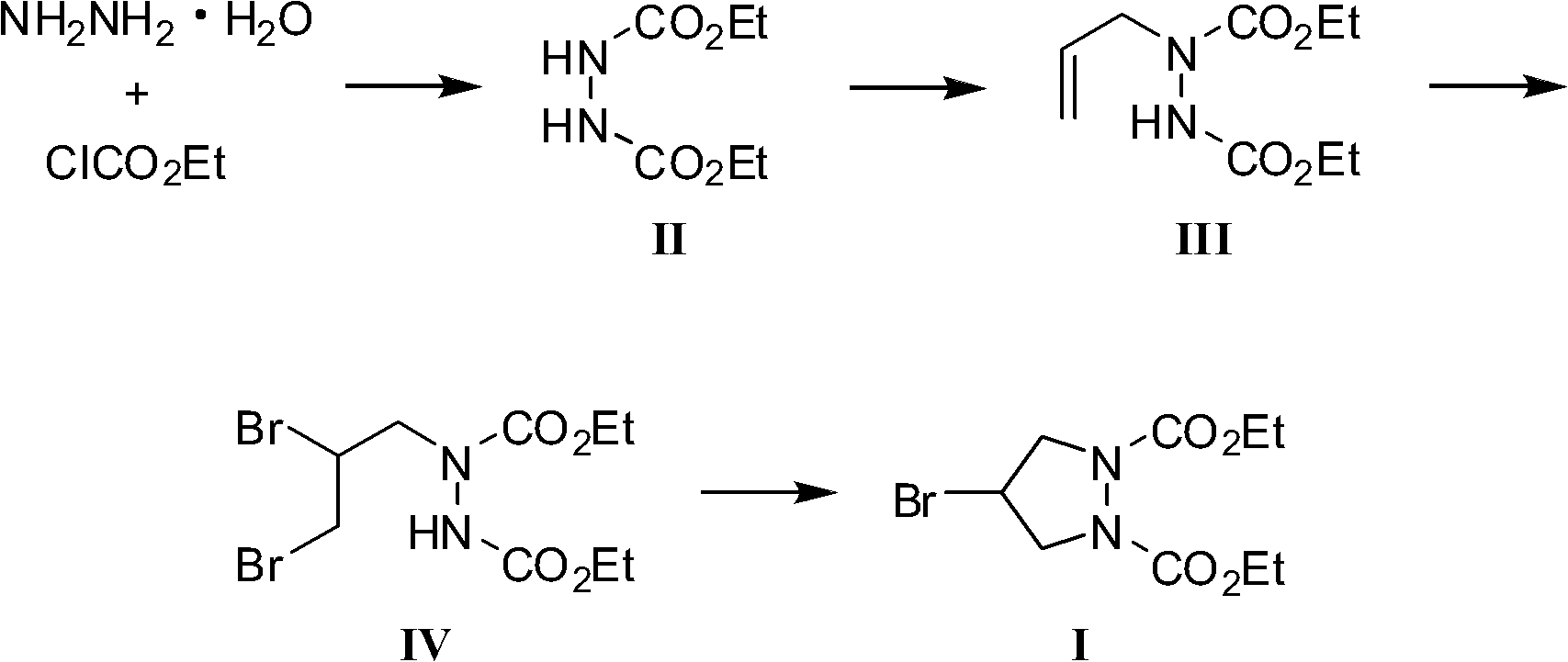
Method for preparing green innovative side chain for biapenem
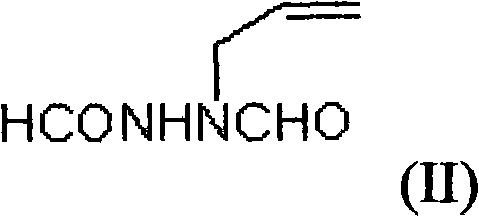
The invention provides a method for preparing a green innovative side chain for biapenem. The method comprises the following steps of: reacting a compound and a compound NH2NH2.H2O under the action of a compound EtOH to obtain a compound HCONHNHCHO(I); under an action of a compound K2CO3 and a compound, reacting the HCONHNHCHO(I) to obtain a compound (II); reacting the compound (II) with compounds KBr and H2O2 in the presence of a catalyst to form a compound (III); and reacting the compound (III) the a compound K2CO3 reacted to obtain a final compound.
