Patents
Literature
110 results about "Squaric acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Squaric acid, also called quadratic acid because its four carbon atoms approximately form a square, is a dibasic organic acid with the chemical formula C₄H₂O₄. The conjugate base of squaric acid is the hydrogensquarate anion C₄HO⁻₄; and the conjugate base of the hydrogensquarate anion is the divalent squarate anion C₄O²⁻₄. This is one of the oxocarbon anions, which consist only of carbon and oxygen.
Acid sensitive ARC and method of use
InactiveUS6110653APhotosensitive materialsSemiconductor/solid-state device manufacturingVinyl etherCross-link
A composition used to form an acid sensitive +E,uns a+EE nti+E,uns r+EE eflective +E,uns c+EE oating (ARC) includes a water soluble resin and a cross-linker. Radiation adsorptive components may be provided as part of the resin or, more preferably, as a separate dye. The composition may be applied on a substrate as a radiation adsorbing layer and additionally cross-linked to form an acid sensitive, water insoluble ARC on which a +E,uns p+EE hoto+E,uns p+EE atterning +E,uns r+EE esist (PPR) layer may be formed. Being acid sensitive, selected portions of an ARC formed from the composition may be removed by a suitable reversal of the cross-linking followed by a develop step, preferably with an aqueous developer, more preferably de-ionized water. The water soluble resin is preferably hydroxystyrene-sulfonated styrene copolymer, poly(2-isopropenyl-2-oxazoline), or poly(acrylic acid), the cross-linker is preferably an acetal diacid or a water soluble divinyl ether, and the dye is preferably 9-anthracene methanol or a squaric acid derivative. If a suitable +E,uns p+EE hoto+E,uns a+EE cid +E,uns g+EE enerator (PAG) is included then an ARC formed from such components may exhibit a photosensitivity similar to or even lower than that of the overlying PPR. The photosensitivity is preferably less than about 900 mJ / cm2, more preferably 100 mJ / cm2 or less.
