Preparation method of biapenem
A biapenem and molar ratio technology, applied in the field of drug synthesis, can solve the problems of unfavorable production safety, low product yield and purity, long synthesis route and the like in the hydrogen absorption reaction, so as to improve the yield of the ring-closing reaction and improve the product quality. Yield and purity, effect of reducing reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
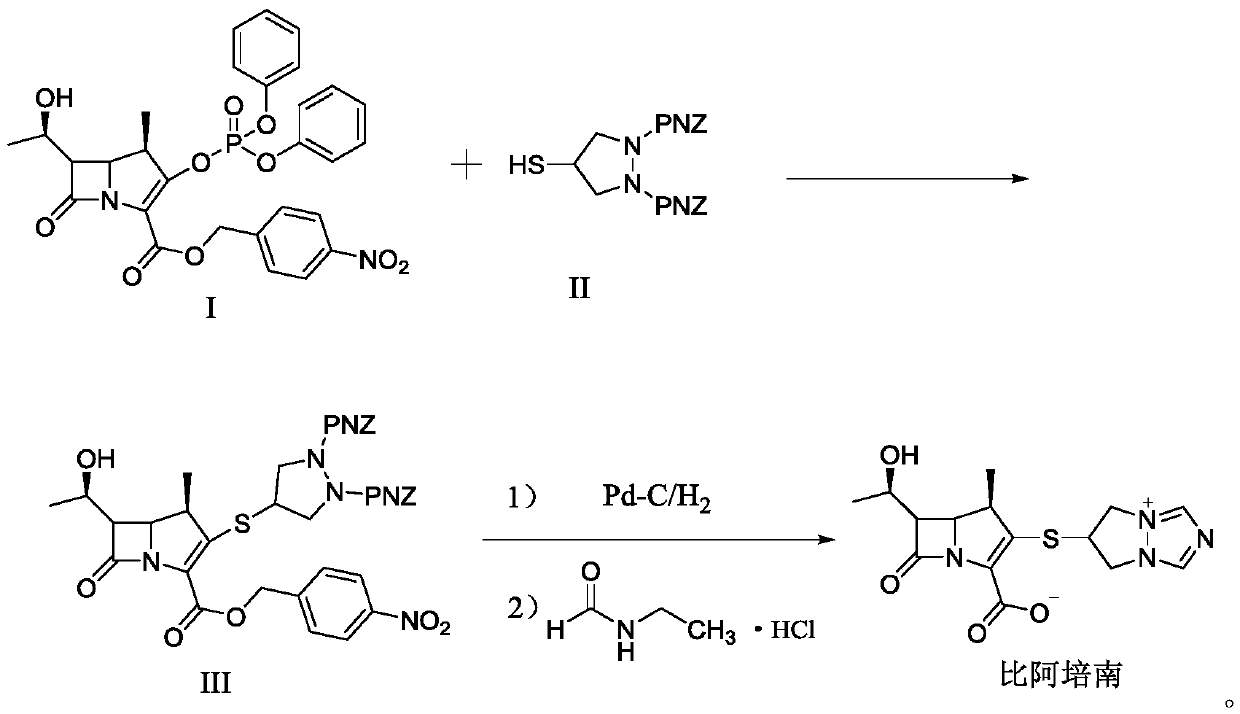
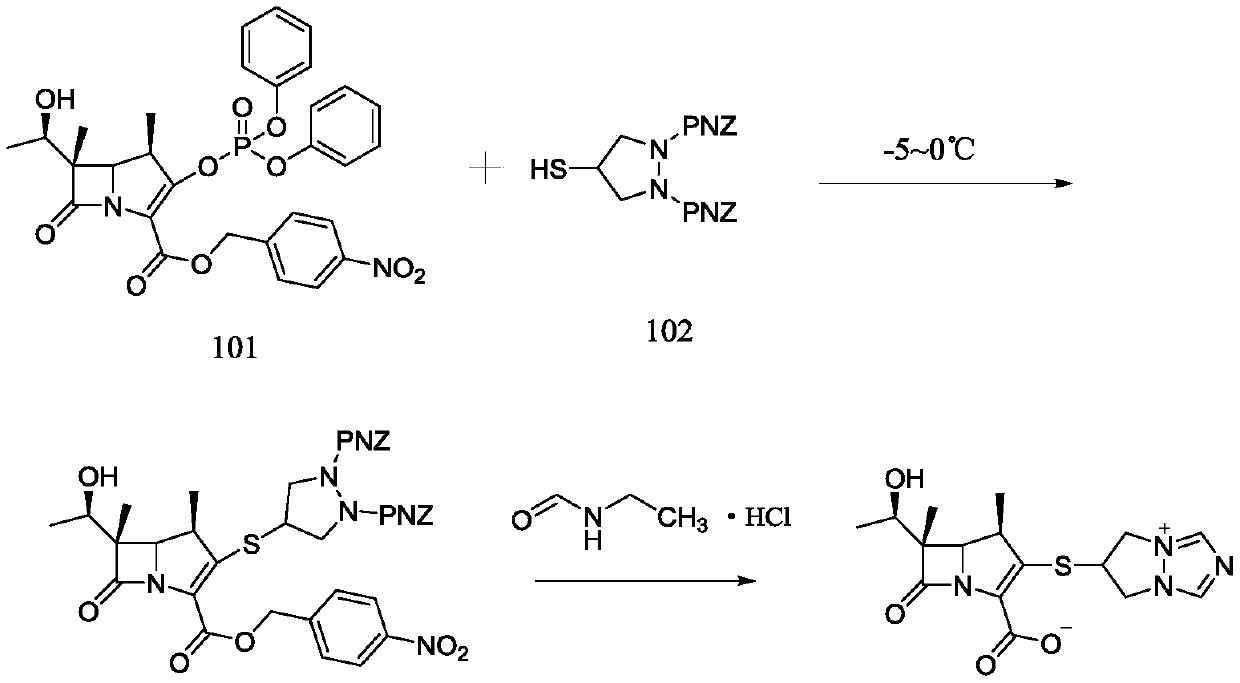
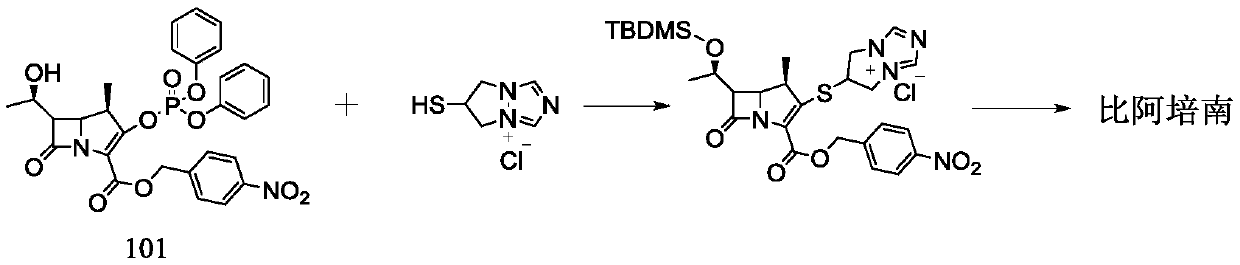
[0034] The preparation of embodiment 1 compound III
[0035] Add 80mL of acetonitrile to a 500mL reaction flask, add 9.24g of 4-mercapto-N,N-bis(p-nitrobenzyloxycarbonyl)pyrazolidine (compound II), add 2.07g of N,N-diisopropylethylamine and Tetraisopropyl titanate 2.27g. Blow nitrogen gas, cool down to -5~0°C, add 11.89g of compound I in batches, complete the addition within 15 minutes, continue to stir and react at -5~0°C for 90 minutes, after the reaction, add 240mL of purified water to crystallize for 2h, suction filter, 40 After vacuum drying at °C for 3 h, 14.17 g of compound III was obtained, with a yield of 87.9%, and a purity of 99.2% by HPLC.
Embodiment 2
[0036] The preparation of embodiment 2 compound III