Owner:湖南欧亚药业有限公司
Novel crystal form of biapenem and synthetic method thereof
InactiveCN102268024BHygroscopic differenceStability differenceAntibacterial agentsOrganic active ingredientsPharmaceutical drugCarboxylic acid
The invention relates to crystal form I of (1R,5S,6S)-2-[(6,7-dihydro-5H-pyrazolo[1,2-alpha]-[1,2,4]triazole-hexabase)]sulfur-6R-1-hydroxyethyl]-1-methyl-carbapenem-3-carboxylate (biapenem), a preparation method of crystal form I, and a pharmaceutical composition containing the crystal form I of biapenem and one or more pharmaceutically acceptable carriers, excipients or diluents. Formula (I) is as described in the specification.
Owner:HARBIN PHARMA GRP CO LTD GENERAL PHARMA FACTORY
Biapenem crystalline compound and composition powder-needle thereof
ActiveCN102584862BImprove stabilityImprove bioavailabilityAntibacterial agentsPowder deliverySodium benzoateBiapenem
The invention relates to a biapenem crystalline compound, which is measured by using a powder X-ray diffraction measurement method. An X-ray powder diffraction pattern indicated by a diffraction angle of 2 theta + / -0.2 degree shows feature diffraction peak at the positions of 5.9 degrees, 6.5 degrees, 10.5 degrees, 14.8 degrees, 15.6 degrees, 16.3 degrees, 17.4 degrees, 19.8 degrees, 22.9 degrees, 23.7 degrees, 25.1 degrees, 28.4 degrees and 34.7 degrees. The invention further relates to a biapenem composition powder-needle comprising the biapenem crystalline compound. Components of the composition powder-needle are 95 to 100 parts of the biapenem crystalline compound and 0.1 to 1 part of sodium benzoate.
Owner:SHANDONG LUOXIN PHARMA GRP CO LTD +2
Biapenem composition freeze-dried powder for injection
InactiveCN103536559AReduced activityImprove antibacterial propertiesAntibacterial agentsOrganic active ingredientsSolubilityChitosan nanoparticles
The invention provides a biapenem composition freeze-dried powder for injection, and belongs to the field of medicine and medicine preparation technology. The biapenem composition freeze-dried powder comprises following raw material ingredients, by weight, 1 part of biapenem, 0.2 to 10 parts of chitosan nanoparticle, and 91.18 to 93.08 parts of injection water. Advantages of the biapenem composition freeze-dried powder are that: the chitosan nanoparticle is used as a freeze-dried skeleton agent of the freeze-dried powder injection instead of mannitol, so that active effects of mannitol on human bodies are avoided, problems of stability and solubility are solved at the same time, antibacterial effect of biapenem is improved significantly, dosage of biapenem in clinic is reduced, and adverse reaction of biapenem is reduced. The biapenem composition freeze-dried powder is successfully developed, and is capable of providing novel ideas for clinical application of biapenem.
Owner:HAINAN WEI KANG PHARMA QIANSHAN
Preparation method of biapenem
The invention discloses a preparation method of biapenem. The preparation method comprises the following steps: (a) by using a compound shown in a formula III as a raw material, adding a palladium catalytic system with the weight ratio of triphenyl phosphine and tri(triphenyl phosphine) palladium of (1-3) to 1 into an organic solvent, and stirring to reaction for 1.5-2 hours at 10-50 DEG C, wherein the addition of tri(triphenyl phosphine) palladium is 1.5-2% of formula III; (b) adding water into a reaction liquid in the step (a) to dilute, washing by dichloroethane or dichloromethane, and decompressing and concentrating to obtain an aqueous solution, wherein the water phase contains biapenem; and (c) adjusting the pH of the biapenem aqueous solution obtained in the step (b) to 5-6 by an organic solvent, adding acetone or ethanol, cooling to below room temperature, stirring and carrying out crystallization for 2-3 hours to separate out biapenem crystals. In the reaction process, hydrogen is not used, and the reaction does not generate hydrogen too. The reaction condition is mild, and the production safety is greatly improved. In the reaction, buffer salt needs not to be added to control the pH of the reaction liquid, so that the preparation method is suitable for industrialized production and simple to operate.