Owner:IBM CORP
Acid sensitive ARC and method of use
InactiveUS6319651B1Photosensitive materialsPhotosensitive materials for photomechanical apparatusVinyl etherCross linker
A composition used to form an acid sensitive antireflective coating (ARC) includes a water soluble resin and a cross-linker. Radiation adsorptive components may be provided as part of the resin or, more preferably, as a separate dye. Being acid sensitive, selected portions of an ARC formed from the composition may be removed by a suitable reversal of the cross-linking followed by a develop step, preferably with an aqueous developer, more preferably de-ionized water. The water soluble resin is preferably hydroxystyrene-sulfonated styrene copolymer, poly(2-isopropenyl-2-oxazoline), or poly(acrylic acid), the cross-inker is preferably an acetal diacid or a water soluble divinyl ether, and the dye is preferably 9-anthracene methanol or a squaric acid derivative.
Owner:INT BUSINESS MASCH CORP
Organic photosensitive devices comprising aryl squaraines and methods of making the same
InactiveUS20120248419A1Controlled diffusionNo net contributionOrganic chemistryMethine/polymethine dyesHeterojunctionAryl
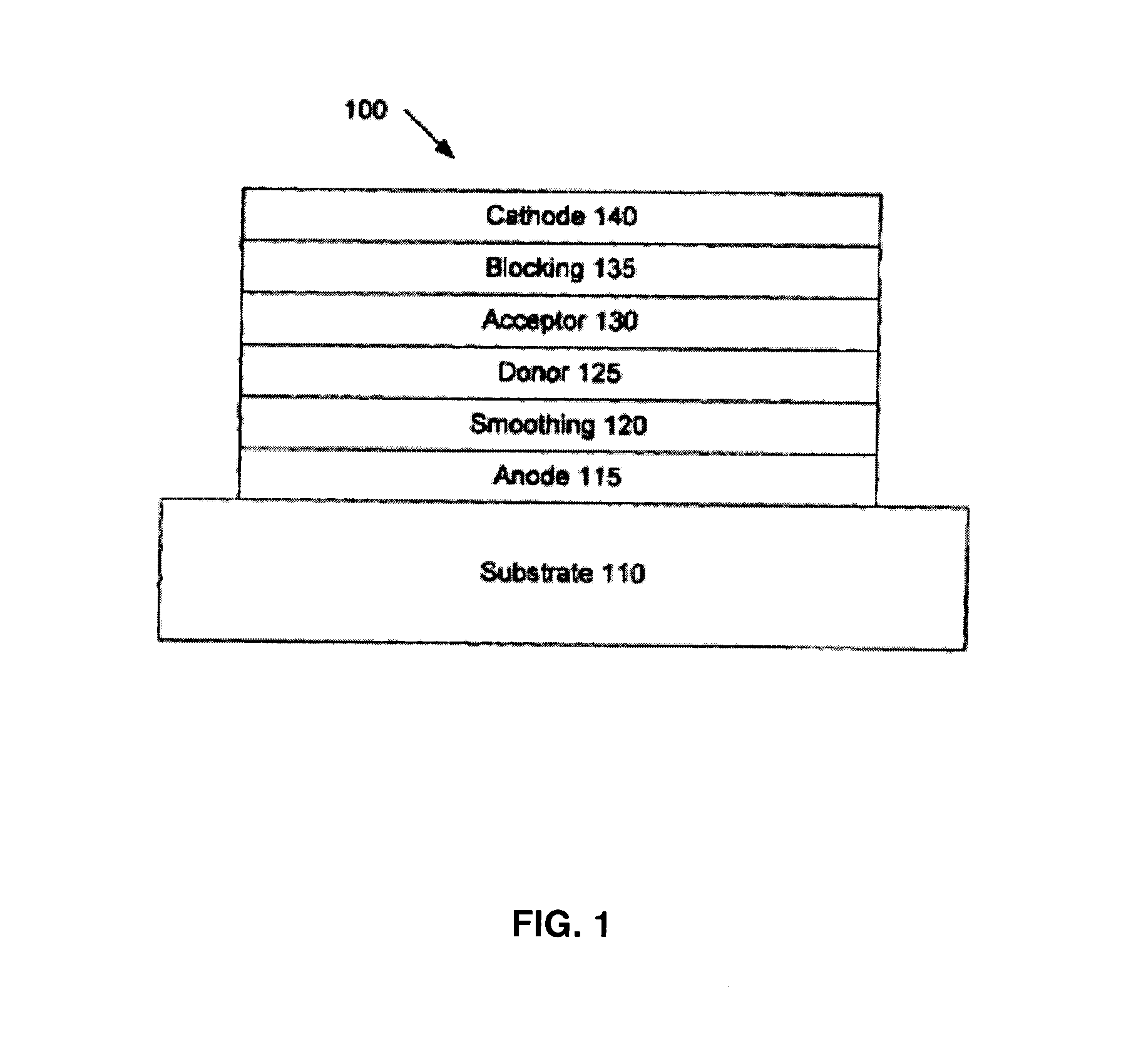
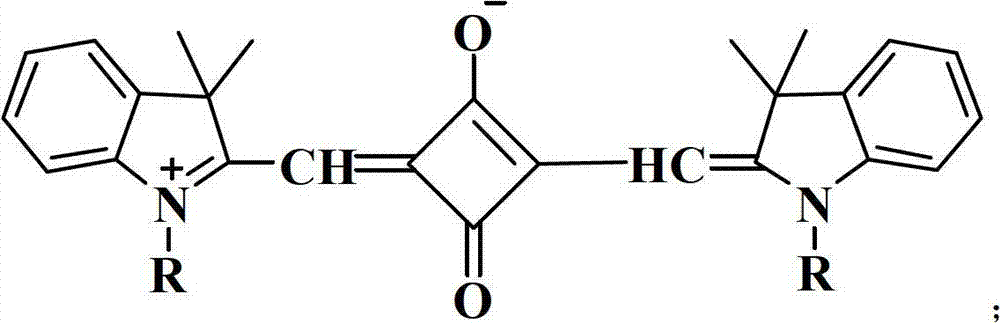
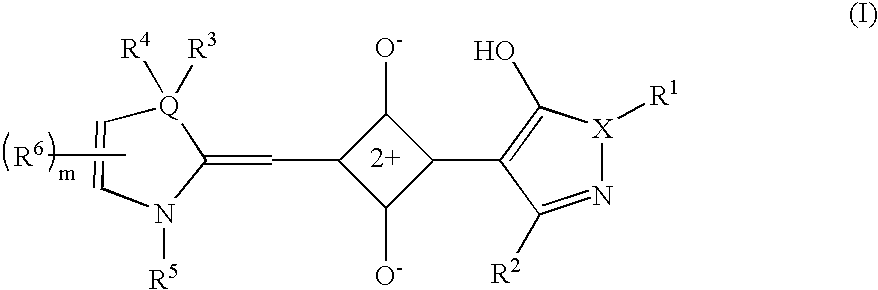
There is disclosed squaraine compounds of formula I:wherein each of Y1 and Y2 is independently chosen from an optionally substituted amino group and an optionally substituted aryl group. Also described are organic optoelectronic devices comprising a Donor-Acceptor heterojunction that is formed from one or more of the squaraine compounds. A method of making the disclosed device, which may include one or more sublimation step for depositing said squaraine compound, is also disclosed.