[0037] Add 80mL of acetonitrile to a 500mL reaction flask, add 9.24g of 4-mercapto-N,N-bis(p-nitrobenzyloxycarbonyl)pyrazolidine (compound II), add 2.33g of N,N-diisopropylethylamine and Tetraisopropyl titanate 2.84g. Blow nitrogen gas, cool down to -5~0°C, add 11.89g of compound I in batches, complete the addition within 15 minutes, continue to stir and react at -5~0°C for 90 minutes, after the reaction, add 240mL of purified water to crystallize for 2h, suction filter, 40 It was dried in vacuo at °C for 3 h to obtain 14.60 g of compound III. The yield was 90.6%, and the purity by HPLC was 99.1%.
Embodiment 3
[0038] The preparation of embodiment 3 compound III
[0039] Add 80mL of acetonitrile to a 500mL reaction flask, add 9.24g of 4-mercapto-N,N-bis(p-nitrobenzyloxycarbonyl)pyrazolidine (compound II), add 2.69g of N,N-diisopropylethylamine and Tetraisopropyl titanate 3.41g. Blow nitrogen gas, cool down to -5~0°C, add 11.89g of compound I in batches, complete the addition within 15 minutes, continue to stir and react at -5~0°C for 90 minutes, after the reaction, add 240mL of purified water to crystallize for 2h, suction filter, 40 It was dried in vacuo at °C for 3 h to obtain 14.89 g of compound III. The yield was 92.4%, and the purity by HPLC was 99.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com