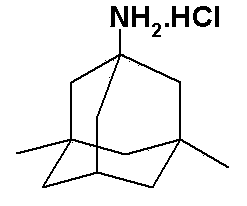
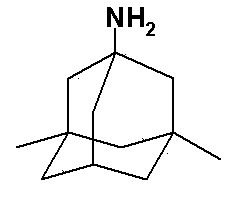
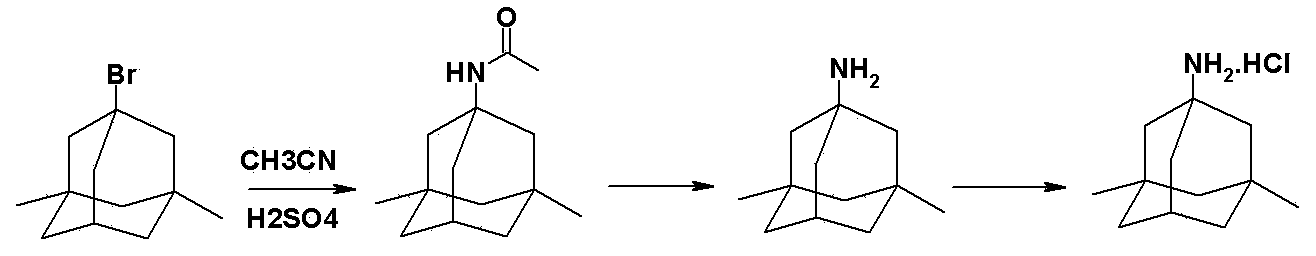
Memantine hydrochloride preparation method
A technology of memantine hydrochloride and hydrochloric acid is applied in the preparation of organic compounds, the preparation of amino compounds, chemical instruments and methods, etc., and can solve the problems of instability, difficult post-processing, and excessively long reaction time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Put 24 grams of 1-bromo-3,5-dimethyladamantane and 8 grams of methyl carbamate into a reaction bottle, add 50 ml of 98% formic acid, stir and dissolve at room temperature, protect with argon, and heat to 90°C for reaction 0.5 hours. Cool to room temperature, distill off formic acid under reduced pressure, then add 24 ml of concentrated hydrochloric acid, stir, heat to 90°C, and react for 3 hours. Cool to 25°C, crystals slowly precipitate out, and after the crystallization is complete, filter to obtain memantine hydrochloride.
[0044] GC purity: 99.5%, yield: 76%;
[0045] GC conditions: instrument Agilent 6960; column: Agilent HP-5; Int:340 o C; Det: 340 o C flow: 3.0 ml / min; GC purity: 99.8%;
[0046] MS(ESI): m / z = 180.2 [M+H + ];
[0047] 1 HNMR (400 MHz, CDCl 3 ):δ 0.793 (s, 6H), 1.161-1.064 (dd, 2H ), 1.283-1.279 (dd, 4H), 1.455-1.372 (dd,4H), 1.621-1.615 (d, 2H), 2.148-2.132 ( t,1H);
[0048] 13 C NMR (400MHz, CDCl 3): δ21.32 (CH3), 27.30 (CH2), 32.51...
Embodiment 2
[0050] Put 24 grams of 1-bromo-3,5-dimethyladamantane and 36 grams of ethyl carbamate into a reaction flask, add 240 ml of 98% formic acid, stir and dissolve at room temperature, protect with argon, and heat to 90°C for reaction 1 hour. Cool to room temperature, evaporate the formic acid under reduced pressure, then add 60 ml of hydrochloric acid with a mass concentration of 10%, stir, heat to 110°C, and react for 4 hours. After cooling to 0°C, crystals precipitate out. After the crystallization is complete, filter to obtain memantine hydrochloride.
[0051] GC purity: 99.0%, yield: 85%.
Embodiment 3
[0053] Put 24 grams of 1-bromo-3,5-dimethyladamantane and 15 grams of methyl carbamate into a reaction flask, add 100 ml of 88% formic acid, stir and dissolve at room temperature, protect with argon, and heat to 140°C for reaction 1.5 hours. Cool to room temperature, evaporate formic acid under reduced pressure, then add 50 ml of 20% hydrochloric acid, stir, heat to 105°C, and react for 3 hours. After cooling to 5°C, crystals precipitate out. After the crystallization is complete, filter to obtain memantine hydrochloride.
[0054] GC purity: 99.2%, yield: 79%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com