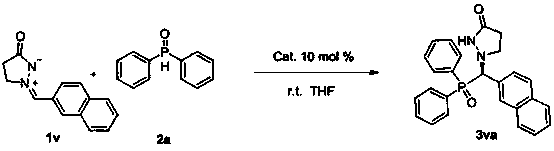
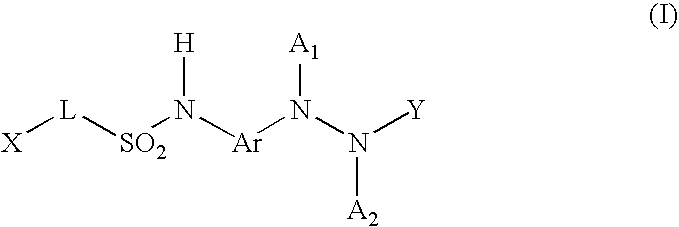
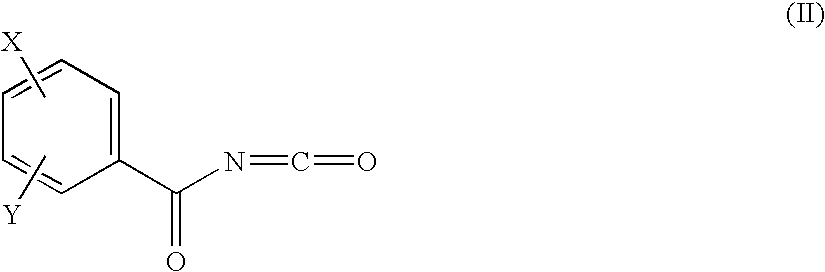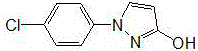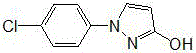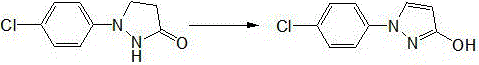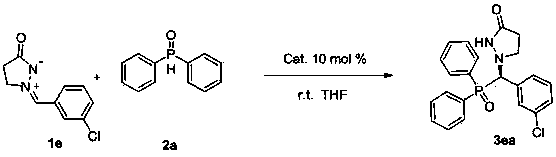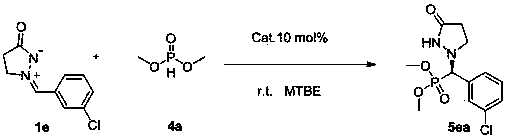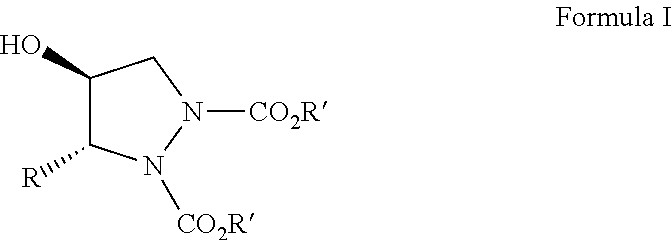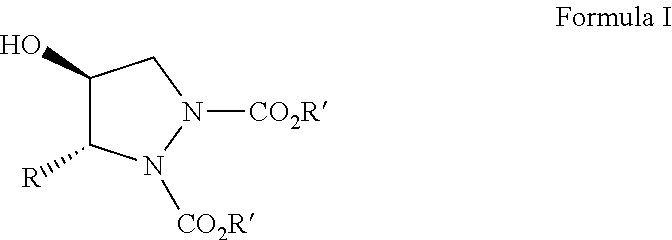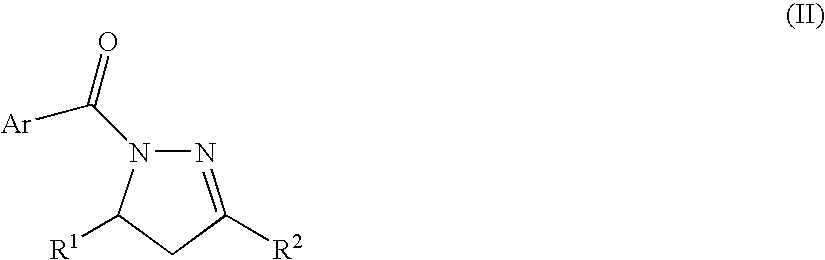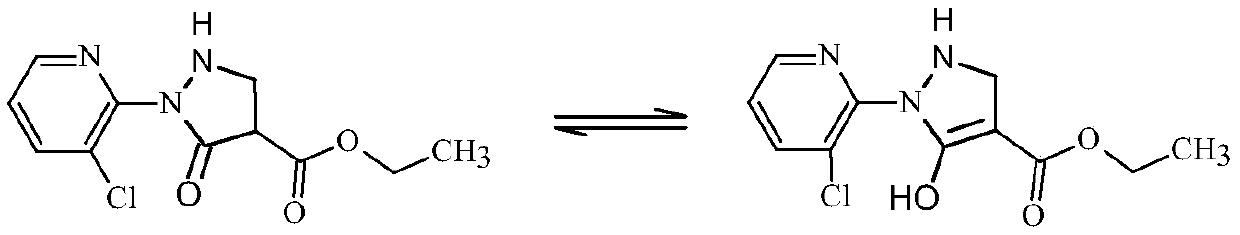Patents
Literature
51 results about "Pyrazolidine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Pyrazolidine is a heterocyclic compound.
Synthetic technology for pyraclostrobin
ActiveCN104211641AFormation reaction is easy to controlSmooth responseOrganic chemistryMethylanilineChlorobenzene
The invention concretely relates to a synthetic technology for pyraclostrobin. The synthetic technology comprises: firstly performing cyclization to obtain 1-(4-chlorophenyl)-pyrazol-3-one, oxidizing the pyrazol ring under the effect of an oxidant to generate 1-(4-chlorophenyl)-3-hydroxypyrazole, then using 2-nitrobenzyl bromide to performing etherification to generate 1-(4-chlorophenyl)-3-[2-(nitrophenyl)methoxy]-1H-pyrazole, then using a reducing agent to perform nitro reducing, so as to generate N-hydroxyl-2-[N'-(4-chlorophenyl)pyrazol-3'-yloxymethyl]aniline, then using ClCO2CH3 to perform N-acylation reaction to generate methyl N-hydroxyl-N-2-{[N'-(4-chlorophenyl)pyrazol-3'-yloxymethyl]phenyl}formate, and finally performing hydroxyl methylation under an alkaline condition to generate pyraclostrobin. The technology enables all operations in the pyraclostrobin preparation process to be relatively controllable, helps to improve the stability of the preparation process and improve the product yield, successfully employs low-cost reagents and substantially reduces production cost, and also the employed reagents are relatively small in toxicity, is relatively beneficial for environment protection, and has no corrosivity on plastic pipes, so that the production safety is improved.
Owner:SHANDONG KANGQIAO BIO TECH CO LTD
Alpha-menaphthyl substituted spiro bis(oxazoline) ligands, synthetic method and application thereof in synthesizing pyrazolidine derivatives
ActiveCN101560191AThe synthesis method is simpleMild conditionsOrganic-compounds/hydrides/coordination-complexes catalystsOrganic cyclisationRegioselectivityChirality
The invention provides spiro bis(oxazoline) ligands with a plurality of chiral centers, a preparation method and application. The ligands are provided with axial chirality of a spiro backbone and central chirality of an oxazoline ring. The ligands can be prepared by condensation of chiral spiro diacid and corresponding alkamine. The invention also provides application of the spiro bis(oxazoline) ligands in synthesizing pyrazolidine derivatives in high regioselectivity and high enantioselectivity.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
Technology for synthesizing pyraclostrobin by six-step method
The invention provides a technology for synthesizing pyraclostrobin by a six-step method. The synthesis technology comprises the following steps: 1) performing a reduction reaction of ortho-nitrotoluene; 2) performing an acylation reaction of hydroxylamine; 3) performing a methylation reaction; 4) obtaining N-methoxyl-N-2-bromomethyl phenyl methyl carbamate through a bromination reaction; and 5) performing synthesis of 1-(4-chlorophenyl)pyrazolidine-3-ketone; and 6) performing synthesis of pyraclostrobin. Compared with the prior art, the preparation method has the advantages of simple process, easily available raw materials with low cost, and mild reaction condition, and the purity and yield of the target products are high.
Owner:ANHUI GUANGXIN AGROCHEM
Ubiquitin E1 inhibitor and preparation method thereof
InactiveCN101961331AEasy to passGood water solubilityOrganic active ingredientsOrganic chemistryFuranProtein Degradations
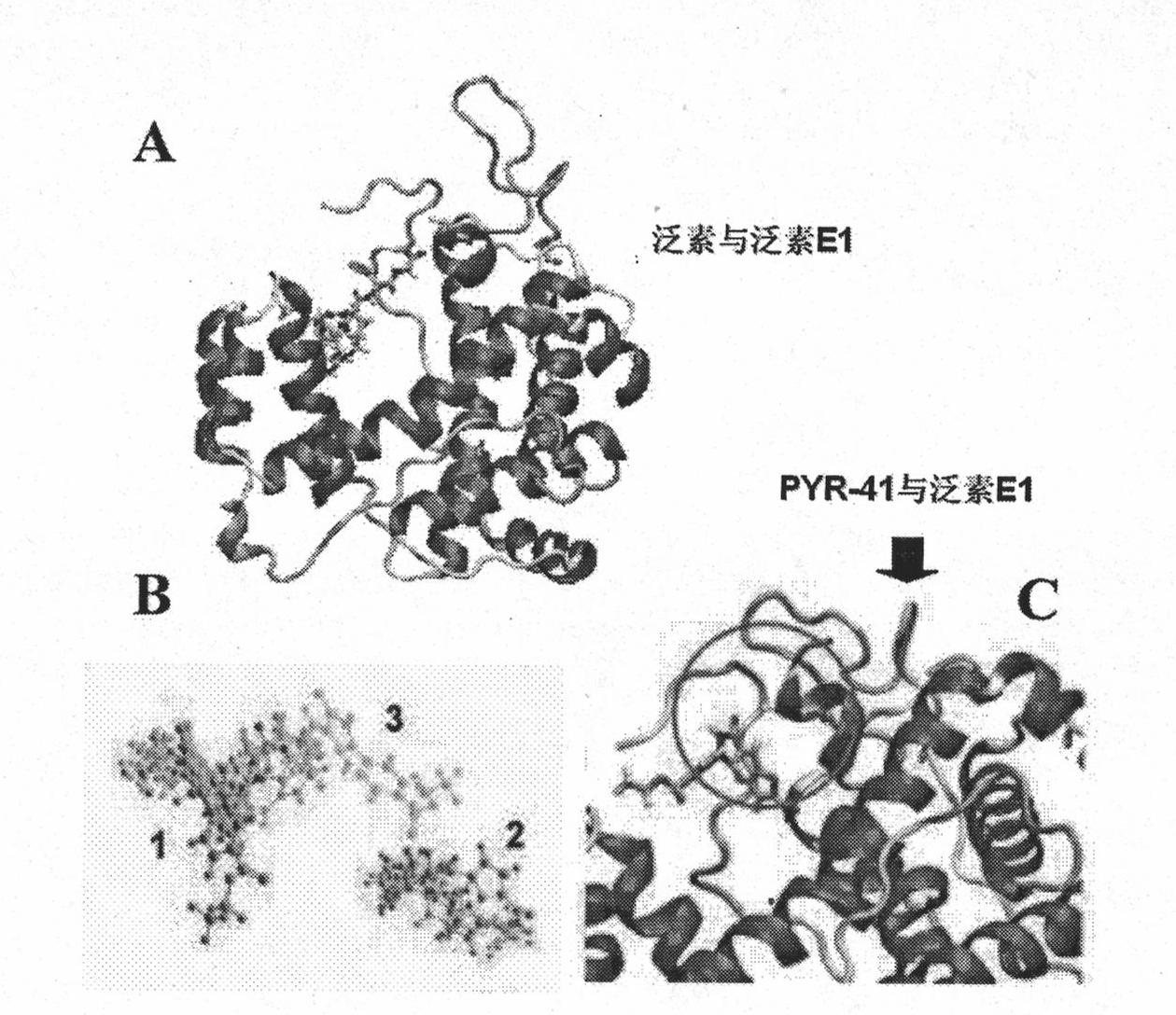
The invention relates to an ubiquitin E1 inhibitor and a preparation method thereof. Series compound Y-1-benzene pyrazole silane-3,5-diketone and relevant derivative (such as 4-(4-ethyoxyl-3-methoxybenzylidene)-1-benzene pyrazole silane-3,5-diketone, and the like) with different alternative groups are prepared by a liquid-phase organic synthesis method according to the structure of PRY-41(4(4-(5-nitro-furan group-methylene]-3,5-dioxo-pyrazolidine-1-radical)-ethyl benzoate). The ubiquitin E1 inhibitor can be used for inhibiting protein degradation mediated by ubiquitination and nondegradation of ubiquitination, and can kill transformed cells containing p53 in the process of blocking up the NFkB activity by the inhibitor, that is to say, the inhibitor possibly has the potential property of treating tumor and can be used as an important tool for researching ubiquitin proteinase system mechanism.
