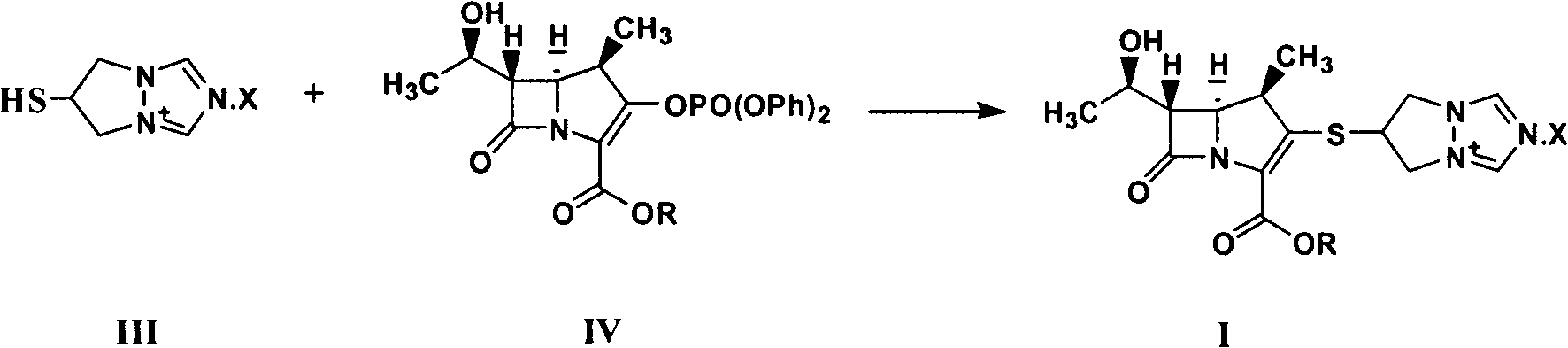
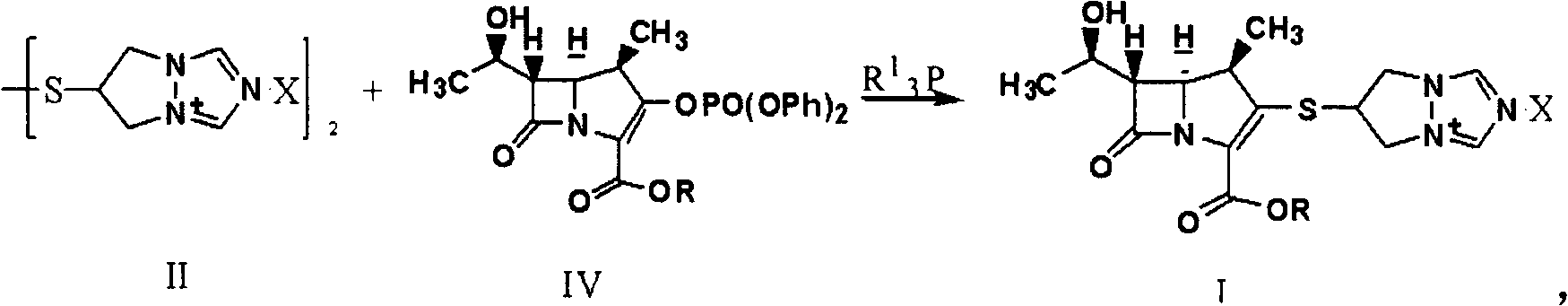Synthesis method of biapenem ester
A technology for apenem ester and compound is applied in the field of synthesis of biapenem ester, can solve the problems of easy moisture absorption of compound, difficult preparation and storage, inconvenient preparation of biapenem ester, etc., and achieves the effect of easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] In the case of air isolation, 500ml of tetrahydrofuran, 8.0g of compound (II) (X is Cl), 10.0g of (R is p-nitrobenzyl) compound (IV), 5.0g of tributylphosphine and 4.0g of Diisopropylethylamine was added to the reactor, reacted at room temperature for 6 hours, monitored by HPLC, the reaction was completed, adjusted to pH=4.7 with hydrochloric acid, cooled to -15°C, added 250ml of dichloromethane, stirred and crystallized for 5 hours, filtered, Wash with 100ml of dichloromethane and dry to obtain 8.1g of target product (I).
Embodiment 2
[0039] In the absence of air, mix 400ml of acetonitrile, 8.0g of compound (II) (X is Cl), 10.0g of (R is p-nitrobenzyl) compound (IV), 5.0g of tributylphosphine and 4.0g of Diisopropylethylamine was added to the reactor, reacted at room temperature for 6 hours, monitored by HPLC, the reaction was completed, adjusted to pH=3.5 with hydrochloric acid, cooled to -15°C, added 250ml of dichloromethane, stirred and crystallized for 5 hours, filtered, Wash with 100ml of dichloromethane and dry to obtain 9.1g of target product (I).
Embodiment 3
[0041] In the case of air isolation, 400ml of acetonitrile, 8.0g of compound (II) (X is Br), 10.0g of (R is p-nitrobenzyl) compound (IV), 5.0g of tributylphosphine and 4.0g of Diisopropylethylamine was added to the reactor, reacted at room temperature for 6 hours, monitored by HPLC, the reaction was completed, adjusted to pH=5.0 with hydrochloric acid, cooled to -25°C, added 250ml of dichloromethane, stirred and crystallized for 2 hours, filtered, Wash with 100ml of dichloromethane and dry to obtain 9.4g of target product (I).
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com