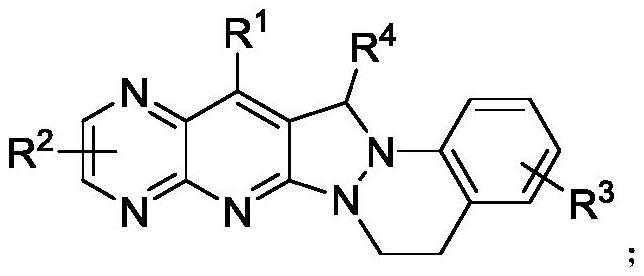
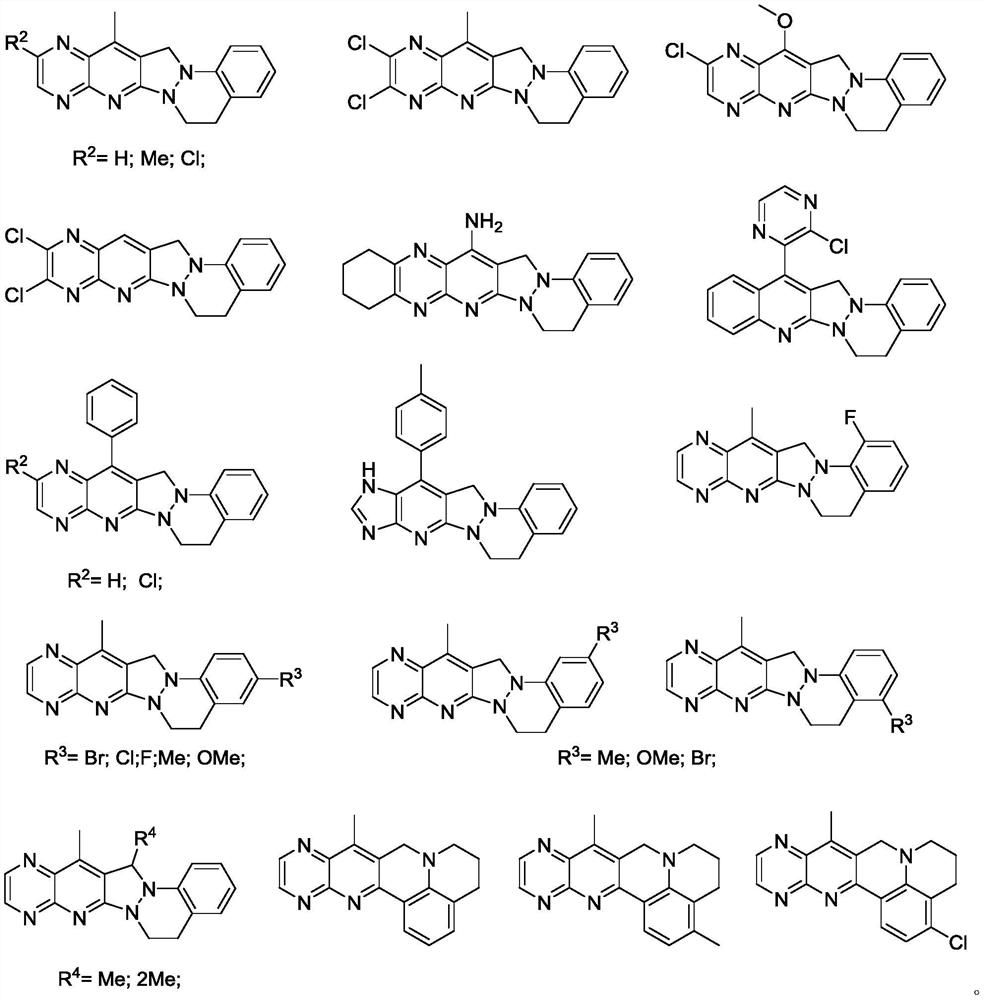
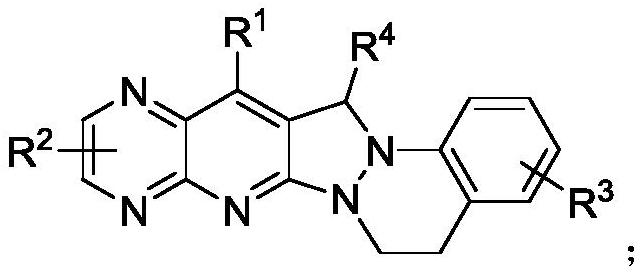
Pyridopyrazolo cinnoline compound, preparation method and application thereof
A technology for pyridopyrazole and compound, which is applied in the field of pyridopyrazolocinnoline compounds and their preparation, can solve the problems of increase, the research lag of compound functionality, etc., and achieves good antibacterial activity, good substrate generalization The effect of adaptability and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Preparation of compound 4aa:
[0063] Add 1mmol of 1-phenyl-2-vinylpyrazolidin-3-one and 1.2mmol of 1-(3-aminopyrazin-2-yl)ethanone into the reaction flask, vacuumize and change nitrogen, and Add 0.01mmol of [Cp*RhCl under nitrogen protection 2 ] 2 , 0.2 mmol of Pd(OAc) 2 And 3.0mmol of AcOH, finally add 5mL of anhydrous xylene, put it in an oil bath at 100°C and heat it for 18h, TLC detects that the reaction is complete, the reaction solution is cooled to room temperature, filtered with diatomaceous earth, washed with ethyl acetate, and the solution is depressurized After concentration, it was separated by silica gel column chromatography, and the developer used ethyl acetate:n-hexane=1:5 to obtain the target compound as a light yellow solid. The structural formula and characterization are as follows:
[0064]
[0065] 13-Methyl-5,6-dihydro-14H-pyrazino[2",3":5',6']pyrido[2',3':3,4]pyrazolo[1,2 -a] cinnoline; 1 H NMR (400MHz, CDCl 3 )δ8.60(m, 2H), 7.19(d, J=9....
Embodiment 2