Owner:西安杰诺瓦生物科技有限公司
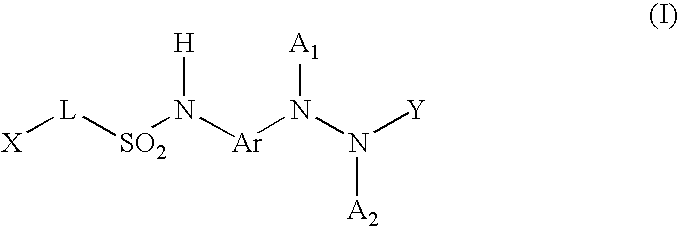
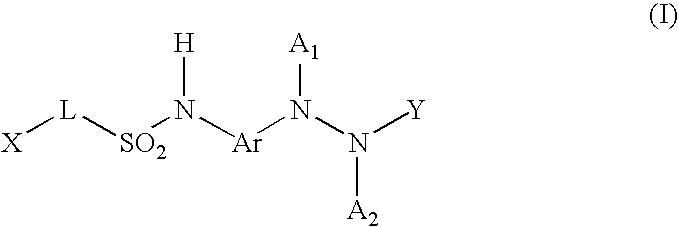
Benzoyl Urea Derivatives
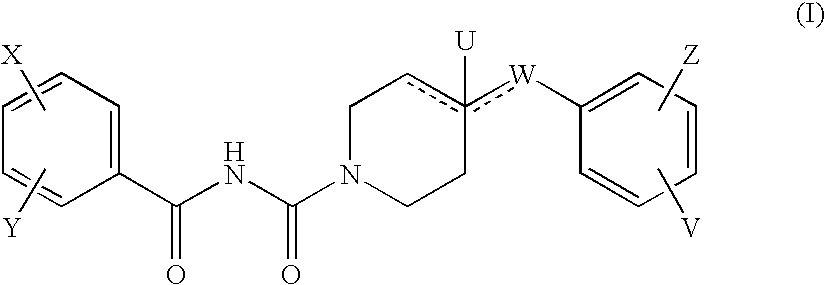
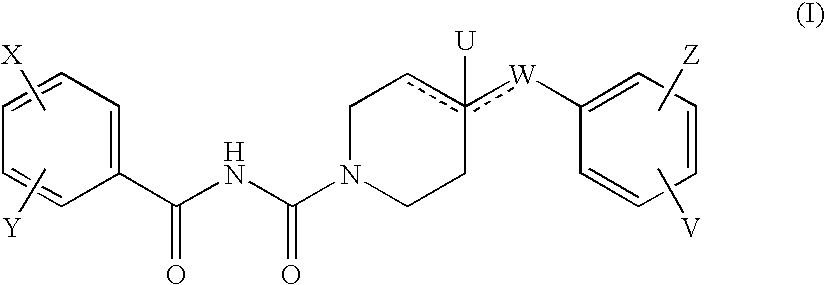
The new benzoyl urea derivatives of formula (I) wherein the meaning of X and Y independently are hydrogen atom, hydroxy, benzyloxy, amino, nitro, C1-C4 alkylsulfonamido optionally substituted with a halogen atom or halogen atoms, C1-C4 alkanoylamido optionally substituted with a halogen atom or halogen atoms, C1-C4 alkoxy, aroyl-carbamoyl optionally substituted with halogen atom or C1-C4 alkyl or C1-C4 alkoxycarbonyl group, or the neighboring X and Y groups optionally form together with one or more identical or different additional hetero atom and —CH═ and / or —CH2— groups an optionally substituted 4-7 membered homo- or heterocyclic ring, preferably morpholine, pyrrole, pyrrolidine, oxo- or thioxo-pyrrolidine, pyrazole, pyrazolidine, imidazole, imidazolidine, oxo- or thioxo-imidazole or imidazolidine, 1,4-oxazine, oxazole, oxazolidine, triazole, oxo- or thioxo-oxazolidine, or 3-oxo-1,4-oxazine ring, V and Z independently are hydrogen or halogen atom, cyano, C1-C4 alkyl, C1-C4 alkoxy, trifluoromethyl, hydroxy or optionally esterized carboxyl group, W is oxygen atom, as well as C1-C4 alkylene, C2-C4 alkenylene, aminocarbonyl, —NH—, —N(alkyl)-, —CH2O—, —CH2S—, —CH(OH)—, —OCH2— group, wherein the meaning of alkyl is a C1-C4 alkyl group—, when the dotted bonds () represent simple C—C bonds then U is hydroxy group or hydrogen atom or when W is C1-C4 alkylene or C2-C4 alkenylene group, then one of the dotted bonds () can represent a further double C—C bond and in this case U means an electron pair, which participate in the double bond and optical antipodes, racemates and the salts thereof are highly effective and selective antagonists of NMDA receptor, and moreover most of the compounds are selective antagonist of NR2B subtype of NMDA receptor. Furthermore objects of the present invention are the pharmaceutical compositions containing new benzoyl urea derivatives of formula (I) or optical antipodes or racemates or the salts thereof as active ingredients and processes for producing these compounds and pharmaceutical compositions.
Owner:RICHTER GEDEON VEGYESZETI GYAR RT
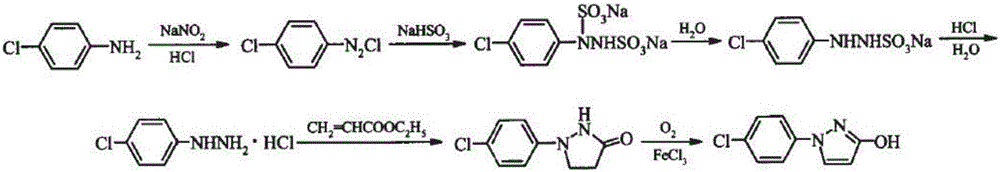
Synthetic process of 1-(4-chlorophenyl)-3-hydroxy-1-h-pyrazole
The invention relates to a synthetic process of 1-(4-chlorophenyl)-3-hydroxy-1-h-pyrazole, which is characterized by comprising the following steps: firstly, preparing heavy nitrogen liquid by using p-chloroaniline as a raw material; then reducing the heavy nitrogen liquid by sodium sulfite solution; next, reacting with dimethyl ester, carrying out acidification by hydrochloric acid so as to obtain a mixture of 1-(4-chlorophenyl) pyrazolidine-3-ketone; finally, reacting with sodium hydroxide solution, carrying out acid modulation, and filtration and rectification, so as to obtain a final product. According to the synthetic process disclosed by the invention, after the heavy nitrogen liquid mixture is obtained in the step 1, the heavy nitrogen liquid mixture is directly used for the next step of reaction without being treated, the process of acidification of the hydrochloric acid is omitted temporarily through the reaction in the step 2, and the hydrochloric acid is directly adopted to carry out acidification and acid modulation in the step 3, so that one acidification process is omitted, and outputs of waste acid and waste water are reduced; synthesis of each ton of the 1-(4-chlorophenyl)-3-hydroxy-1-h-pyrazole can reduce generation of 10 tons of waste water and reduce consumption of 5 tons of hydrochloric acid.
Owner:ANHUI GUANGXIN AGROCHEM
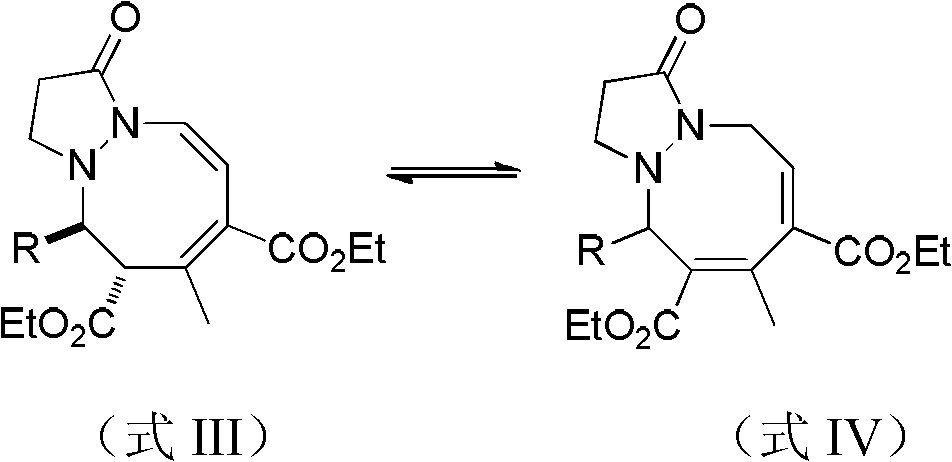
1,2-oxinane pyrazolidone compounds and preparation method and applications thereof
The invention discloses 1,2-oxinane pyrazolidone compounds and a preparation method and applications thereof. The 1,2-oxinane pyrazolidone compounds are shown in a formula III or a formula IV. The preparation method comprises the following steps: uniformly mixing a compound shown in a formula I and a compound shown in a formula II in a solvent and reacting in the presence of a phosphine catalyst to obtain the compounds shown in the formula III or the formula IV. The invention provides a new, simple and feasible method for synthesizing the 1,2-oxinane pyrazolidone compounds; the synthesized compounds are new compounds which are not reported in literatures; in the method disclosed by the invention, the cycloaddition reaction is adopted and the method belongs to the atom economic reaction; and the method uses organic phosphine as the catalyst rather than a transition metal catalyst, thus the product does not have heavy metal residual pollutants and has weeding effect on crabgrass and barnyard grass.