Owner:SHANGHAI NEW ASIA PHARMA +1
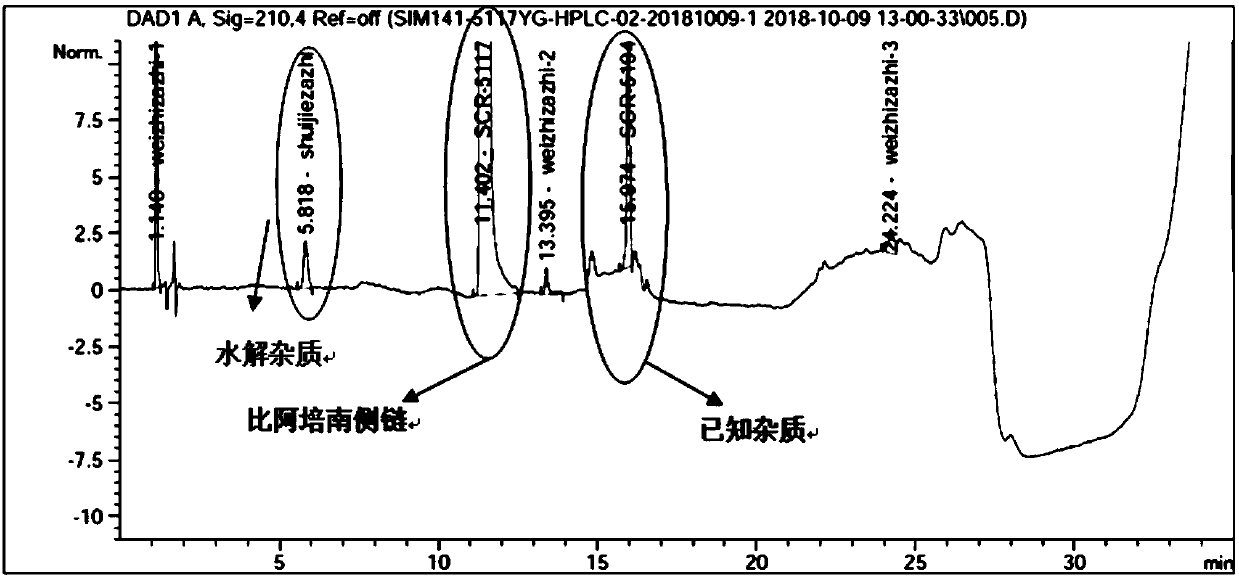
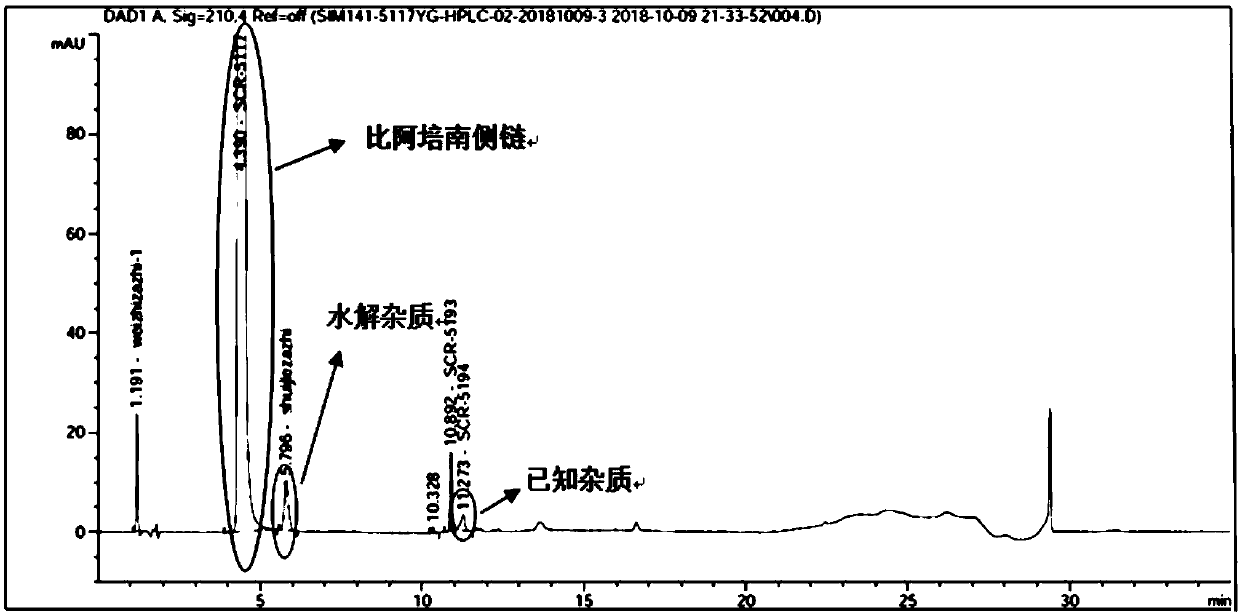
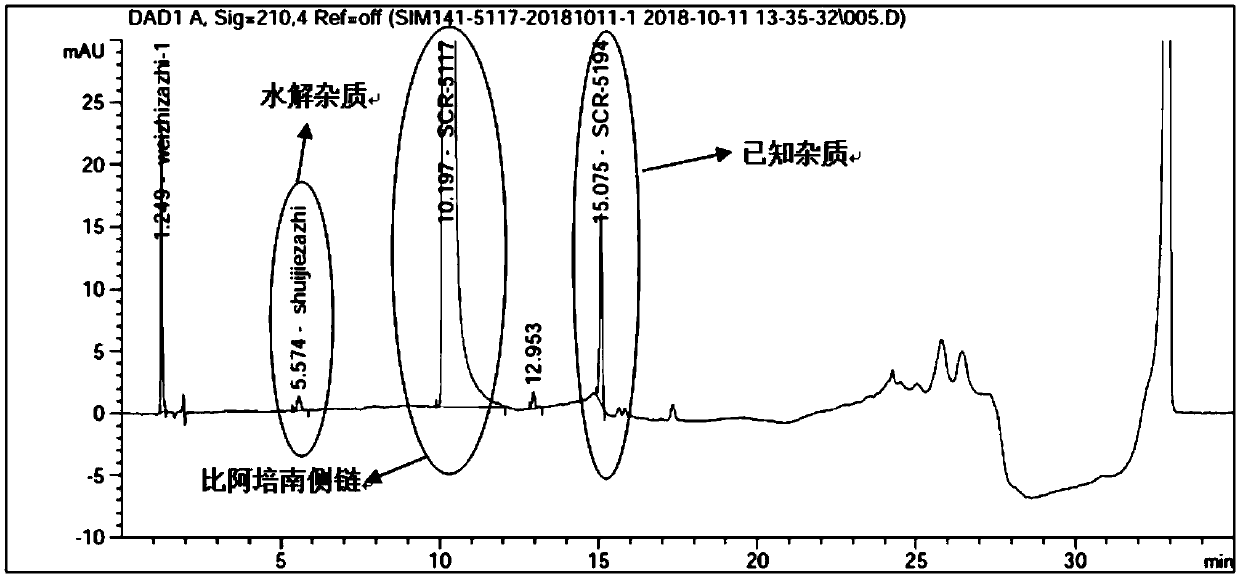
HPLC detection method for biapenem side chain related substances
ActiveCN111366644AHigh detection sensitivityImprove accuracyComponent separationSide chainOrganosolv
The invention discloses an HPLC detection method of biapenem side chain related substances. Linear gradient elution is carried out by using octadecylsilane chemically bonded silica as a filler and using a mixed solution of a buffer solution of which the pH value is 3.0-4.5 and an organic solvent as a mobile phase. The detection sensitivity of the detection method provided by the invention is obviously improved compared with that of related substances of the