[0067] Preparation of compound 4ba: specific experimental operation process with reference to Example 1, 1-phenyl-2-vinylpyrazolidin-3-one of 1mmol, 1-(3-amino-6-methylpyrazine of 1.2mmol -2-yl) ethyl ketone was added in the reaction flask, vacuumed for nitrogen, and 0.01 mmol of [Cp*RhCl was added under nitrogen protection 2 ] 2 , 0.2 mmol of Pd(OAc) 2 And 3.0mmol of AcOH, finally add 5mL of anhydrous xylene, put it in a 100°C oil bath and heat for 18h, the product obtained is a light yellow oily liquid, the product structure and characterization are as follows:
[0068]
[0069] 11,13-Dimethyl-5,6-dihydro-14H-pyrazino[2",3":5',6']pyrido[2',3':3,4]pyrazolo[ 1,2-a]cinnoline; 1 H NMR (400MHz, CDCl 3)δ8.57(s,1H),7.12–7.03(m,2H),6.76(t,J=11.2Hz,1H),6.58(d,J=7.3Hz,1H),4.35(s,2H), 3.39(m,2H),3.09(m,2H),2.68(s,3H),2.59(s,3H). 13 C NMR (101MHz, CDCl 3 )δ160.31, 151.65, 149.03, 148.92, 144.50, 144.05, 138.12, 129.95, 127.07, 121.84, 120.33, 116.90, 116.47, 56.35, 47.70, 26.0...
Embodiment 3
[0071] Preparation of compound 4ca: specific experimental operation process with reference to Example 1, 1mmol of 1-phenyl-2-vinylpyrazolidin-3-one, 1.2mmol of 1-(3-amino-6-chloropyrazine- 2-yl) ethyl ketone was added in the reaction flask, vacuumed for nitrogen, and 0.01 mmol of [Cp*RhCl was added under nitrogen protection 2 ] 2 , 0.2 mmol of Pd(OAc) 2 And 3.0mmol of AcOH, finally add 5mL of anhydrous xylene, put it in a 100°C oil bath and heat it for 18h, the product obtained is a light yellow solid, the structural formula and characterization of the product are as follows:
[0072]
[0073] 11-Chloro-13-methyl-5,6-dihydro-14H-pyrazino[2",3":5',6']pyrido[2',3':3,4]pyrazolo [1,2-a]cinnoline; 1 H NMR (400MHz, CDCl 3 )δ8.81(s,1H),7.07–6.99(m,2H),6.73(t,J=8.4Hz,1H),6.55(d,J=7.4,1H),4.32(s,2H),3.76 (m,2H),3.39(m,2H),2.49(s,3H). 13 C NMR (101MHz, CDCl 3 )δ159.87, 151.34, 149.55, 146.15, 145.82, 145.68, 141.48, 127.33, 126.35, 123.04, 122.88, 121.24, 117.45, 57.40, 49.08,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com