Owner:CHINA AGRI UNIV
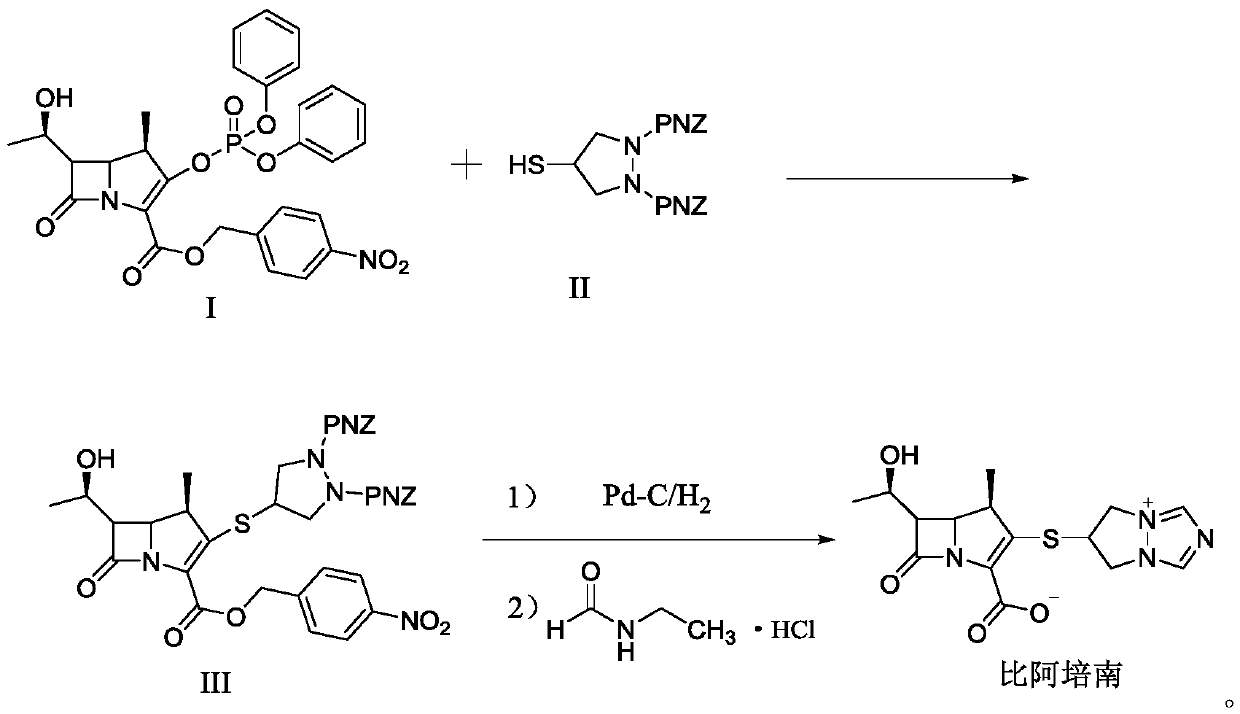
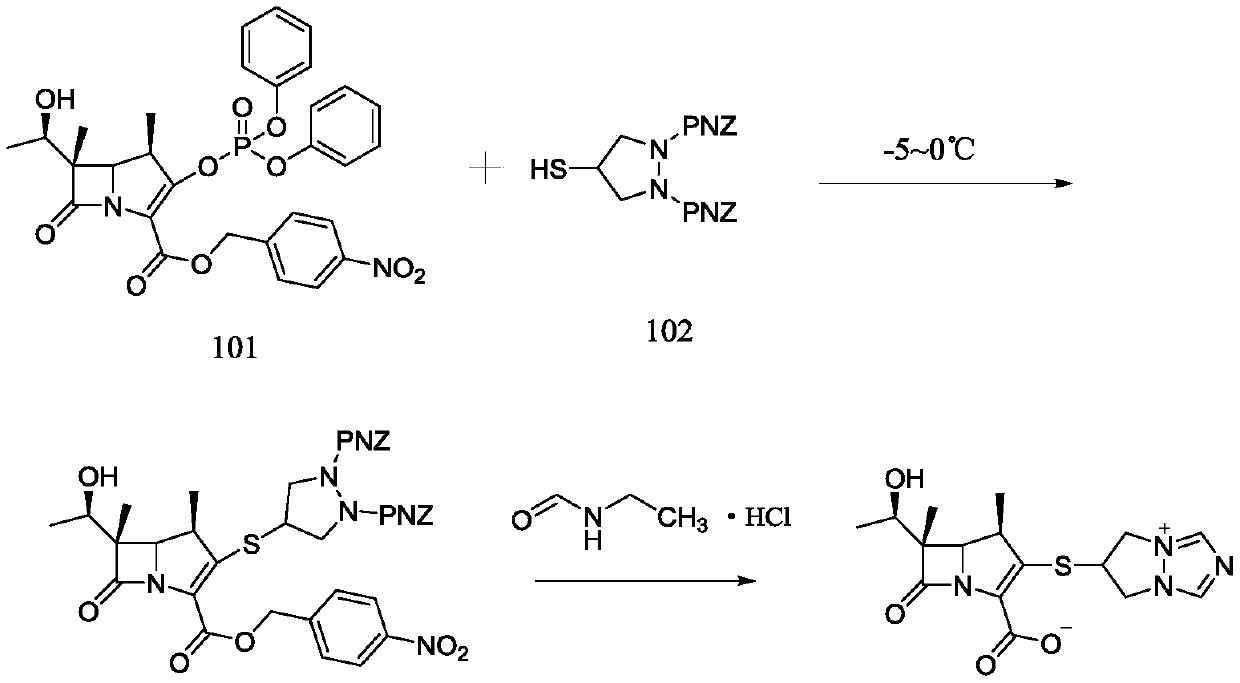
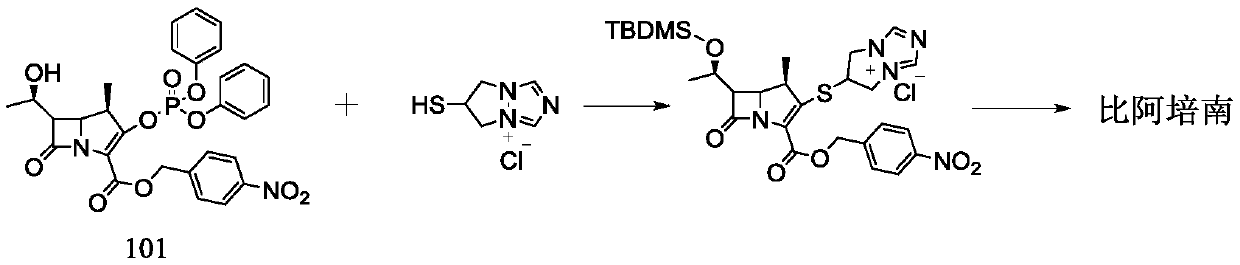
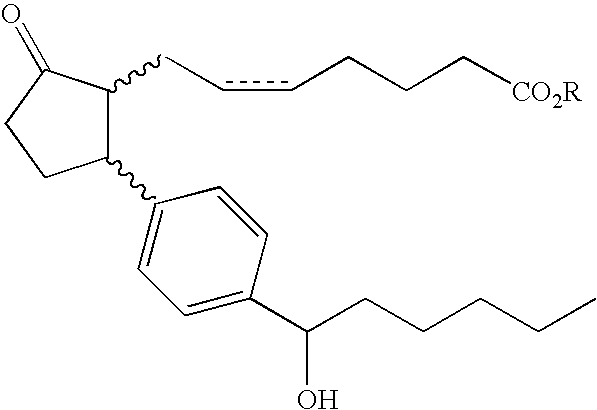
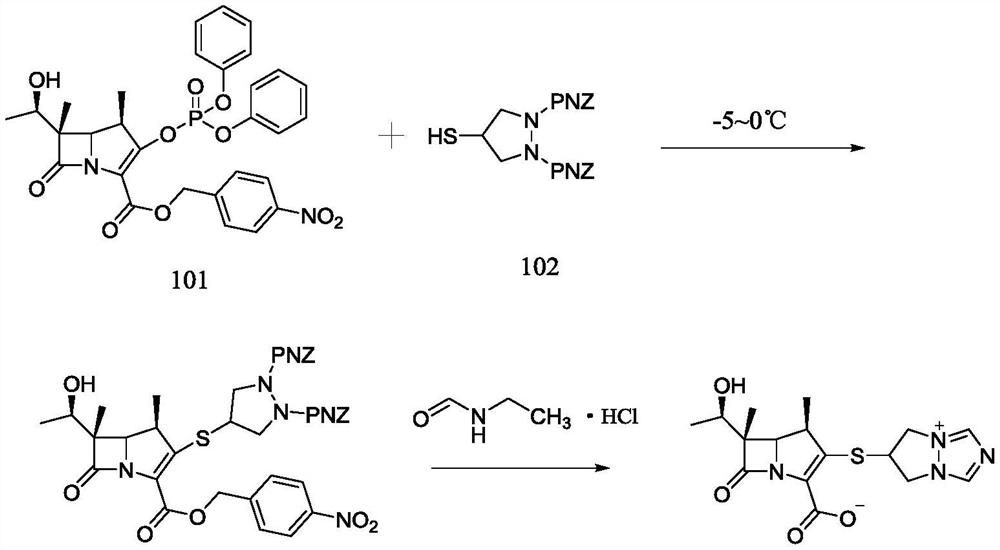
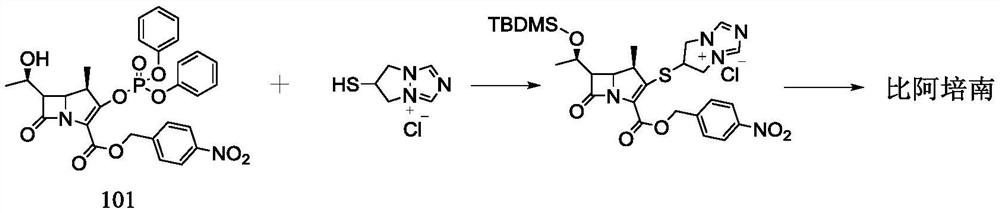
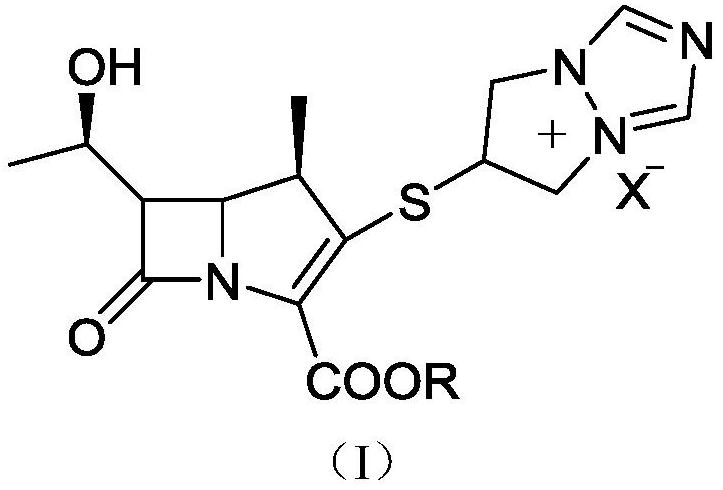
Preparation method of biapenem
The invention belongs to the technical field of medicines, and discloses a preparation method of biapenem. The method comprises the following steps: p-nitrobenzyl (1R,5R,6S)-6-[(1R)-1-hydroxyethyl]-2-[(diphenylphosphono)oxy]-1-methylcarboxyl pen-2-em-3-carboxylate and 4-sulfydryl-N,N-bis(p-nitrobenzyloxycarbonyl)pyrazolidine are used as raw materials, substitution is performed, hydrogenation is performed and cyclization is performed to synthesize the biapenem. The method provided by the invention overcomes the defects of a long reaction route, easy degradation of raw materials in the reactionprocess, a low yield and the like in the prior art, and is more suitable for industrial production.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD +2
Preparation method of 1-(4-chlorophenyl)-3-pyrazole alcohol
InactiveCN106008350AHomogeneous reactionLow energy consumption for desolvationOrganic chemistryAlcoholSolvent
The invention relates to a preparation method of 1-(4-chlorophenyl)-3-pyrazolol, which belongs to the technical field of preparation of pesticide intermediates. Included steps: first acidic solvent is added in the device, under stirring, add 1-(4-chlorophenyl) pyrazolidin-3-ketone, iron trichloride, heat up to completely dissolve, ventilate and react, and the reaction ends Finally, remove most of the solvent under reduced pressure, add water and stir, and a large amount of products are precipitated. After adjusting the pH value with sodium hydroxide solution, continue stirring for 0.5h, and filter to obtain 1-(4-chlorophenyl)-3-pyrazolol. Advantages: the acidic solvent is cheap and easy to obtain, the reaction is homogeneous, the energy consumption of desolventization is low, the yield is high, the production cost is low, and it is suitable for industrial production.
Owner:SHANDONG EFIRM BIOCHEMISTRY & ENVIRONMENTAL PROTECTION CO LTD
Method for industrially producing bromo-pyrazolidinic acid through micro-channel
PendingCN111440144AWell mixedEmission reductionOrganic chemistryChemical/physical/physico-chemical microreactorsAklanonic acidPollutant emissions
Owner:山东华科化工有限公司
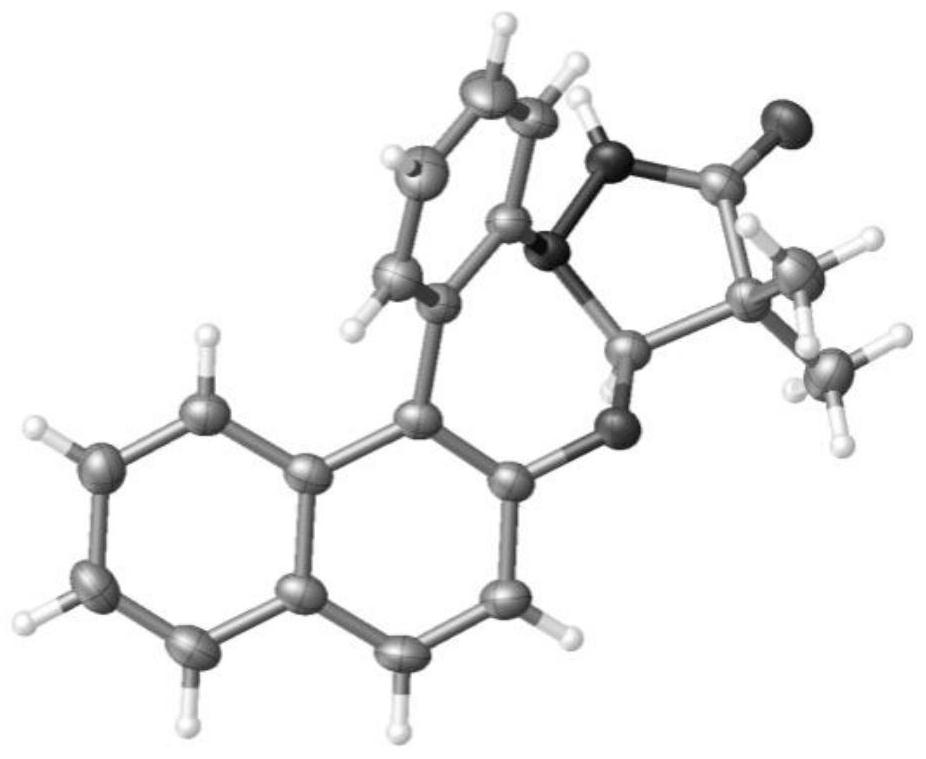
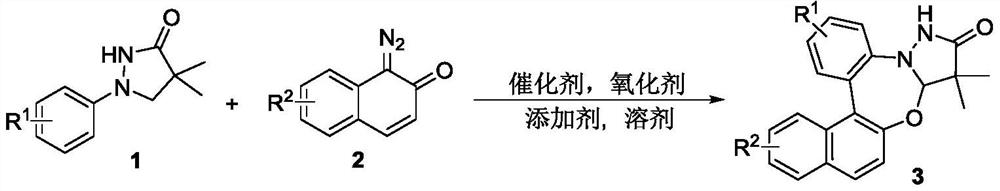
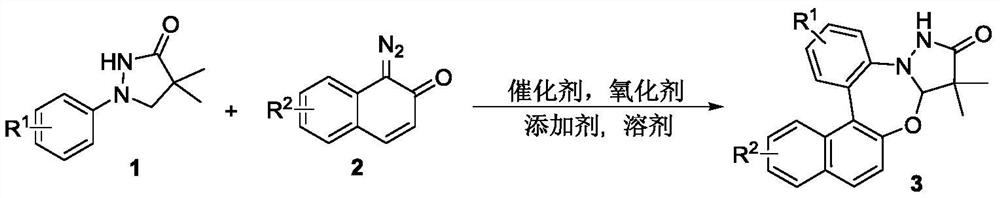
Synthetic method of pyrazolidinone-fused benzo 1,3-oxazepine compound
The invention discloses a synthesis method of a pyrazolidinone-fused benzo 1,3-oxazepine compound, and belongs to the technical field of organic synthesis. 1-aryl pyrazolidinone 1 and a diazonaphthalene ketone compound 2 are used as raw materials, and in the presence of a catalyst, an oxidizing agent and an additive, a heating reaction is performed in an organic solvent to obtain the pyrazolidinone-fused benzo 1,3-oxazepine compound 3. According to the method, the pyrazolidinone-fused benzo 1,3-oxazepine compound is efficiently and regioselectively synthesized through cascade reaction between the 1-aryl pyrazolidinone compound and the diazonaphthalene ketone compound, and the method has the advantages that the raw materials are simple and easy to obtain, the operation is simple and convenient, the condition is mild, the selectivity is good, the substrate application range is wide, and the like.
