Preparation method of biapenem
A technology of biapenem and organic solvent, applied in the field of preparation of biapenem, can solve the problems of complex equipment, low production efficiency, water removal, etc., to improve the crystallization yield and purity, reduce equipment investment, and reduce water effect of dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
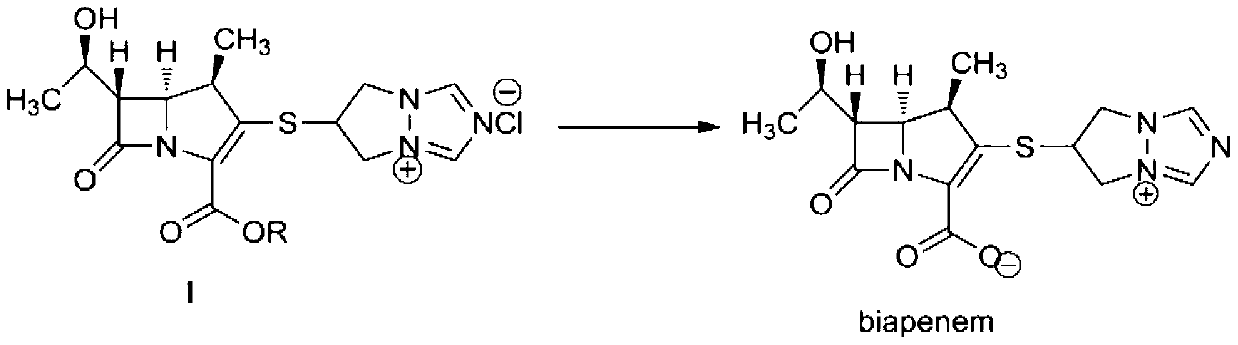
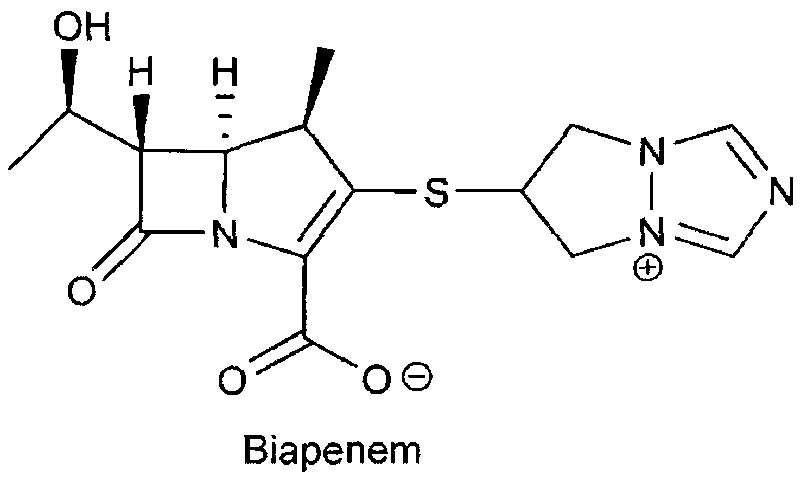
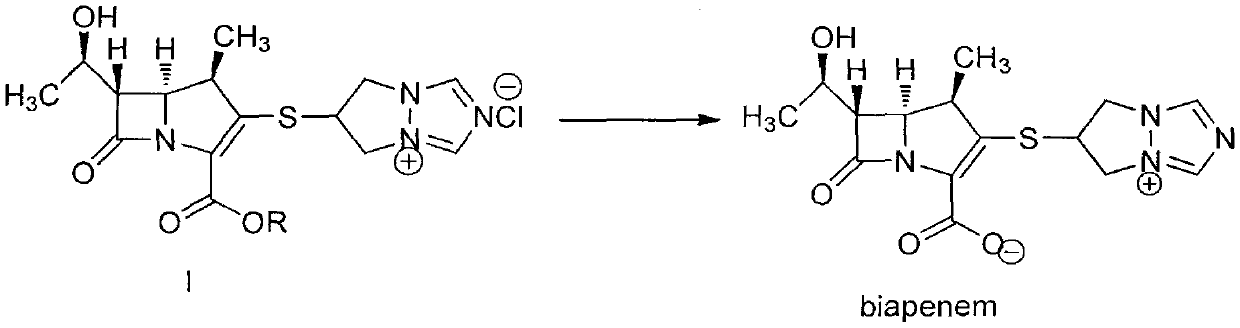
[0049] 6-[(4R, 5S, 6S)-2-(4-nitrobenzyloxycarbonyl)-6-[(1R)-1-hydroxyethyl]-4-methyl-7-oxo-1-nitrogen Heterobicyclo[3,2,0]hept-2-en-3-yl]thio-6,7-dihydro-5H-pyrazolo[1,2-α][1,2,4]tri Azol-4-ium chloride (I, R=O 2 NPhCH 2 ) 300g (0.58mol), add into 1.5L of water and 1.0L of tetrahydrofuran, stir to dissolve, add 70g of palladium carbon (10%), control hydrogenation pressure 4~5Kg / cm 2 , react at 10-20°C for 2.5 hours; filter off palladium-carbon immediately after the reaction, adjust the pH to 3.0-6.0 with 4-dimethylaminopyridine, separate the liquids, add 4.0L of acetone to the water phase, and stir at 10-20°C for 3.0 hours. Crystals were precipitated, filtered, the solid was washed with 300 ml of acetone, and dried under reduced pressure to obtain 180 g of biapenem (yield: 90%).
Embodiment 2