apixem side chain, the separation degree of each impurity and a main peak is very good, and the increase of hydrolyzed impurities can be effectively controlled.
Owner:JIANGSU SIMCERE PHARMA +1
Preparation method of biapenem intermediate
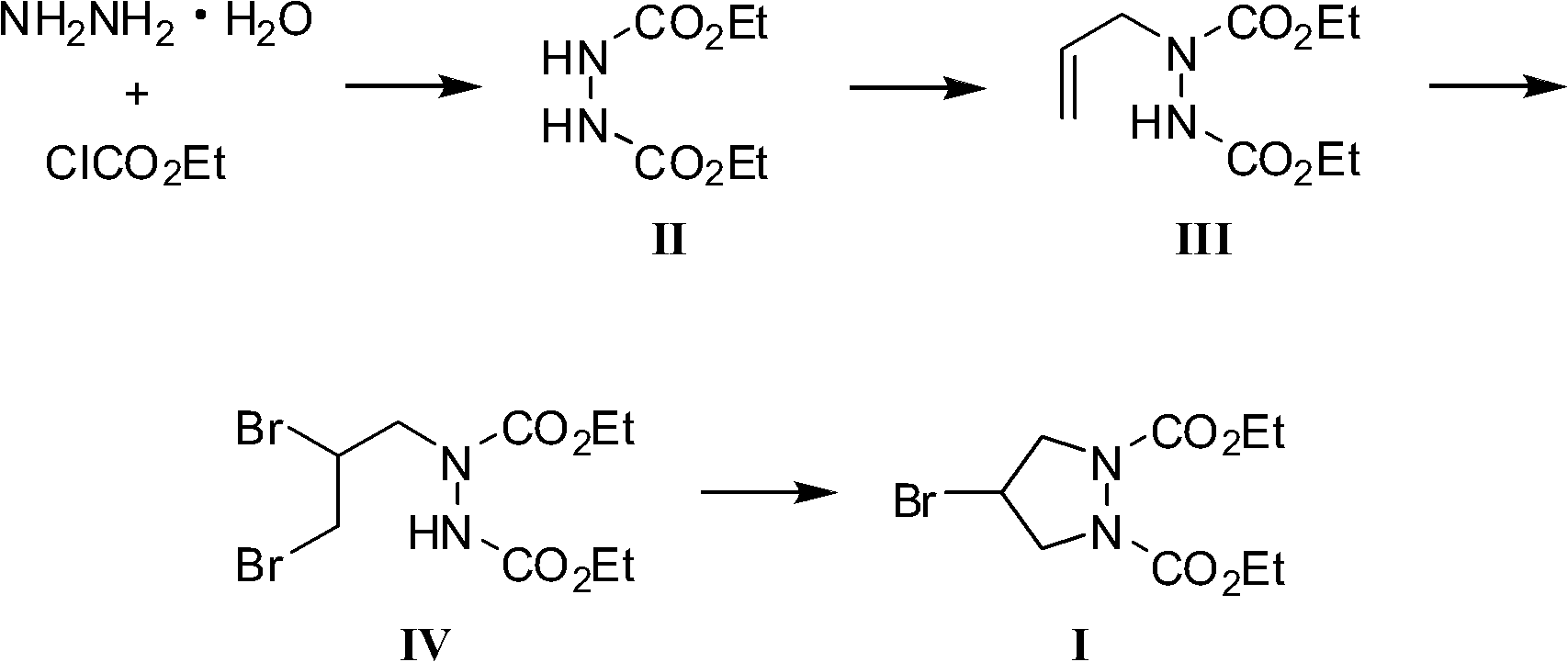
InactiveCN102180831AWide variety of sourcesSufficient supplyOrganic chemistryEthyl chloroformateHydrazine compound
The invention provides a preparation method of a biapenem intermediate and relates to a preparation method of an antibiotic medicament biapenem intermediate 4-bromine-1,2-ethoxycarbonyl pyrazolidine. The method comprises the following steps: successively carrying out a reaction on hydrazine hydrate as a raw material and ethyl chloroformate and allyl bromide; and then carrying out addition reaction