Owner:HENAN NORMAL UNIV
Antibacterial and anti-ultraviolet finishing agent and method for preparing same
InactiveCN105648776AImprove antibacterial propertiesImproves UV resistanceBiochemical fibre treatmentLight resistant fibresBenzoic acidMeth-
The invention discloses an antibacterial and anti-ultraviolet finishing agent. The antibacterial and anti-ultraviolet finishing agent comprises, by weight, 10-20 parts of vinyl chloride-vinyl acetate copolymerization emulsion, 15-25 parts of 1, 3, 5-trimethyltris-1, 5-(3, 3-trifluoropropyl)-cyclotrisiloxane, 5-10 parts of citric acid, 6-15 parts of 2, 4-dichlorobenzoic acid, 10-16 parts of 4-nitro-1, 2-diamino-3, 5-pyrazolidine diketone, 4-8 parts of poly-hydroxypropyl dimethyl ammonium chloride, 18-28 parts of polyoxyethylene stearate, 9-14 parts of tetra-tert-butyl orthotitanate, 3-9 parts of methoxyl zinc acrylate and 120-180 parts of deionized water. The antibacterial and anti-ultraviolet finishing agent has the advantage of excellent antibacterial and anti-ultraviolet effects.
Owner:WUJIANG LINWANG WEAVING MILL
Preparation method of biapenem intermediate
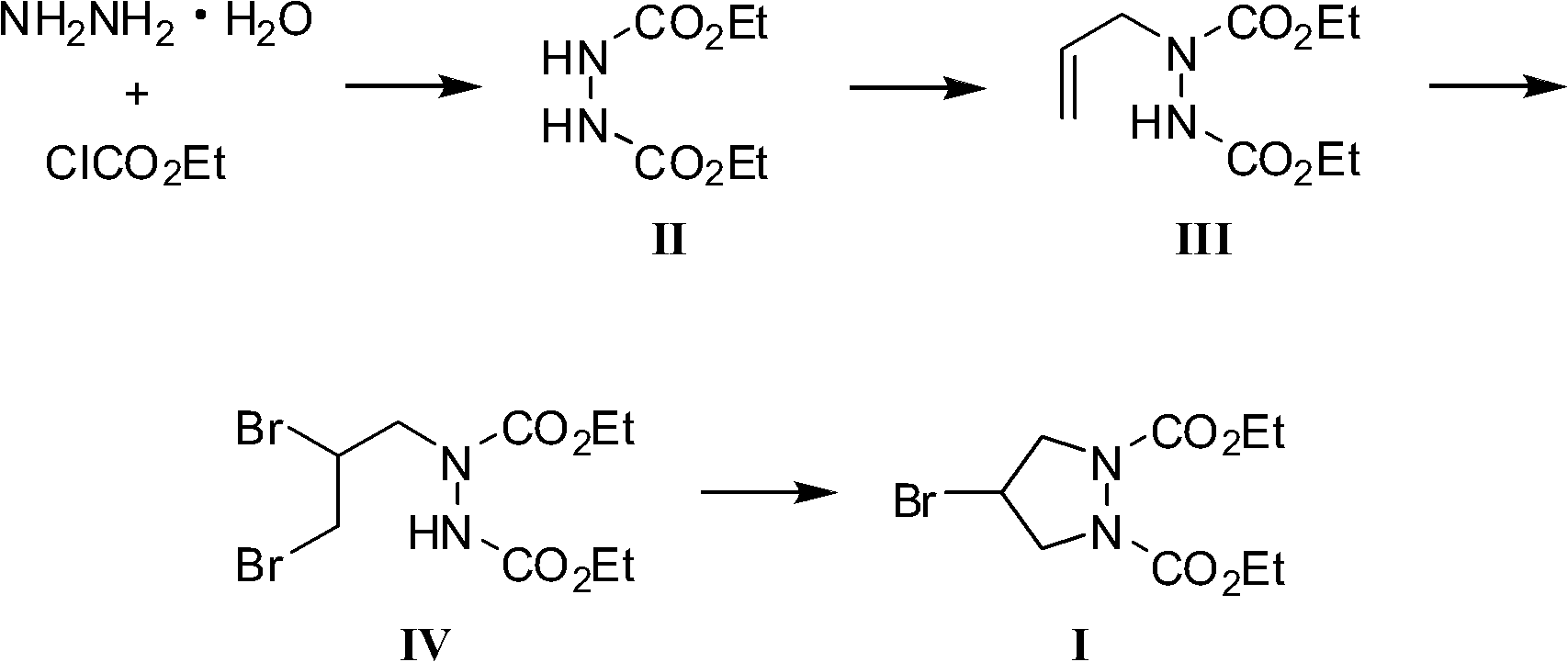
InactiveCN102180831AWide variety of sourcesSufficient supplyOrganic chemistryEthyl chloroformateHydrazine compound
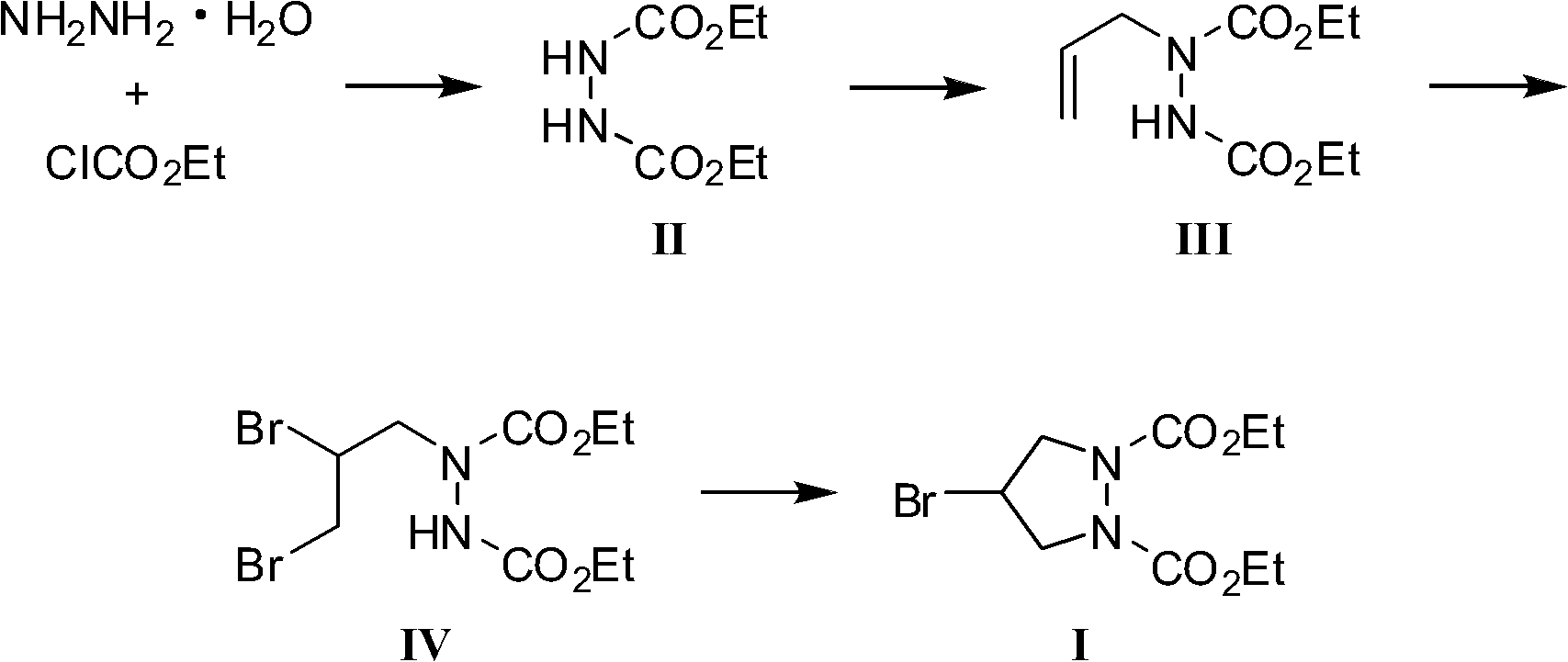
The invention provides a preparation method of a biapenem intermediate and relates to a preparation method of an antibiotic medicament biapenem intermediate 4-bromine-1,2-ethoxycarbonyl pyrazolidine. The method comprises the following steps: successively carrying out a reaction on hydrazine hydrate as a raw material and ethyl chloroformate and allyl bromide; and then carrying out addition reaction with bromide, and finally, carrying out cyclization reaction under the alkaline condition so as to obtain the product. The invention provides a bran-new synthesis route; and used raw material sources are wide and abundant, the prices of the raw materials are cheap, reaction condition is mild, process is simple, reactions in various steps are conventionally operated, and product cost is reduced.
Owner:TONGJI UNIV
Diagnostic radiographic silver halide photographic film material
A single-side coated silver halide photographic film material has been disclosed, said film material comprising a support, at least one light-sensitive emulsion layer and a substantially light-insensitive protective hydrophilic colloid layer farther away from said support than said emulsion layer, wherein said emulsion layer contains a silver halide emulsion rich in silver bromide with cubic crystals having an average numerical diameter in the range from 0.4 up to 0.8 mum, wherein at least 95 mole % of bromide ions are present, and wherein said hydrophilic colloid layer or another substantially light-insensitive hydrophilic colloid layer essentially comprises a hydrazide represented by the general formula (I) having a silver halide adsorbing group or a masked silver halide adsorbing group; besides a method for forming a diagnostic image comprising the steps of contacting said photographic film material with an intensifying screen, forming a film / screen assembly, and exposing said assembly to X-ray radiation with an energy lower than or equal to 70 kVp, and processing said film material during a time of 90 seconds or less in a processing cycle following the steps of developing, fixing, rinsing and drying, and wherein the developing proceeds in a radiographic developer composition essentially comprising a hydroquinone and a phenidone (a 1-phenyl-3-pyrazolidine-1-one compound) as a developing agent and a heteroatomic nitro-indazol.
Owner:T2PHARMA GMBH
Preparation method of key intermediate of 3-bromo-1-(3-chloro-2-pyridyl)-1H-imidazole-5-carboxylic acid
ActiveCN113024509AIncrease productivityReduce manufacturing costOrganic chemistryCarboxylic acidHydrolysis
The invention discloses a preparation method of a key intermediate of 3-bromo-1-(3-chloro-2-pyridyl)-1H-imidazole-5-carboxylic acid. The method comprises the following steps: by taking 2,3-dichloropyridine as an initial raw material, carrying out a hydrazine hydrate reflux reaction, and then conducting cooling and centrifuging to obtain a 3-chloro-2-pyridyl wet product; then, enabling the 3-chloro-2-pyridyl wet product to react with diethyl maleate and sodium ethoxide to generate 2-(3-chloro-2-pyridyl)-5-oxo-3-pyrazolidine; then carrying out bromination reaction on the 2-(3-chloro-2-pyridyl)-5-oxo-3-pyrazolidine and phosphorus oxybromide to produce a 3-bromo-1-(3-chloro-2-pyridyl)-4,5-dihydro-1H-pyrazole-5-carboxylic acid ethyl ester wet product; putting the 3-bromo-1-(3-chloro-2-pyridyl)-4,5-dihydro-1H-pyrazole-5-carboxylic acid ethyl ester wet product into water, adding sodium persulfate, and carrying out a reaction so as to obtain 3-bromo-1-(3-chloro-2-pyridyl)-1H-pyrazole-5-carboxylic acid ethyl ester; and conducting hydrolysis in water to obtain 3-bromo-1-(3-chloro-2-pyridyl)-1H-imidazole-5-carboxylic acid. The method can greatly improve the production efficiency and reduce the production cost, and can be directly used for the next reaction without drying treatment.
