Synthesis method for biapenem
A synthesis method and biapenem technology, applied in the field of antibiotic drug synthesis, can solve the problems of difficult synthesis, unsuitable by-products for labor protection, low yield and the like, so as to save synthesis time, reduce production cost and increase yield. rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
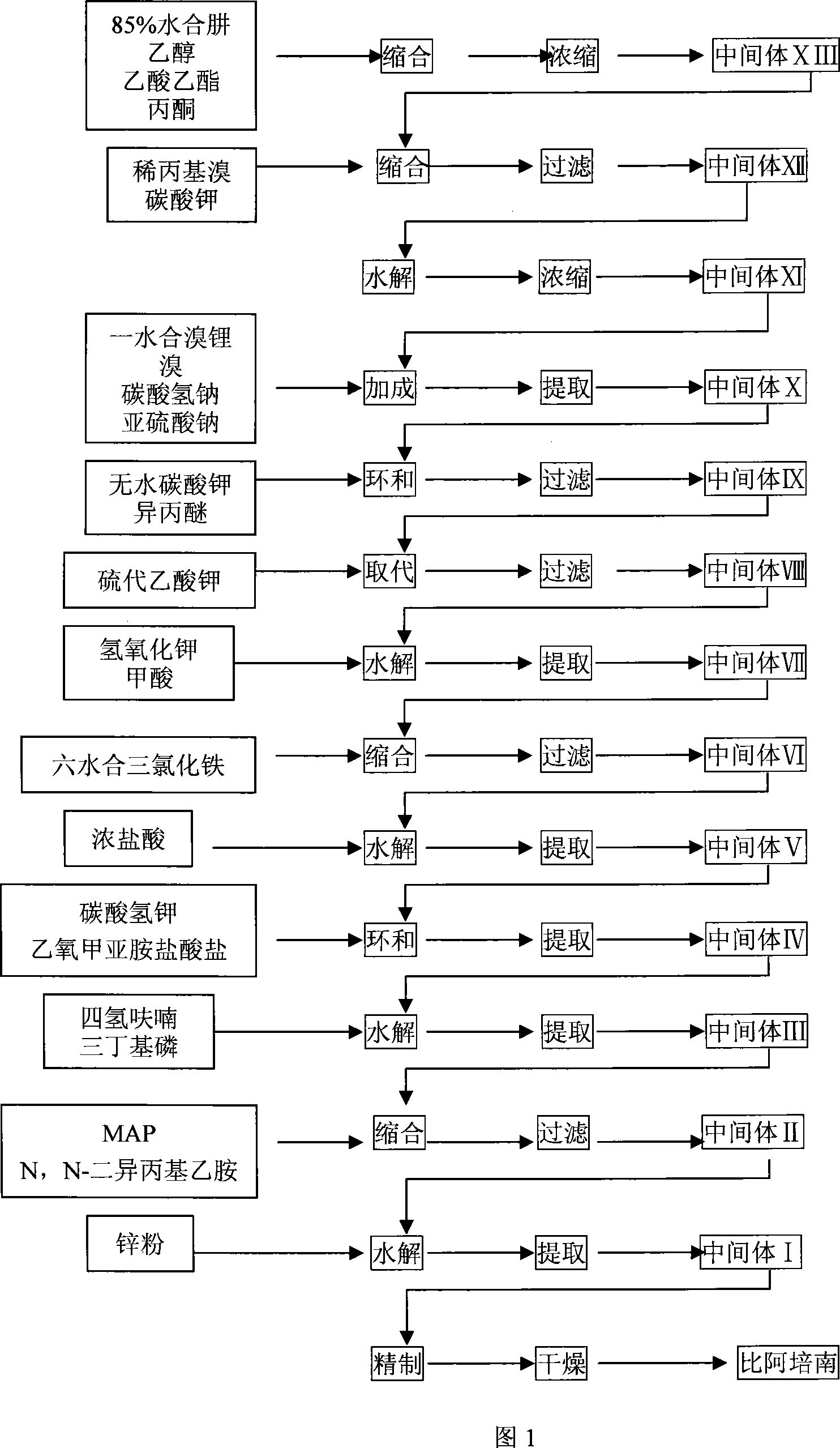
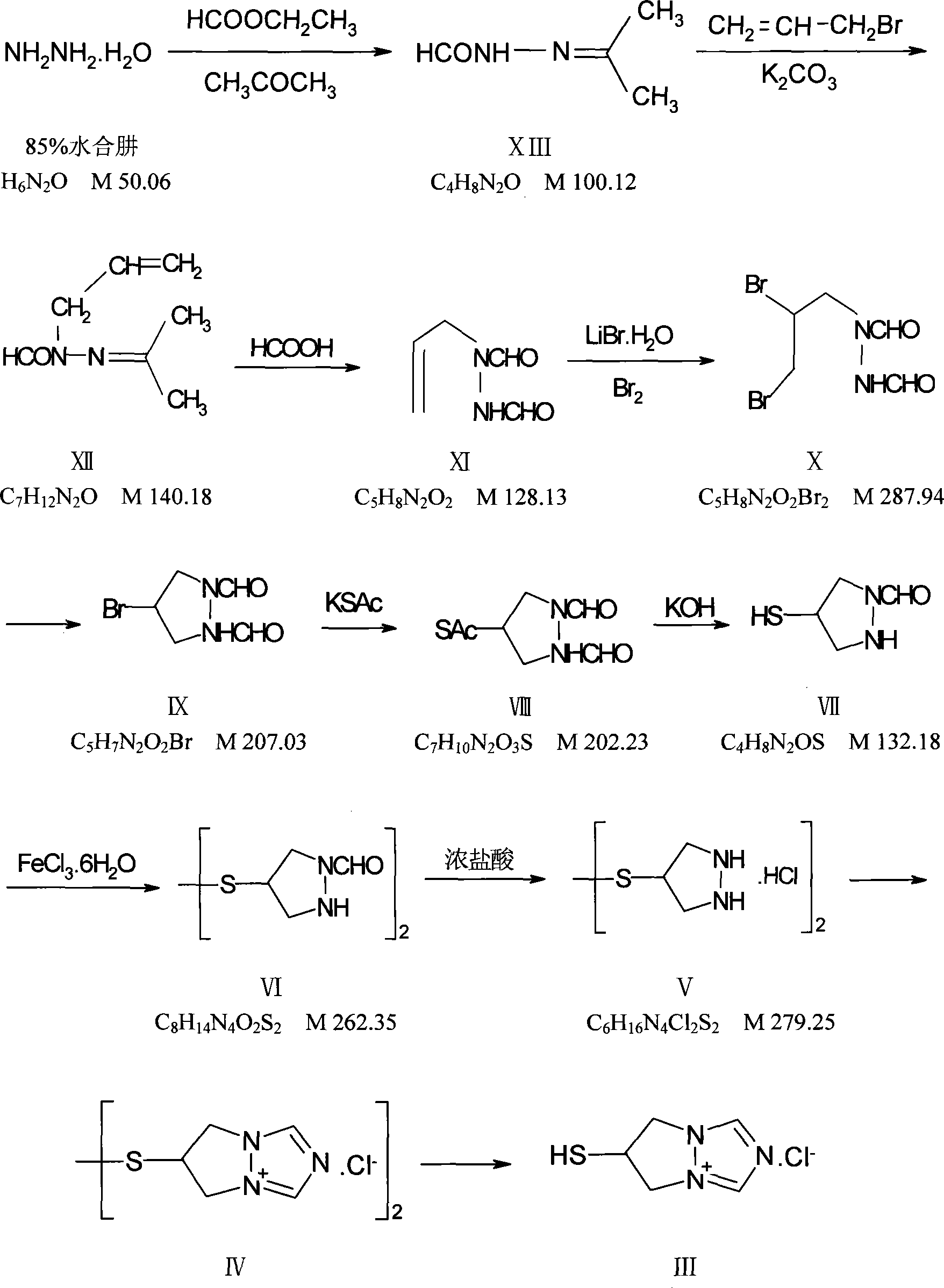
Image
Examples
Embodiment 1
[0052] Example 1: Preparation of Biapenem
[0053] Detailed synthesis process:
[0054] 1. Synthesis of 1-formaldehyde-2-isopropylidene hydrazine (yield: 85.9%)
[0055] Feed ratio: 85% hydrazine hydrate: ethyl formate: acetone = 1: 1.64: 2.65 (W / V / V)
[0056] After mixing 2639g of 85% hydrazine hydrate with 7L of ethanol, cool in an ice-salt bath to -5°C, then add 4319ml of ethyl formate dropwise, keep the temperature from -5°C to 0°C, drop it for about 1 hour, and continue at -4°C Stir for 0.5 hours, then gradually rise to room temperature, stir for 14 hours, the reaction solution is colorless and transparent, add 7L of acetone dropwise, drop it in 30 minutes, then stir at room temperature for 30 minutes to obtain a light yellow solution, concentrate under reduced pressure to obtain a light yellow solid , after recrystallization from ethanol, 3853 g of white crystals (intermediate XIII) were obtained; melting point: 67~68°C
[0057] Quality control standard: Melting point...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com