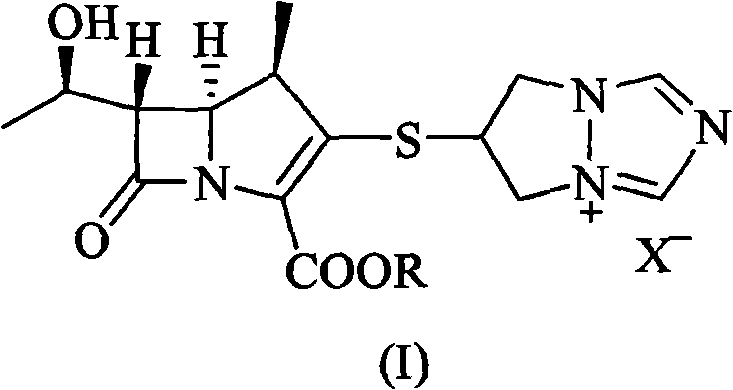
Method for preparing biapenem
A biapenem and aprotic technology, which is applied in the field of synthesis of pharmaceutical compounds, can solve the problems of increasing the cost of recycling solvents, slowing down production progress, and being unfavorable for industrial scale production, so as to facilitate large-scale production, improve production capacity, easy separation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
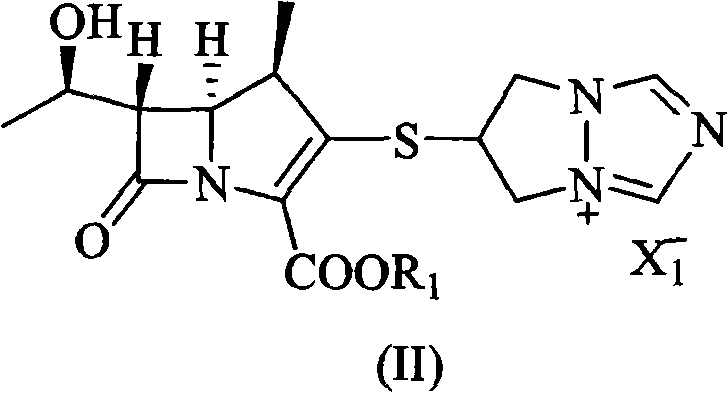
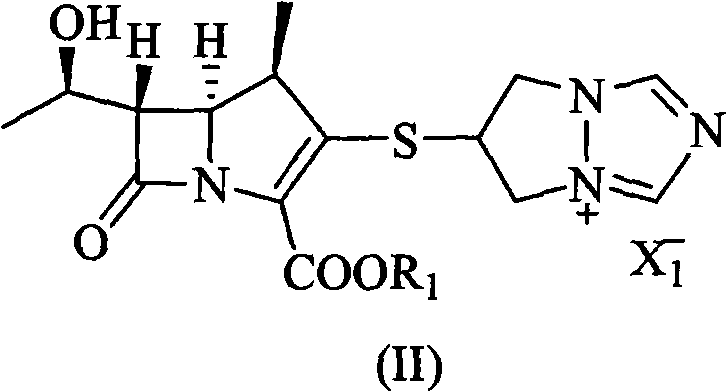
[0032] 6-[[(4R,5S,6S)-2-(4-nitrobenzyloxycarbonyl)-6-[(R)-1-hydroxyethyl]-4-methyl-7-oxo-1- Azabicyclo[3.2.0]hept-2-en-3-yl]thio]-6,7-dihydro-5H-pyrazolo[1,2-a][1,2,4]tri Add 30g (57.5mmol) of azolium chloride salt, add 80ml of N,N-dimethylformamide, stir to dissolve, add 8g (5%) of platinum carbon, and control the hydrogen pressure at 8Kg / cm 2 , reacted at 30°C for 2.5h, added the reaction mixture to 700ml of water, filtered, added 2500ml of ethanol to the filtrate, stirred for 1h, filtered, washed the solid with 2×30ml of ethanol, dried under reduced pressure to obtain 15.3g of white solid of biapenem (43.7 mmol), yield 72.9%.
[0033] Elemental analysis: theoretical value C: 51.42; H: 5.18; N: 15.99
[0034] Test value C: 51.48; H: 5.27; N: 15.87
[0035] IR(KBr)cm -1 : 3345.2, 3124.2, 1750.7, 1601.0, 1563.5
[0036] 1 HNMR (D 2 O, internal standard TMS): δ1.23 (3H, d, J = 7.2Hz); 1.28 (3H, d, J = 6.4Hz); 3.36-3.40 (1H, m); 3.51 (1H, dd, J = 6.0Hz, 2.8Hz); 4.23-4.30(2...
Embodiment 2
[0038] 6-[[(4R,5S,6S)-2-(4-nitrobenzyloxycarbonyl)-6-[(R)-1-hydroxyethyl]-4-methyl-7-oxo-1- Azabicyclo[3.2.0]hept-2-en-3-yl]thio]-6,7-dihydro-5H-pyrazolo[1,2-a][1,2,4]tri Add 30g (57.5mmol) of azolium chloride salt, add 110ml of N-methylpyrrolidone, stir to dissolve, add 3g of platinum dioxide, and control the hydrogen pressure to 4Kg / cm 2 , reacted at 35°C for 4h, added the reaction mixture to 800ml of water, filtered, added 1600ml of acetone to the filtrate, stirred for 1h, filtered, washed the solid with 2×30ml of acetone, dried under reduced pressure to obtain 16g (45.7mmol) of biapenem as a white solid , yield 76.2%.
Embodiment 3
[0040] 6-[[(4R,5S,6S)-2-(4-methoxybenzyloxycarbonyl)-6-[(R)-1-hydroxyethyl]-4-methyl-7-oxo-1 -Azabicyclo[3.2.0]hept-2-en-3-yl]thio]-6,7-dihydro-5H-pyrazolo[1,2-a][1,2,4] Triazolium chloride salt 30g (59.2mmol), add N,N-dimethylacetamide 90ml, stir to dissolve, add palladium carbon (5%) 8g, control hydrogen pressure 6Kg / cm 2 , react at room temperature for 3h, add the reaction mixture to 900ml of water, filter, add 2500ml of tert-butanol to the filtrate, stir for 1h, filter, wash the solid with 2×30ml of tert-butanol, and dry under reduced pressure to obtain 15.9g of white solid Biapenem (45.4 mmol), yield 75.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com