Owner:杭州新桂实业有限公司
Synthesis method of chiral phosphorus-contained pyrazolone compound
ActiveCN103923121ASimple and fast operationMild reaction conditionsGroup 5/15 element organic compoundsPhosphorous acidEnantio selectivity
The invention discloses a synthesis method of a chiral phosphorus-contained pyrazolone compound. The synthesis method concretely comprises the step: with pyrazolidinone imine and diaryl phosphorus oxide or dimethyl phosphite as a reactant, synthesizing the chiral phosphorus-contained pyrazolone compound in a solvent under the analysis of multifunctional chiral squaric acid amide. The method disclosed by the invention is simple and easy to obtain raw material, mild in reaction condition, simple and convenient for after-treatment, wide in applicable substrate range, high in yield and high in enantioselectivity; in addition, the chiral phosphorus-contained pyrazolone compound synthesized by using the method can be used for synthesizing an intermediate of a drug and an insecticide.
Owner:SUZHOU UNIV
Diagnostic radiographic silver halide photographic film material
InactiveUS20040009423A1Improved gradationQuality improvementX-ray/infra-red processesRadiation applicationsX-rayKetone
A single-side coated silver halide photographic film material has been disclosed, said film material comprising a support, at least one light-sensitive emulsion layer and a substantially light-insensitive protective hydrophilic colloid layer farther away from said support than said emulsion layer, wherein said emulsion layer contains a silver halide emulsion rich in silver bromide with cubic crystals having an average numerical diameter in the range from 0.4 up to 0.8 mum, wherein at least 95 mole % of bromide ions are present, and wherein said hydrophilic colloid layer or another substantially light-insensitive hydrophilic colloid layer essentially comprises a hydrazide represented by the general formula (I) having a silver halide adsorbing group or a masked silver halide adsorbing group; besides a method for forming a diagnostic image comprising the steps of contacting said photographic film material with an intensifying screen, forming a film / screen assembly, and exposing said assembly to X-ray radiation with an energy lower than or equal to 70 kVp, and processing said film material during a time of 90 seconds or less in a processing cycle following the steps of developing, fixing, rinsing and drying, and wherein the developing proceeds in a radiographic developer composition essentially comprising a hydroquinone and a phenidone (a 1-phenyl-3-pyrazolidine-1-one compound) as a developing agent and a heteroatomic nitro-indazol.
Owner:T2PHARMA GMBH
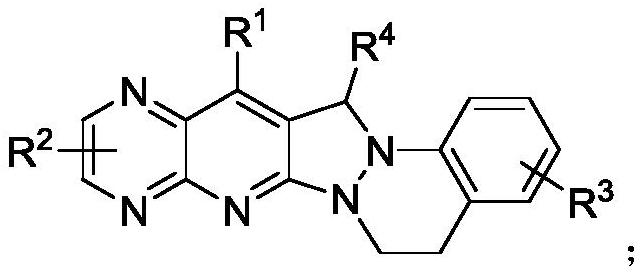
Pyridopyrazolo cinnoline compound, preparation method and application thereof
PendingCN111892601AEasy to prepareGood substrate universalityAntibacterial agentsOrganic chemistryEscherichia coliAntimicrobial drug
The invention provides a pyridopyrazolo cinnoline compound and a preparation method thereof. According to the invention, the pyridopyrazolo cinnoline compound is prepared from a 1-aryl-2-vinyl pyrazolidone compound and an aminopyrazinone compound through a Friedlander reaction and an arylhydroalkylation reaction at the same time under the action of a catalyst and an additive; the preparation method is simple, and the compound has good substrate universality; the pyridopyrazolo cinnoline compound provided by the invention is a brand-new synthetic method provided on the basis of the prior art; and the synthesized pyridopyrazolo cinnoline compound has good antibacterial activity on escherichia coli, pseudomonas aeruginosa, staphylococcus aureus and bacillus subtilis, and a new choice is provided for research and clinical use of antibacterial drugs.
Owner:朱继强
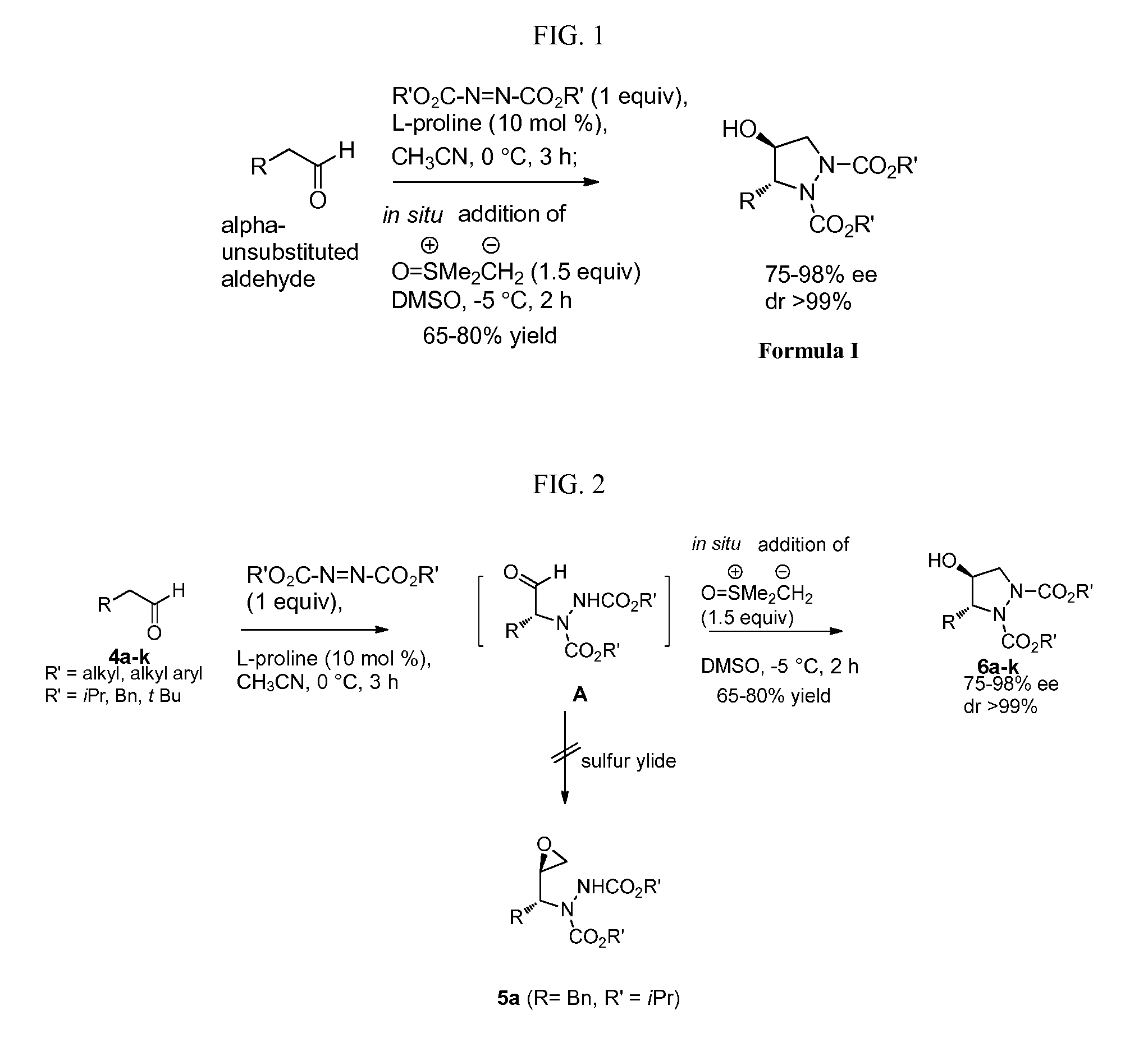
