New crystal form of biapenem and its synthesis method
A technology of crystal form ratio and crystallization, which can be used in pharmaceutical formulations, organic active ingredients, medical preparations containing active ingredients, etc., and can solve unclear problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
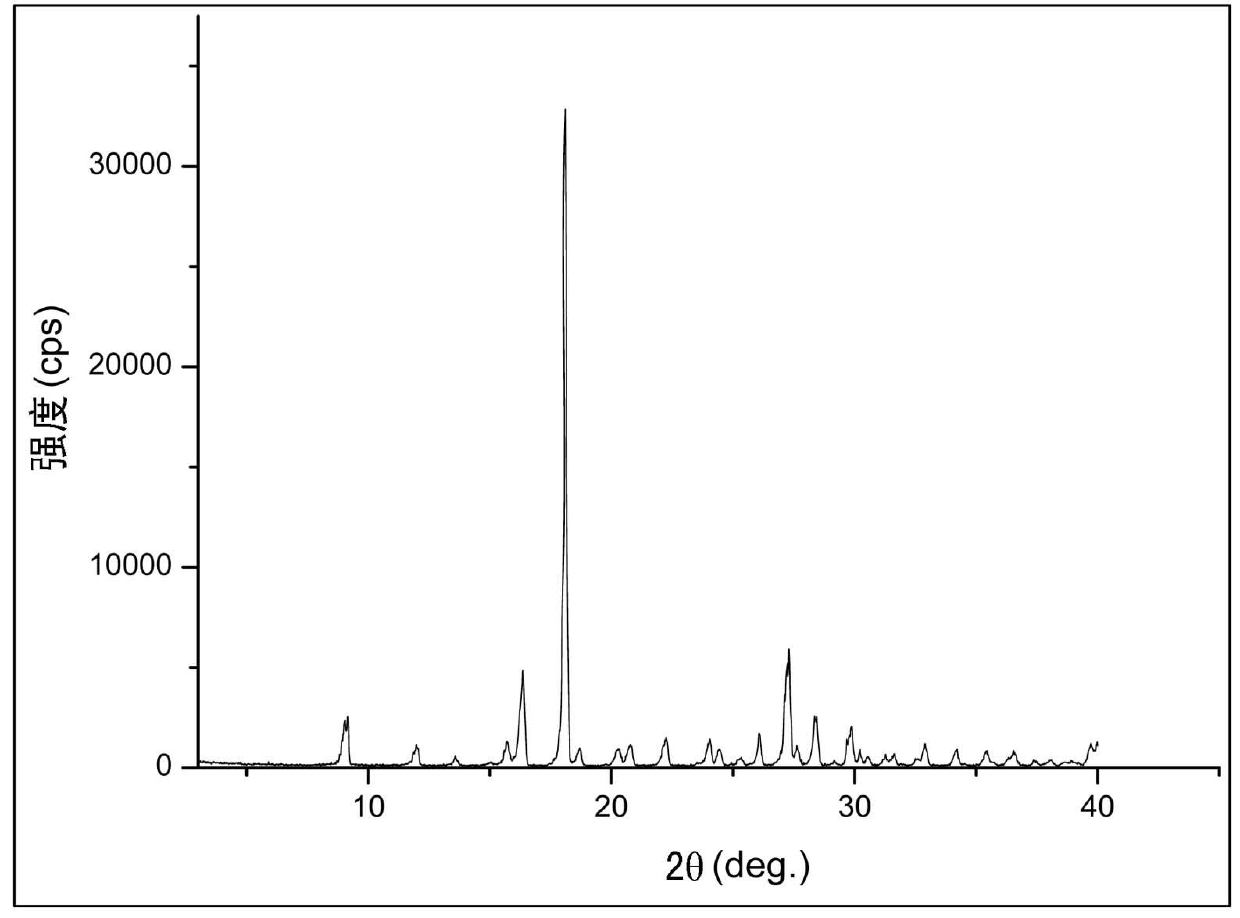
[0040] Example 1: Preparation of Biapenem Form I
[0041] Add 300ml of water into the dissolution reaction bottle, control the temperature at 20°C, add 10g of crude biapenem under stirring, add 1g of activated carbon after dissolution, control the temperature at 40°C, stir for 20 minutes, filter, and transfer the filtrate to the crystallization reaction bottle , add 800ml crystallization solvent acetone and 0.1g seed crystal to the crystallization reaction bottle under stirring for crystallization, after the crystallization solvent is added, slowly cool down to 20°C, continue to stir and grow crystals for 2 hours, filter, wash with 80ml acetone, and drain Vacuum drying was carried out to obtain 9.20 g of biapenem crystals. (Yield 92.0%).
[0042] This crystal is used as a sample, and powder X-ray diffraction (hereinafter referred to as XRD) is measured to obtain figure 1 The X-ray diffraction pattern shown. From this result, it can be seen that X-ray diffraction peaks ap...
