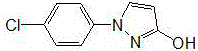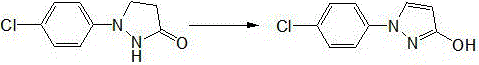Preparation method of 1-(4-chlorophenyl)-3-pyrazole alcohol
A technology of chlorophenyl and pyrazolidine, which is applied in the field of preparation of 1--3-pyrazolol, which can solve the problems of high temperature, large energy consumption, high cost of raw materials, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] First add 60g of acetic acid into the device, add 19.66g (0.1mol) of 1-(4-chlorophenyl)pyrazolidin-3-one and 0.0162g (0.0001mol) of ferric chloride under stirring, and heat up to 50°C , react with air, remove most of the solvent under reduced pressure after 4h, add water and stir, a large amount of product precipitates, adjust the pH value to 7 with sodium hydroxide solution, continue stirring for 0.5h, filter to obtain 1-(4-chlorophenyl)- 3-pyrazolol 19.4g, yield 99.7%, content 99.6% (LC).
Embodiment 2
[0023] First add 100g of 88% formic acid to the device, add 19.66g (0.1mol) of 1-(4-chlorophenyl)pyrazolidin-3-one and 1.62g (0.01mol) of ferric chloride under stirring, and heat up To 100°C, react with air, remove most of the solvent under reduced pressure after 4h, add water and stir, a large amount of product precipitates, adjust the pH value to 5 with sodium hydroxide solution, continue stirring for 0.5h, and filter to obtain 1-(4-chlorobenzene Base)-3-pyrazolol 19.32g, yield 99.3%, content 99.1% (LC).
Embodiment 3
[0025] First add 80g of propionic acid into the device, add 19.66g (0.1mol) of 1-(4-chlorophenyl)pyrazolidin-3-one and 0.81g (0.005mol) of ferric chloride under stirring, and heat up to 80 After 4 hours, remove most of the solvent under reduced pressure, add water and stir, a large amount of product precipitates, adjust the pH value to 8 with sodium hydroxide solution, continue stirring for 0.5h, and filter to obtain 1-(4-chlorophenyl) -3-pyrazolol 19.28g, yield 99.1%, content 99.7% (LC).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com