Organocatalytic synthesis of chiral pyrazolidines and their analogues
Disclosed herein is a highly enantio- (75 to 98% ee) and diastereoselective (99 to 100% de) synthesis of functionalized pyrazolidines via tandem a-amination-Corey Chaykovsky reaction of alpha unsubstituted aldehydes.
Owner:COUNCIL OF SCI & IND RES
Synthesis and antimicrobial uses of dinuclear silver(i) pyrazolates
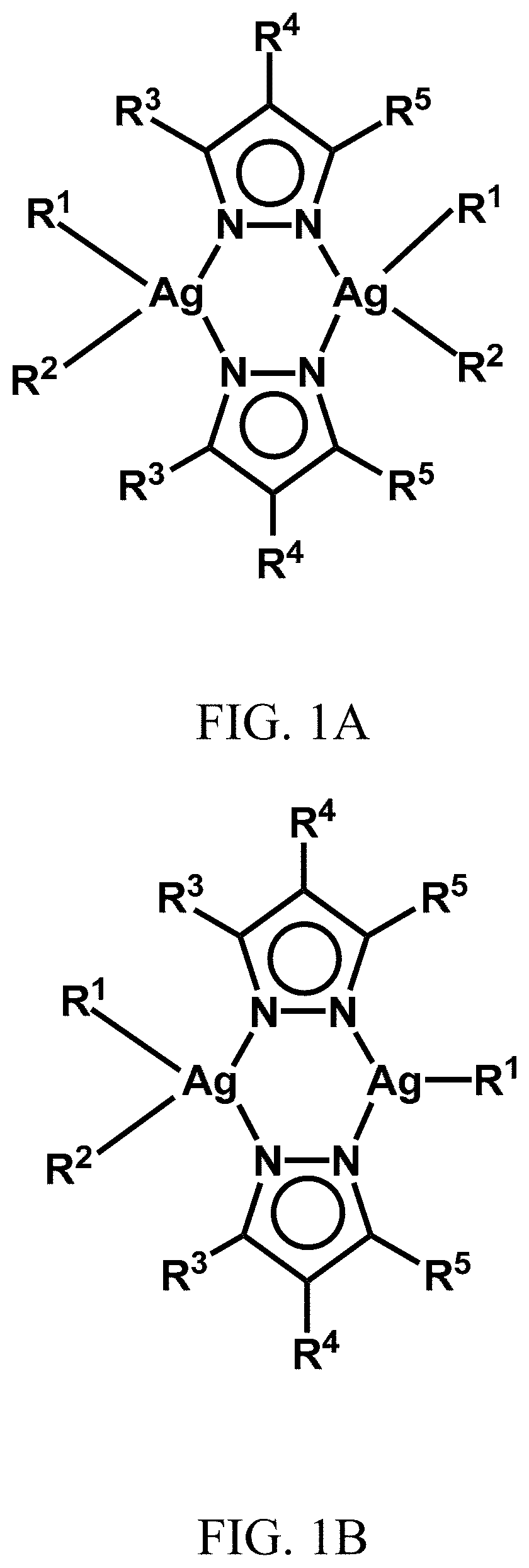
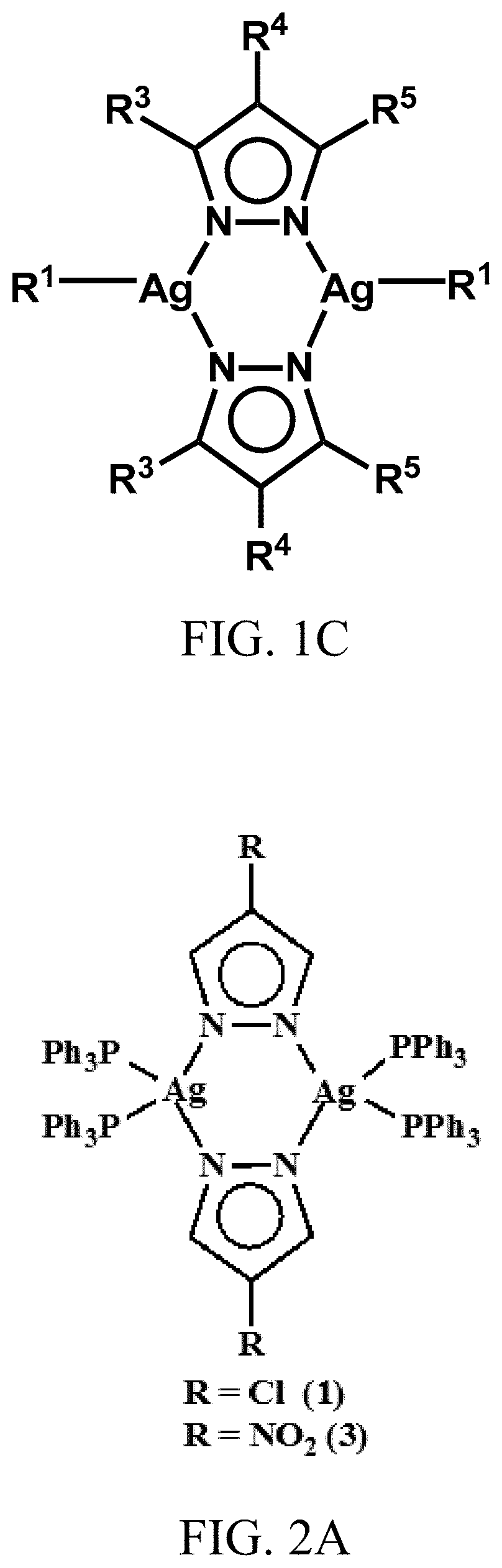
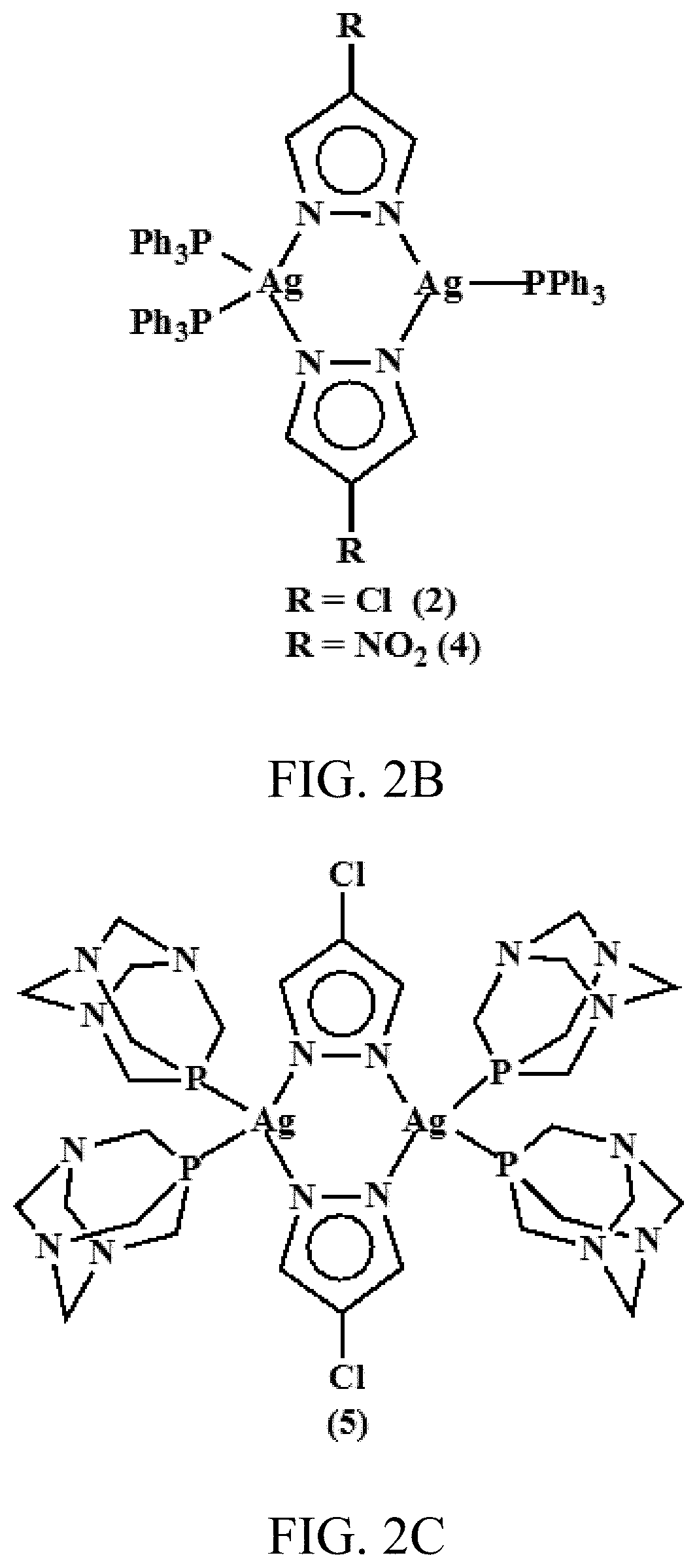
ActiveUS20200061110A1Excellent aqueous solubilityImprove lipophilicityAntibacterial agentsGroup 1/11 organic compounds without C-metal linkagesBiotechnologyAntibiosis
Novel dinuclear silver(I) pyrazolido complexes and methods of synthesizing them are provided. Advantageously, the novel silver(I) pyrazolido complexes have excellent antimicrobial activity and methods of using said complexes to treat bacterial, fungal, and viral infections are also provided.
Owner:FLORIDA INTERNATIONAL UNIVERSITY
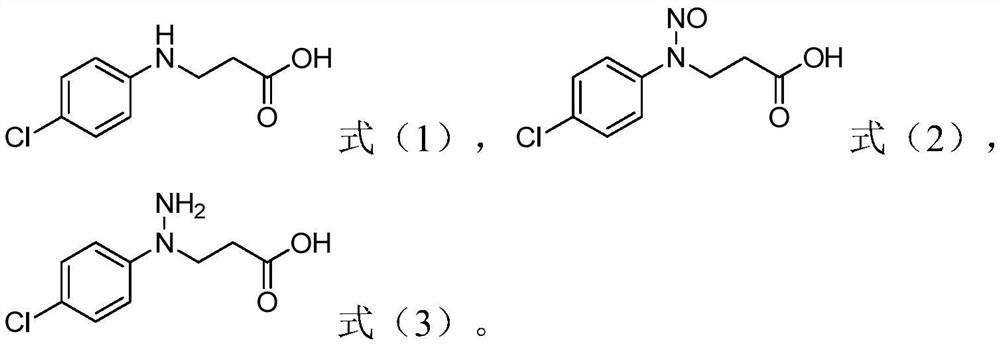
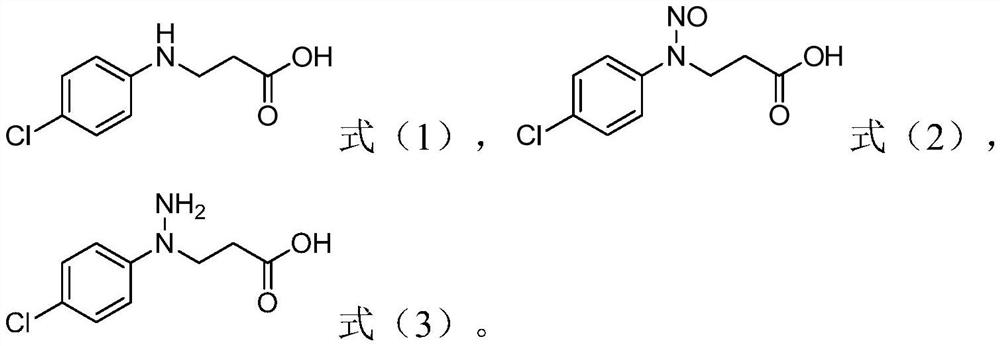
Method for preparing 1-(4-chlorphenyl)-pyrazolidine-3-one
ActiveCN112341392AAvoid steps such as restoreHigh reaction safetyOrganic chemistryP-chloroanilinePalladium on carbon
The invention relates to the field of bactericide synthesis, and discloses a method for preparing 1-(4-chlorphenyl)-pyrazolidine-3-one. The method comprises the following steps: 1) reacting parachloroaniline with acrylic acid to obtain an addition reaction product; 2) purifying the addition reaction product, and carrying out nitrosation reaction on the purified addition reaction product and nitrite, or directly carrying out nitrosation reaction on the addition reaction product and nitrite to obtain a nitrosation reaction product; 3) performing reduction by taking palladium on carbon as a catalyst and hydrogen as a reducing agent to obtain a reduction product; and 4) removing the catalyst from the reduction product, and carrying out heat treatment to obtain 1-(4-chlorphenyl)-pyrazolidine-3-one. According to the method, the steps of diazotization, sodium sulfite reduction and the like are avoided, the reaction safety is high, the amount of three wastes is small, and the method is suitable for industrial production.
Owner:NUTRICHEM LAB CO LTD
A kind of preparation method of fipronil intermediate
The invention relates to a preparation method of a fipronil intermediate-5-amino-1-(2,6-dichloro-4-(trifluoromethyl)phenyl)-4-(trifluoromethylthio)-1H-pyrazoles-3-nitrile. According to the invention, a brand new reaction mechanism is used for using 5-amino-3cyan-1-(2,6-dichloro4-(trifluoromethyl)phenyl)-pyrazolidine sulfenyl to prepare 5-amino-1-(2,6-dichloro-4-(trifluoromethyl)phenyl)-4-(trifluoromethylthio)-1H-pyrazoles-3-nitrile. In the reaction process, usage of severe toxic substances such as PCI can be avoided, phosphorous oxychloride with little toxicity is taken as a chloridizing agent, security of the preparation process is greatly increased, and the purity of the products can reach more than 95%.
Owner:安徽美诺华药物化学有限公司
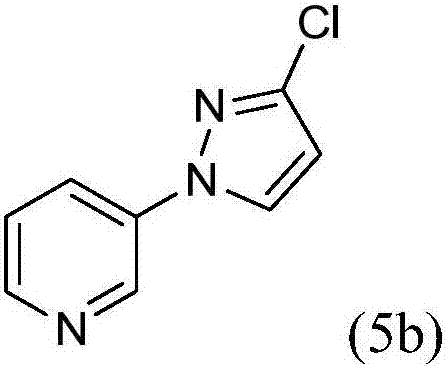
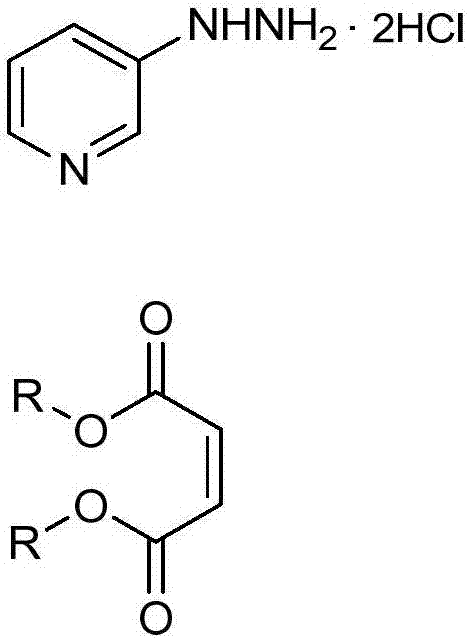
Process for the preparation of 3-(3-chloro-1h-pyrazol-1-yl)pyridine
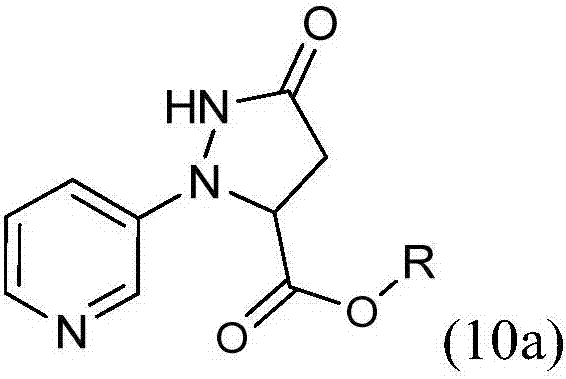
3-(3-chloro-1H-pyrazol-1-yl)pyridine is prepared by cyclizing 3-hydrazinopyridine-*dihydrochloride with a dialkyl maleate to provide an alkyl 5-oxo-2-(pyridin-3-yl)pyrazolidine-3-carboxylate, by chlorinating to provide an alkyl 3-chloro-1-(pyridin-3-yl)-4,5-dihydro-1H-pyrazole-5-carboxylate, by oxidizing to provide an alkyl 3-chloro-1-(pyridin-3-yl)-1H-pyrazole-5-carboxylate, by converting the ester to the carboxylic acid by hydrolysis to provide 3-chloro-1-(pyridin-3-yl)-1H-pyrazole-5-carboxylic acid hydrochloride, and by removing the carboxylic acid by a decarboxylation reaction.