Embodiment 2
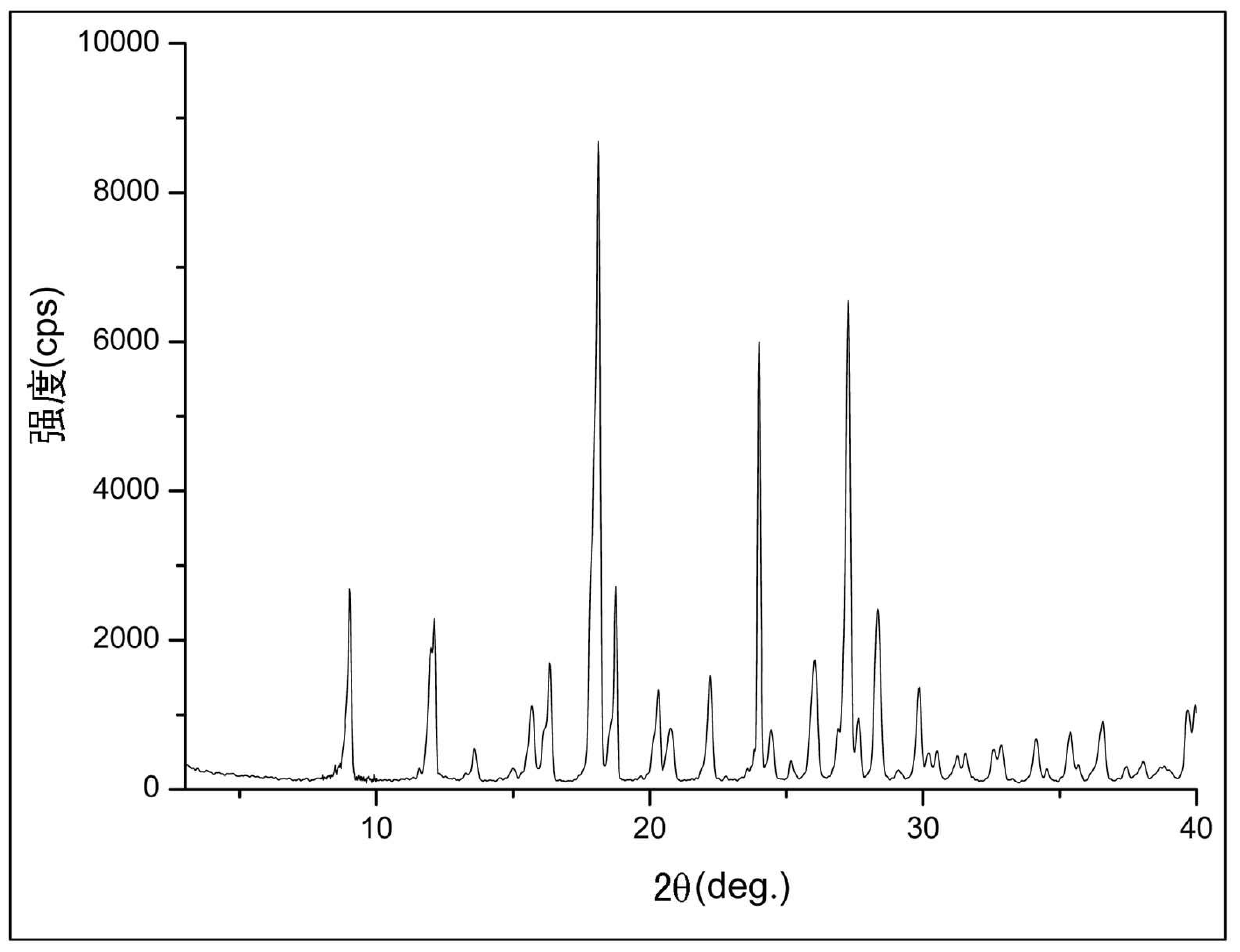
[0047] Example 2: Preparation of Biapenem II Crystal Form
[0048] Add 500ml of water into the dissolution reaction bottle, control the temperature at 20°C, add 10g of crude biapenem under stirring, add 1g of activated carbon after dissolution, control the temperature at 20°C, stir for 20 minutes, filter, and transfer the filtrate to the crystallization reaction bottle , add 1500ml of crystallization solvent methanol to the crystallization reaction bottle under stirring for crystallization. After the crystallization solvent is added, the temperature is controlled at 10°C, and the crystal is grown for 2 hours, filtered, washed with 80ml of methanol, and the product is vacuum-dried after pumping dry 9.11 grams of biapenem crystals. (Yield 91.1%).
[0049] This crystal is used as a sample, and powder X-ray diffraction (hereinafter referred to as XRD) is measured to obtain figure 1 The X-ray diffraction pattern shown. It can be seen from the results that X-ray diffraction pe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com