with bromide, and finally, carrying out cyclization reaction under the alkaline condition so as to obtain the product. The invention provides a bran-new synthesis route; and used raw material sources are wide and abundant, the prices of the raw materials are cheap, reaction condition is mild, process is simple, reactions in various steps are conventionally operated, and product cost is reduced.
Owner:TONGJI UNIV
Application of biapenem in preparation of medicine for preventing and treating bovine enterovirus infection
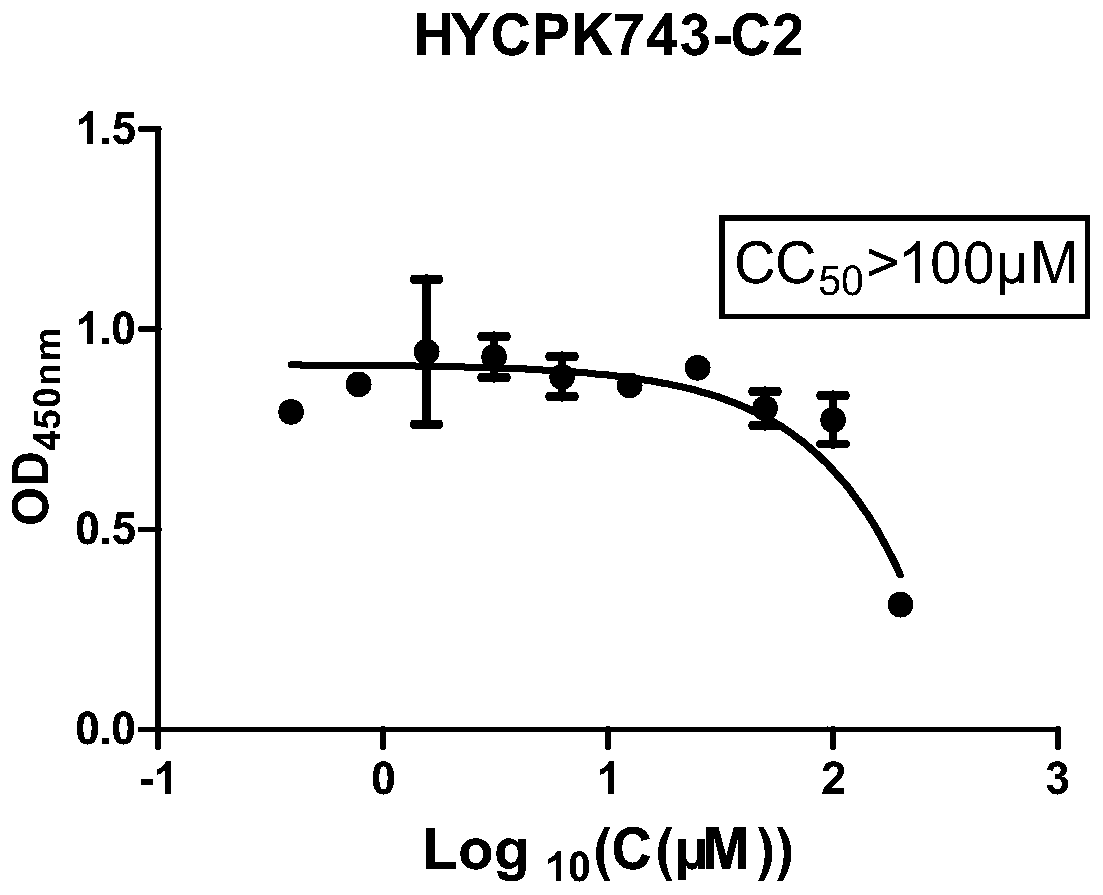
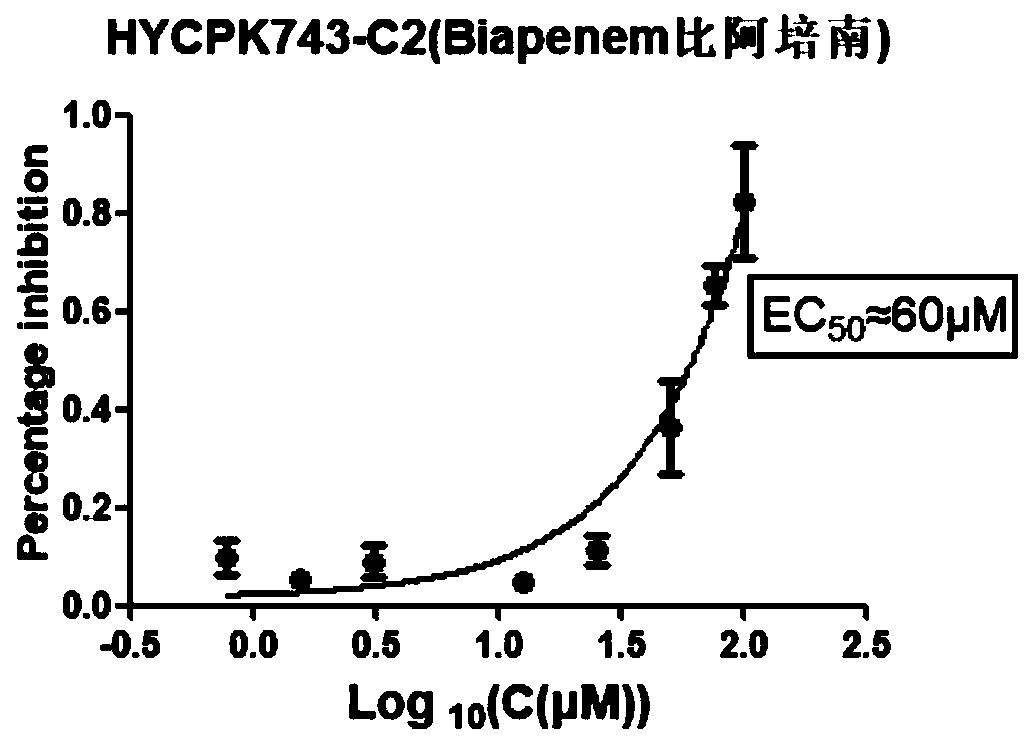
ActiveCN110812357APrevent proliferationLow toxicityOrganic active ingredientsAntiviralsBovine enterovirusChemical compound
The invention provides an application of biapenem in preparation of a medicine for preventing and treating bovine enterovirus infection. The compound biapenem can effectively inhibit proliferation ofbovine enterovirus and has low toxicity to cells, and experiments prove that the median toxicity concentration (CC50) of biapenem to MDBK cells is greater than 100 [mu]M, and the median effective concentration (EC50) of biapenem to bovine enterovirus is 60 [mu]M; the treatment index of biapenem to bovine enterovirus is greater than 1.67, which indicates that biapenem has a prospect of being developed into a drug for preventing and / or treating bovine enterovirus infection, opens up the new drug application for biapenem, lays an experimental foundation for developing efficient and specific anti-BEV drugs, and provides a new visual field.