Owner:DOW AGROSCIENCES LLC
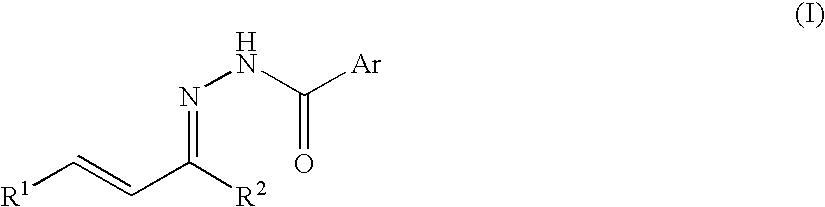
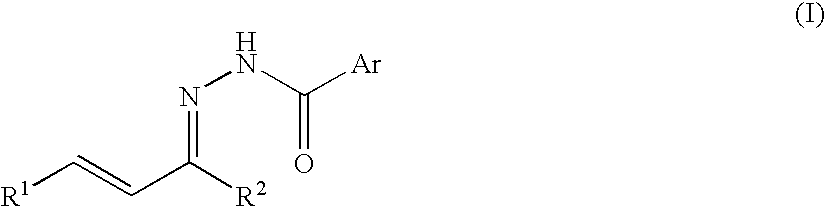
Process for producing nitrogenous 5-membered cyclic compound
A method of the intramolecular and intermolecular cyclization of an N-acylhydrazone for obtaining a pyrazoline skeleton or pyrazolidine skeleton under ordinary conditions with high stereoselectivity and in high yield. An N-acylhydrazone represented by the following formula (I): (wherein R1 and R2 are the same or different and each represents hydrogen or a hydrocarbon group and Ar represents an optionally substituted aromatic hydrocarbon group) is converted to an N-acylpyrazoline derivative with high stereoselectivity in the presence of a Lewis acid catalyst or asymmetric Lewis acid catalyst.
Owner:JAPAN SCI & TECH CORP
Synthetic method of pyraclostrobin intermediate pyrazole alcohol
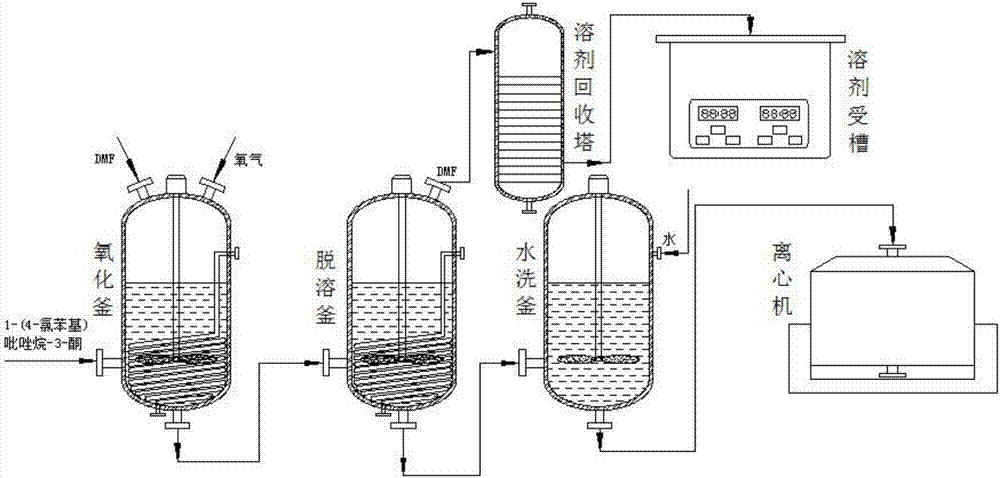
The invention discloses a synthetic method of a pyraclostrobin intermediate pyrazole alcohol. The process method specifically comprises the following steps: inputting 1-(4-chlorphenyl)-pyrazolidine-3-one in a reaction kettle; adding a solvent DMF and introducing oxygen for 5-6 hours; keeping the temperature for 1 hour; after oxidizing reaction, transferring the solution into a desolventizing kettle; evaporating and recovering DMF; after desolventization, adding water to wash and centrifugalize; and obtaining a solid phase light yellow solid 1-(4-chlorphenyl)-3-pyrazole. The synthetic method can effectively recover the solvent after initial reaction of the pyraclostrobin intermediate pyrazole alcohol, so that the synthetic cost is lowered, the economical benefit is improved, the synthetic process design of the pyraclostrobin intermediate pyrazole alcohol is optimized, and the demand on the synthetic process design of the pyraclostrobin intermediate pyrazole alcohol is met.
Owner:ANHUI GUANGXIN AGROCHEM
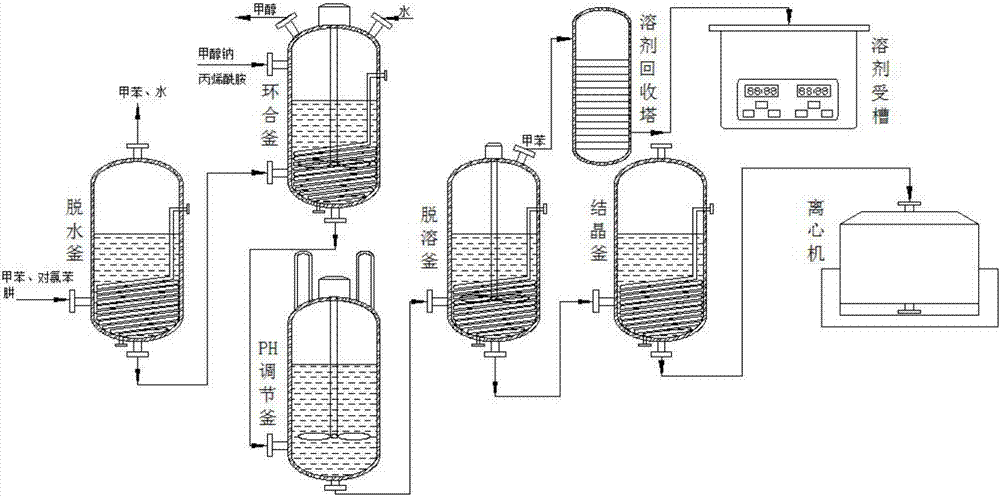
Synthesis process of pyraclostrobin intermediate pyrazolidone
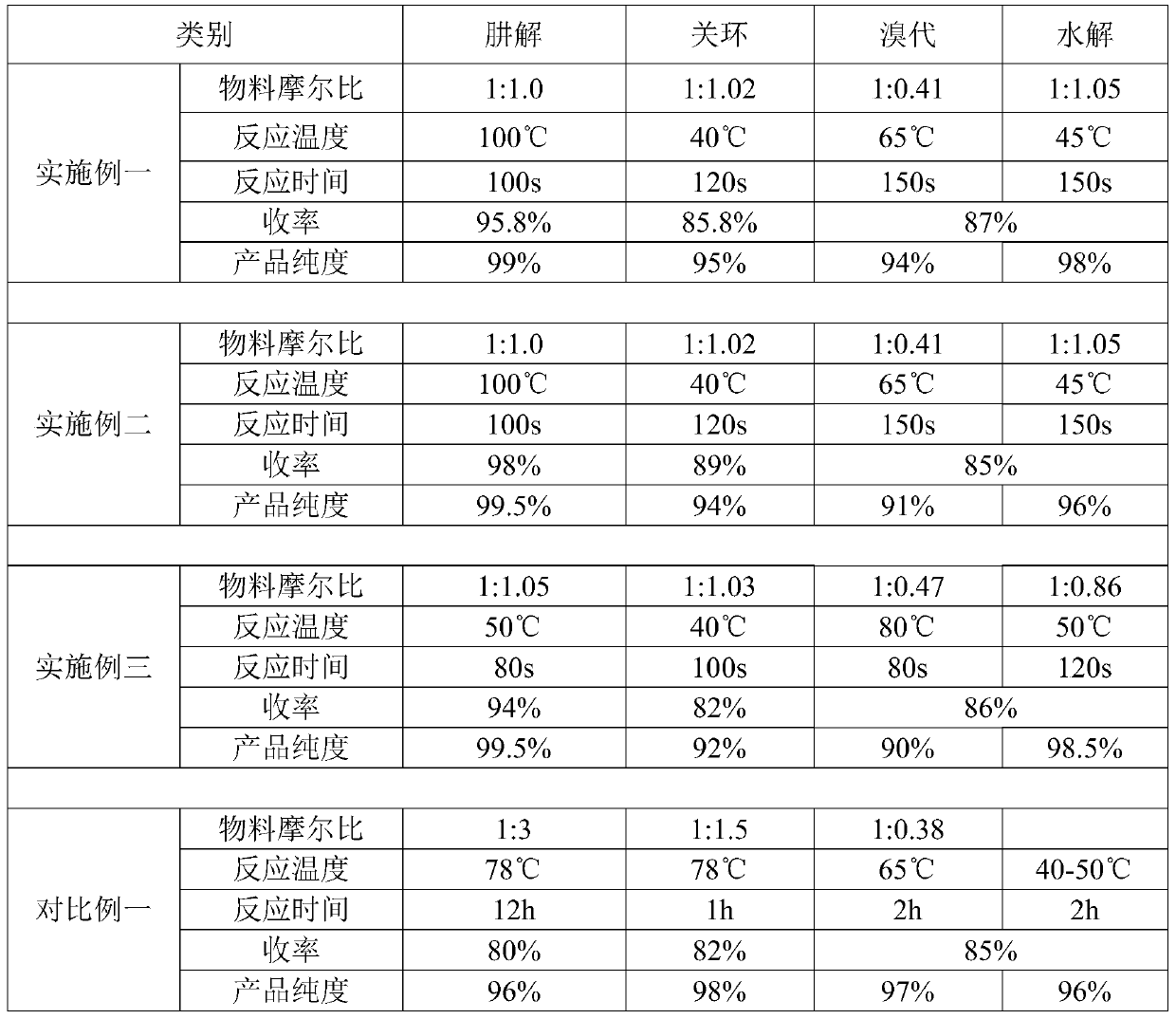
The invention discloses a synthesis process of pyraclostrobin intermediate pyrazolidone. The process method particularly comprises the following steps: putting a p-chlorophenylhydrazine toluene solution into a dehydration kettle, performing temperature-rising azeotropy to take out toluene and water, transferring to a cyclization kettle after dehydration is completed, putting sodium methoxide solid into the cyclization kettle, adding quantitative acrylic amide, performing heat-preserving reaction at 60 to 70 DEG C for 2 to 3 hours, distilling and recovering methanol after the reaction, adding water to terminate the reaction, adding quantitative hydrochloric acid to adjust the pH value to be 5 to 6, transferring the materials to a desolvation kettle after the pH value is adjusted, evaporating and recovering the solvent toluene, transferring the materials to a crystallization kettle after desolvation is completed, cooling, crystallizing, centrifuging and performing solidoid to obtain 1-(4-chlorphenyl)pyrazolidine-3-ketone. By the synthesis process, the solvent can be effectively recovered after the initial reaction of the pyraclostrobin intermediate pyrazolidone is completed, the synthesis cost is reduced, the economic benefit is improved, the synthesis process design of the pyraclostrobin intermediate pyrazolidone is optimized, and the design requirements are met.
Owner:ANHUI GUANGXIN AGROCHEM
A kind of preparation method of biapenem
The invention belongs to the technical field of medicines, and discloses a preparation method of biapenem. The method comprises the following steps: p-nitrobenzyl (1R,5R,6S)-6-[(1R)-1-hydroxyethyl]-2-[(diphenylphosphono)oxy]-1-methylcarboxyl pen-2-em-3-carboxylate and 4-sulfydryl-N,N-bis(p-nitrobenzyloxycarbonyl)pyrazolidine are used as raw materials, substitution is performed, hydrogenation is performed and cyclization is performed to synthesize the biapenem. The method provided by the invention overcomes the defects of a long reaction route, easy degradation of raw materials in the reactionprocess, a low yield and the like in the prior art, and is more suitable for industrial production.
Owner:SHANDONG LUOXIN PHARMA GRP CO LTD +2
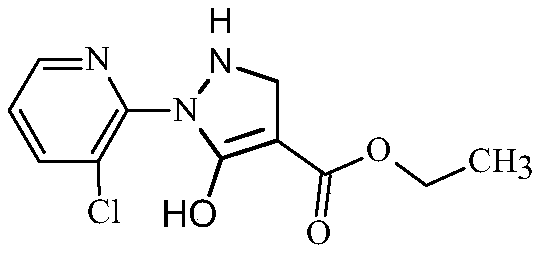
Synthesis method of 2-(3-chloropyridine-2-yl)-5-hydroxy-3-pyrazolidine ethyl formate
InactiveCN110483480AMild reaction conditionsNot easy to decomposeOrganic chemistrySynthesis methodsSolvent
The invention discloses a synthesis method of 2-(3-chloropyridine-2-yl)-5-hydroxy-3-pyrazolidine ethyl formate, which comprises the following steps: dissolving 3-chloro-2-aminopyridine in a mixed solution of an organic acid or inorganic acid and a solvent A, and carrying out diazotization at -5 to 10 DEG C in the presence of a diazotization reagent to obtain a diazonium solution; dropwise adding the obtained diazonium liquid into diethyl succinate (diethyl n-succinate) for reaction, and adding alkali and preserving heat in the reaction.. Through the route of diazotization, coupling and cyclization, the reaction conditions are mild; meanwhile, the diazonium salt of the 3-chloro-2-aminopyridine is relatively stable and is not easy to decompose and explode, and the synthesis is safe and reliable. Metal sodium or sodium ethoxide is not used, so that the safety risk in the synthesis process is reduced. The yield of the target product can reach 72% or above and is higher than that of a current common process method.