[0051] 6-[(4R, 5S, 6S)-2-(4-nitrobenzyloxycarbonyl)-6-[(1R)-1-hydroxyethyl]-4-methyl-7-oxo-1-nitrogen Heterobicyclo[3,2,0]hept-2-en-3-yl]thio-6,7-dihydro-5H-pyrazolo[1,2-α][1,2,4]tri Azol-4-ium chloride (I, R=O 2 NPhCH 2 ) 600g (1.16mol), was added into 3.0L of water and 2.0L of tetrahydrofuran, stirred and dissolved, and 120g of palladium hydroxide (10%) was added, and the hydrogenation pressure was controlled at 10-15Kg / cm 2 , react at 10-15°C for 1.0 hour; after the reaction, use N-methylmorpholine to adjust the pH to 3.0-6.0, filter off the palladium hydroxide, separate the liquids, add 8.0L ethanol to the water phase, stir at 0-5°C for 0.5 hours, Crystals were precipitated, filtered, the solid was washed with 600 ml of ethanol, and dried under reduced pressure to obtain 364 g of biapenem (yield: 91.0%).
Embodiment 3
[0053] 6-[(4R, 5S, 6S)-2-(4-nitrobenzyloxycarbonyl)-6-[(1R)-1-hydroxyethyl]-4-methyl-7-oxo-1-nitrogen Heterobicyclo[3,2,0]hept-2-en-3-yl]thio-6,7-dihydro-5H-pyrazolo[1,2-α][1,2,4]tri Azol-4-ium chloride (I, R=O 2 NPhCH 2 ) 600g (1.16mol), was added to 3.0L of water and 2.0L of tetrahydrofuran, stirred and dissolved, and 200g of platinum carbon (5%) was added, and the hydrogenation pressure was controlled to 20Kg / cm 2 , react at 25-30°C for 0.5 hours; filter the platinum carbon within 10 minutes after the end of the reaction, adjust the pH to 4.0-6.0 with N-methylpiperidine, separate the liquid, add 8.0L ethanol to the water phase, stir at 5-10°C for 2.0 After hours, crystals were precipitated, filtered, the solid was washed with 600 ml of ethanol, and dried under reduced pressure to obtain 360 g of biapenem (yield: 90%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com