Owner:DAIRY CATTLE RES CENT SHANDONG ACADEMY OF AGRI SCI +1
Preparation method of biapenem
ActiveCN102731534ASolve dissolveReduce degradationOrganic chemistryBulk chemical productionBiapenemSolvent
The invention relates to a preparation method of biapenem as shown in the formula 1. The method comprises the following steps of: using a biapenem intermediate I as a raw material, using a single solvent--water as a reaction solvent and conducting a hydrogenated deprotection reaction in the presence of alkali and a catalyst. The single solvent water is used as a reaction solvent, thus solving the problem of dissolving the catalyst by the reaction solvent, simplifying post-treatment operational step, reducing product degradation and raising product purity. In addition, the preparation method is economical, safe and environmentally friendly, and is more suitable for industrial operation at large scale.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD +1
Refining method of biapenem crude product
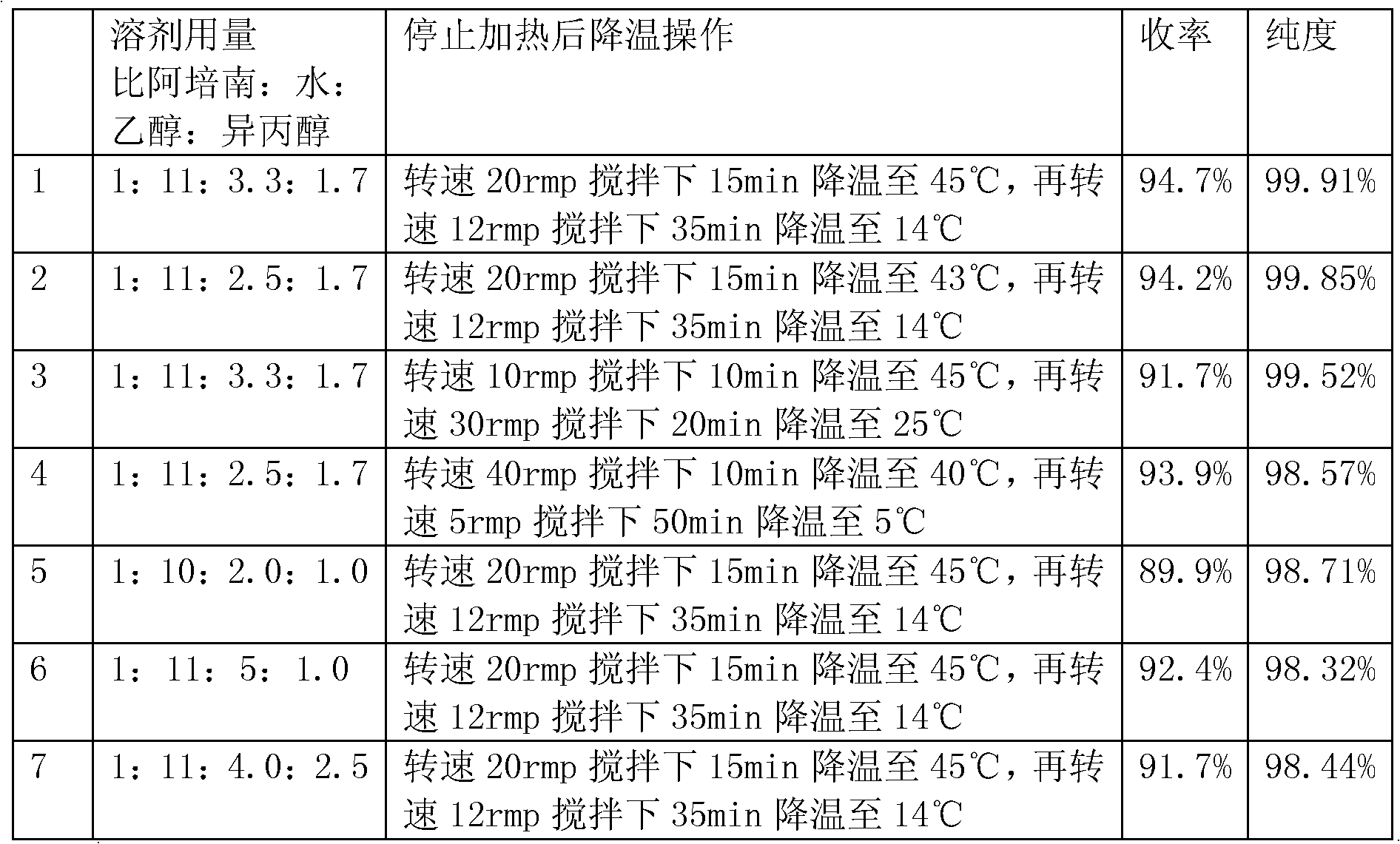
The invention belongs to the technical field of chemical pharmacy, and particularly relates to a refining method of a biapenem crude product. According to the invention, a biapenem refining process is optimized, and crystallization is carried out by stages under different temperature conditions by adopting a reverse crystallization mode of dropwise adding a feed liquid into a crystallization agent. The temperature of first crystallization is-10 to 10 DEG C, crystallization is carried out at a low temperature, crystals can be rapidly separated out, the form of the crystals is easy to control, and the granularity of the crystals of the obtained product is small; the temperature of secondary crystallization is 5-30 DEG C, and crystallization is carried out at a relatively high temperature, so that solvent residues of the product can be reduced. The product prepared by the method disclosed by the invention is short in redissolution time, high in purity, high in yield and good in stability, the crystal form of the product is consistent with that of an original product, and a powerful guarantee is provided for product consistency evaluation.
Owner:ZHUHAI UNITED LAB
Biapenem dimer and preparation method thereof