Owner:江苏优普生物化学科技股份有限公司
Substituted thiazoles for preventing and/or treating cell or tissue necrosis
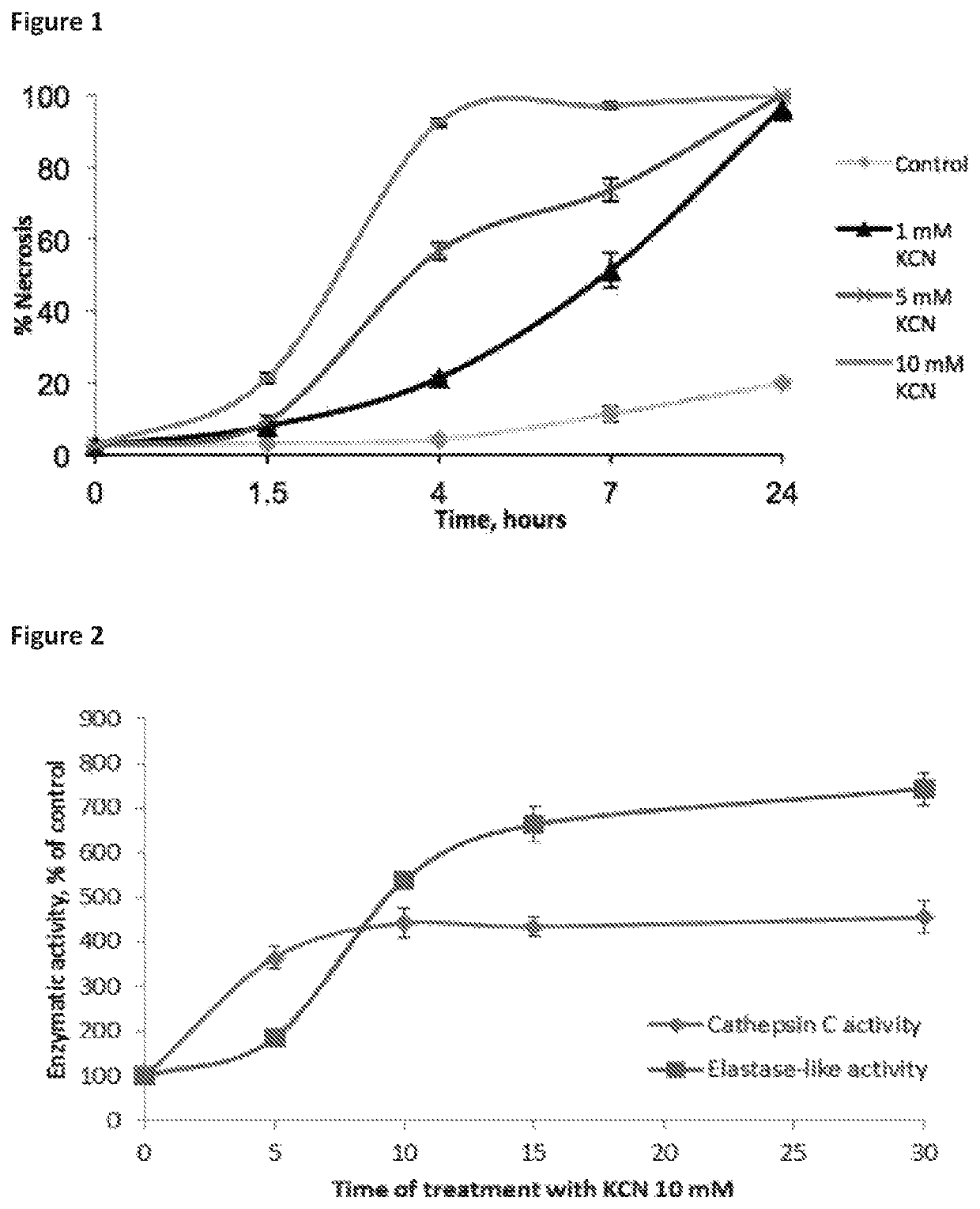
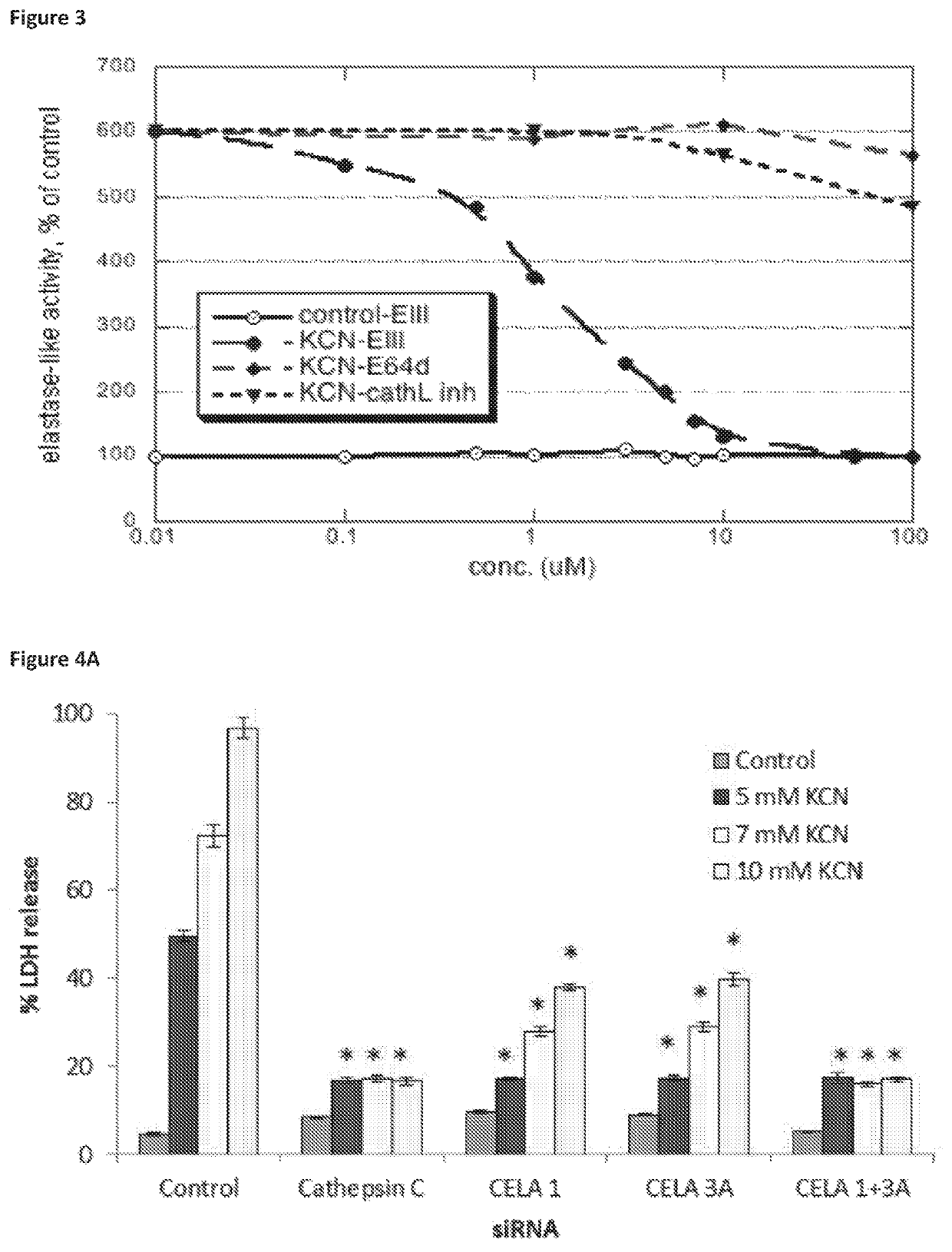
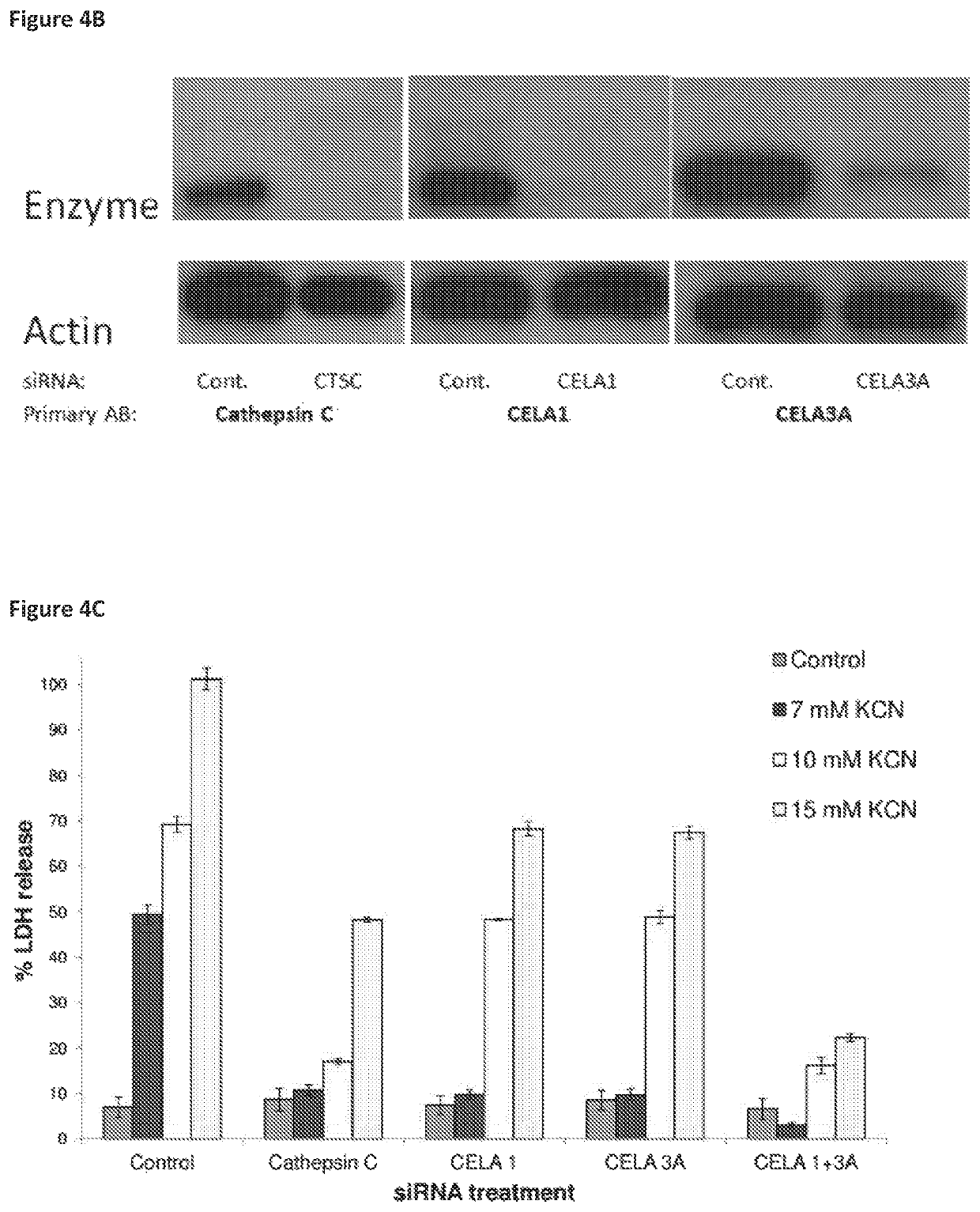
ActiveUS10968185B2Preventing and treating and halting/abrogating necrosisGood lookingAntibacterial agentsOrganic active ingredientsCathepsin CCombination therapy
Inhibitor compounds and agents of Cathepsin C, CELA1, CELA3A and / or structurally related molecules thereto, compositions comprising same and uses thereof in the inhibition and / or prevention of cell and / or tissue necrosis are described. The compounds include thiazoles of Formula II,where G1 is an optionally substituted pyrrolidine, an optionally substituted pyridine, an optionally substituted aryl, an optionally substituted piperidine, an optionally substituted piperazine, an optionally substituted imidazolidine, or an optionally substituted pyrazolidine. G3 isor G3 is an optionally substituted alkyl, an optionally substituted aryl or an optionally cyloalkyl. G2 is an optionally substituted alkyl, an optionally substituted aryl, an optionally substituted cyloalkyl or an optionally substituted heterocycle. Various applications for the described compounds, and combination therapies are described as well.
Owner:ELA PHARMA LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com