Synthesis method of 4-nitrobenzene ethane
A synthetic method, the technology of nitroethylbenzene, which is applied in the field of synthesis of 4-nitroethylbenzene, can solve the problems of no ortho-para selectivity, unsatisfactory yield, high price, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
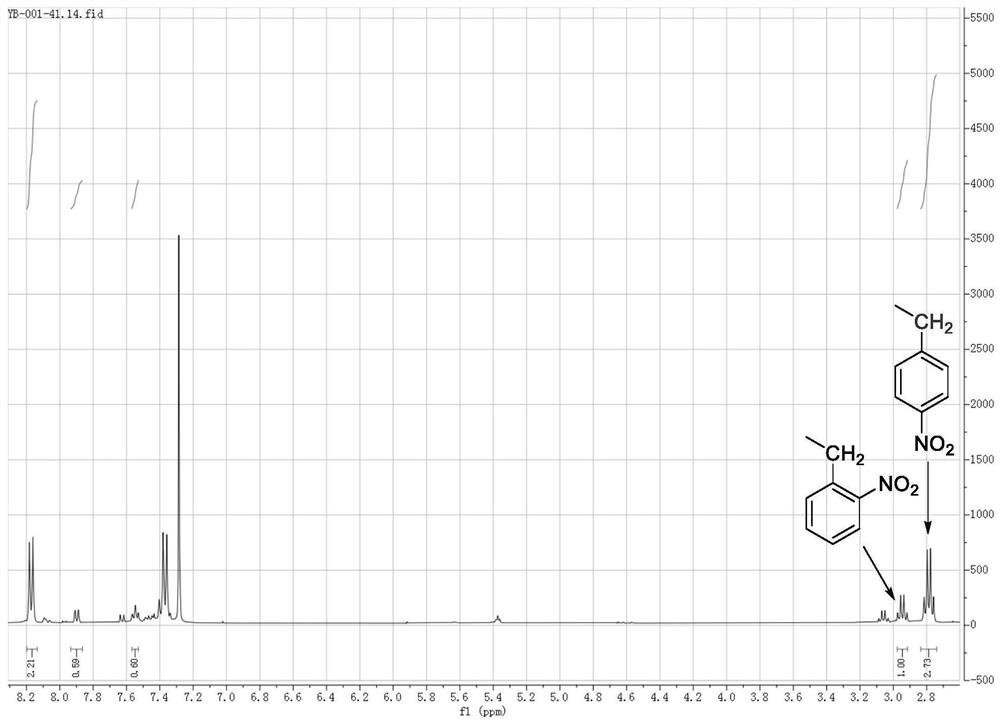
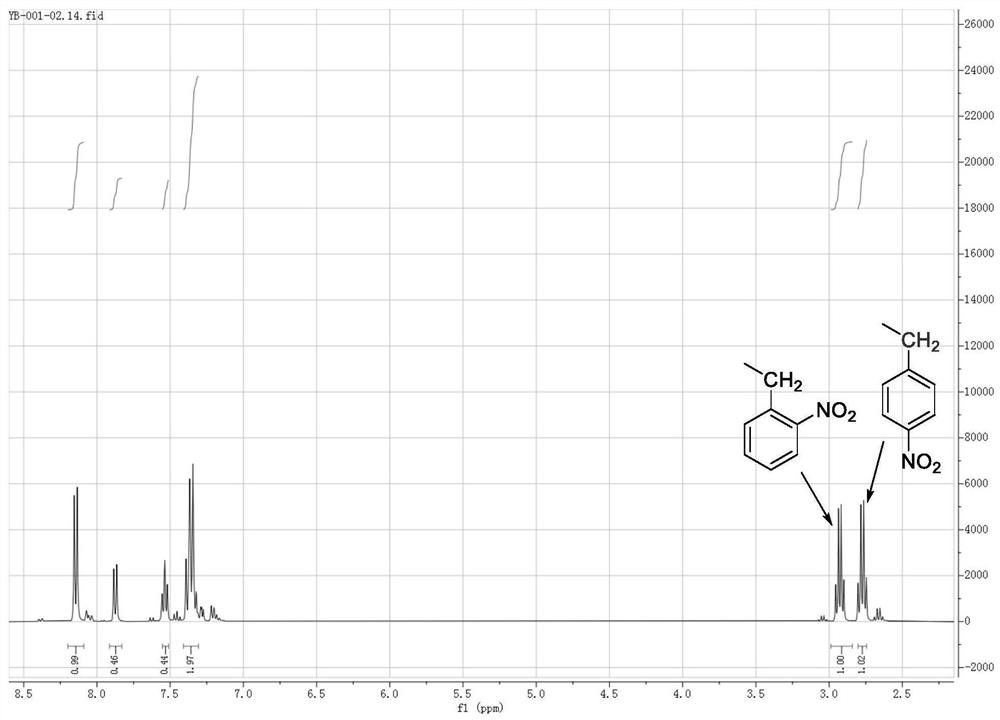
[0025] Ethylbenzene (106.2 g, 1mol) was dissolved in nitromethane (150ml), stirred, and the fumetic acid (63g, 1.0mol) and urea (0.3 g, 0.005mol) were added dropwise, respectively, the reaction temperature was controlled at 45 °C, the reaction was 2.0 hours, and the product was nuclear magnetic resonance hydrogen spectrogram as shown Figure 1 As shown, 148 °C distillation collected product 4-nitroethylbenzene 111.9g, yield 74%, distillation to recover the solvent nitromethane.
[0026] Figure 1 In the middle, the integration height shows 2-nitroethylbenzene: 4-nitroethylbenzene = 1:3.72, showing good reaction selectivity, and the arrows on the figure indicate the chemical displacement and integration of ethyl, Figure 1 Description, the present invention provides a method product of only a very small amount of dinitroethylbenzene generated, indicating that the synthesis method of the present invention is very selective, the target 4- nitroethylbenzene is the dominant product.
[0...
Embodiment 2
[0029] Ethylbenzene (106.2 g, 1mol) was dissolved in acetic acid (90ml), stirred, and the fuming nitric acid (63g, 1mol) and potassium nitrate (0.425 g, 0.005 mol) were added dropwise, the reaction temperature was controlled at 40 °C, the reaction was 2.5 hours, the solvent acetic acid was recovered by distillation, and the collection product 4-nitroethylbenzene was distilled at 148 °C at 90.7 g, with a yield of 60%.
Embodiment 3
[0031] Ethylbenzene (106.2 g, 1mol) was dissolved in acetic acid (85ml), stirred, and smoked nitric acid (63g, 1mol) and urea (0.425 g, 0.005 mol) were added dropwise, respectively, the reaction temperature was controlled at 40 °C, the reaction was 2.5 hours, the solvent acetic acid was recovered by distillation, and the collection product 4-nitroethylbenzene 105.8g was distilled at 148 °C, with a molar yield of 70%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com