The invention relates to a biapenem dimer with a structure shown in the following formula and a preparation method and an application thereof. The preparation method comprises the steps of: weighing biapenem raw material, dissolving in acidic water solution as solvent to obtain a solution; standing at 25-100DEG C (at 25-50DEG C for 24 hours and at 51-100DEG C for 1-4 hours), and filtering to obtain filtrate; and separating the filtrate with reversed phase high performance liquid chromatography, and preparing the dimer. The biapenem dimer can be used as a reference substance of biapenem impurities to facilitate the control of the amount of biapenem and related preparations thereof.
Owner:NANJING SIMCERE DONGYUAN PHARM CO LTD +1
Preparation method of biapenem
ActiveCN102212077BReduce dosageEasy to operateOrganic chemistryBulk chemical productionOrganic baseKetone
The invention discloses a preparation method of biapenem. The method comprises the following steps of: undergoing a catalytic hydrogenation reaction on a compound which is shown as a formula I and serves as a raw material and H2 in a mixed solvent of water and an organic solvent; adding an organic base immediately for regulating the pH value after the reaction; adding an organic solvent for precipitating biapenem crystals; and recrystallizing the crystals in water, organic acid and ethanol or ketone to obtain a refined biapenem product. The preparation method has the advantages of easiness for operating, no need of adjusting the pH value with a buffer salt during hydrogenation, no need of resin purification after hydrogenation, no need of special equipment, greatly-lowered water consumption, high yield and high product purity, and is suitable for industrial production.
Owner:SHANGHAI INST OF PHARMA IND CO LTD +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com

![Crystal form of 6, 7-dihydro-6-mercapto-5H-pyrazolo[1,2-alpha][1,2,4] triazoliumchloride and preparation method thereof Crystal form of 6, 7-dihydro-6-mercapto-5H-pyrazolo[1,2-alpha][1,2,4] triazoliumchloride and preparation method thereof](https://images-eureka.patsnap.com/patent_img/18c1e736-fe04-47e4-bfc2-a858f5e0ca1e/H2008100797329E0000011.PNG)
![Crystal form of 6, 7-dihydro-6-mercapto-5H-pyrazolo[1,2-alpha][1,2,4] triazoliumchloride and preparation method thereof Crystal form of 6, 7-dihydro-6-mercapto-5H-pyrazolo[1,2-alpha][1,2,4] triazoliumchloride and preparation method thereof](https://images-eureka.patsnap.com/patent_img/18c1e736-fe04-47e4-bfc2-a858f5e0ca1e/H2008100797329E0000021.PNG)
![Crystal form of 6, 7-dihydro-6-mercapto-5H-pyrazolo[1,2-alpha][1,2,4] triazoliumchloride and preparation method thereof Crystal form of 6, 7-dihydro-6-mercapto-5H-pyrazolo[1,2-alpha][1,2,4] triazoliumchloride and preparation method thereof](https://images-eureka.patsnap.com/patent_img/18c1e736-fe04-47e4-bfc2-a858f5e0ca1e/H2008100797329E0000031.PNG)