Owner:UNIV OF SOUTHERN CALIFORNIA +1
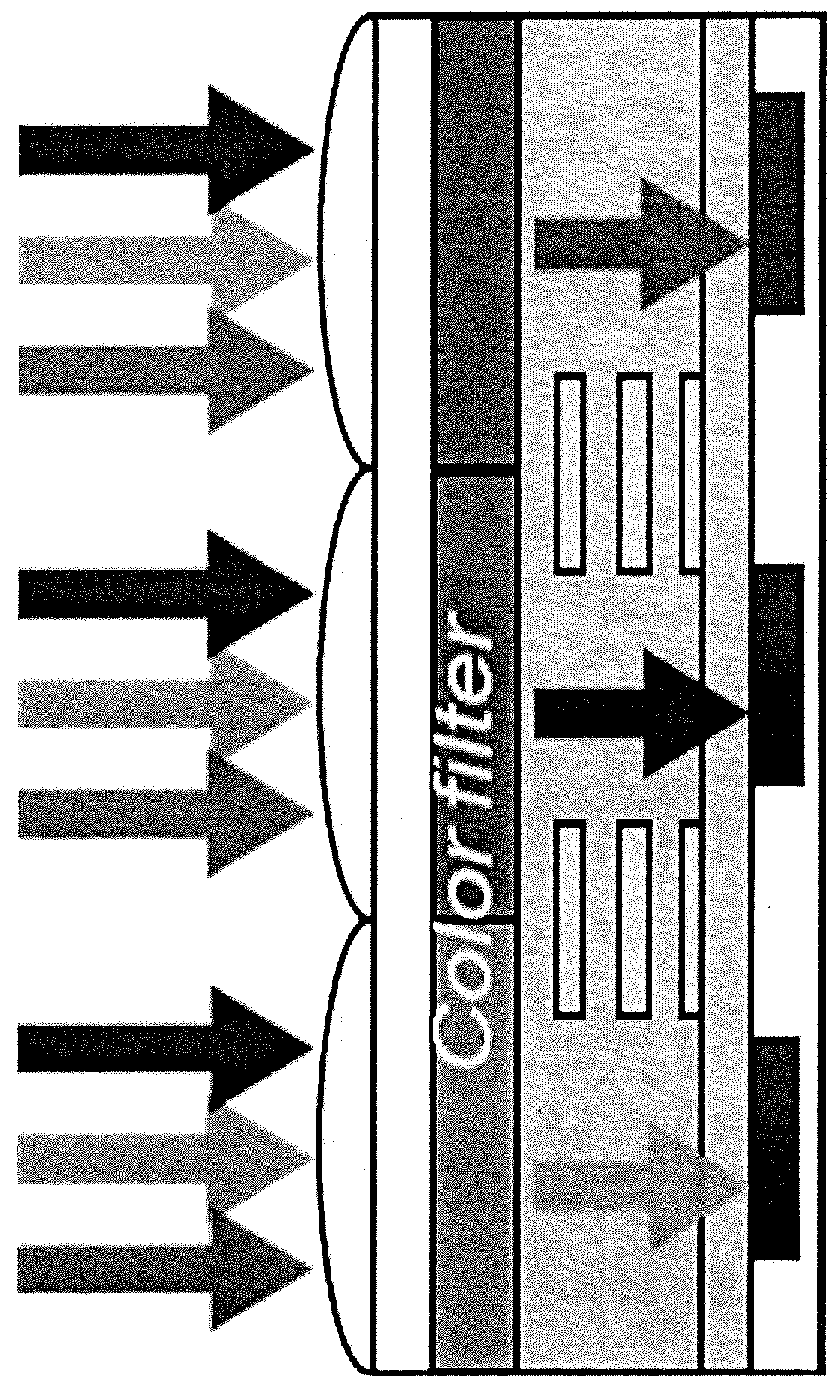
Optical filter, solid state image-capturing device using same, and camera module using same
ActiveCN103858028AReduce scattered lightExcellent transmittance characteristicsMethine/polymethine dyesSolid-state devicesFluorescenceTransmittance
The present invention addresses the problem of providing an optical filter having exceptional transmittance characteristics in which the drawbacks of conventional near-infrared-cutting filters and other optical filters are overcome, and light scattering is minimal even during light absorption. This optical filter is characterized by containing a squarylium compound and a compound for absorbing or quenching fluorescence. This optical filter preferably contains a near-infrared absorbing dye that contains a squarylium compound (A), and at least one compound (B) selected from the group consisting of a phthalocyanine compound (B-1), and a cyanine compound (B-2).
Owner:JSR CORPORATIOON
Hypochlorite ion fluorescence probe, and synthetic method and application thereof
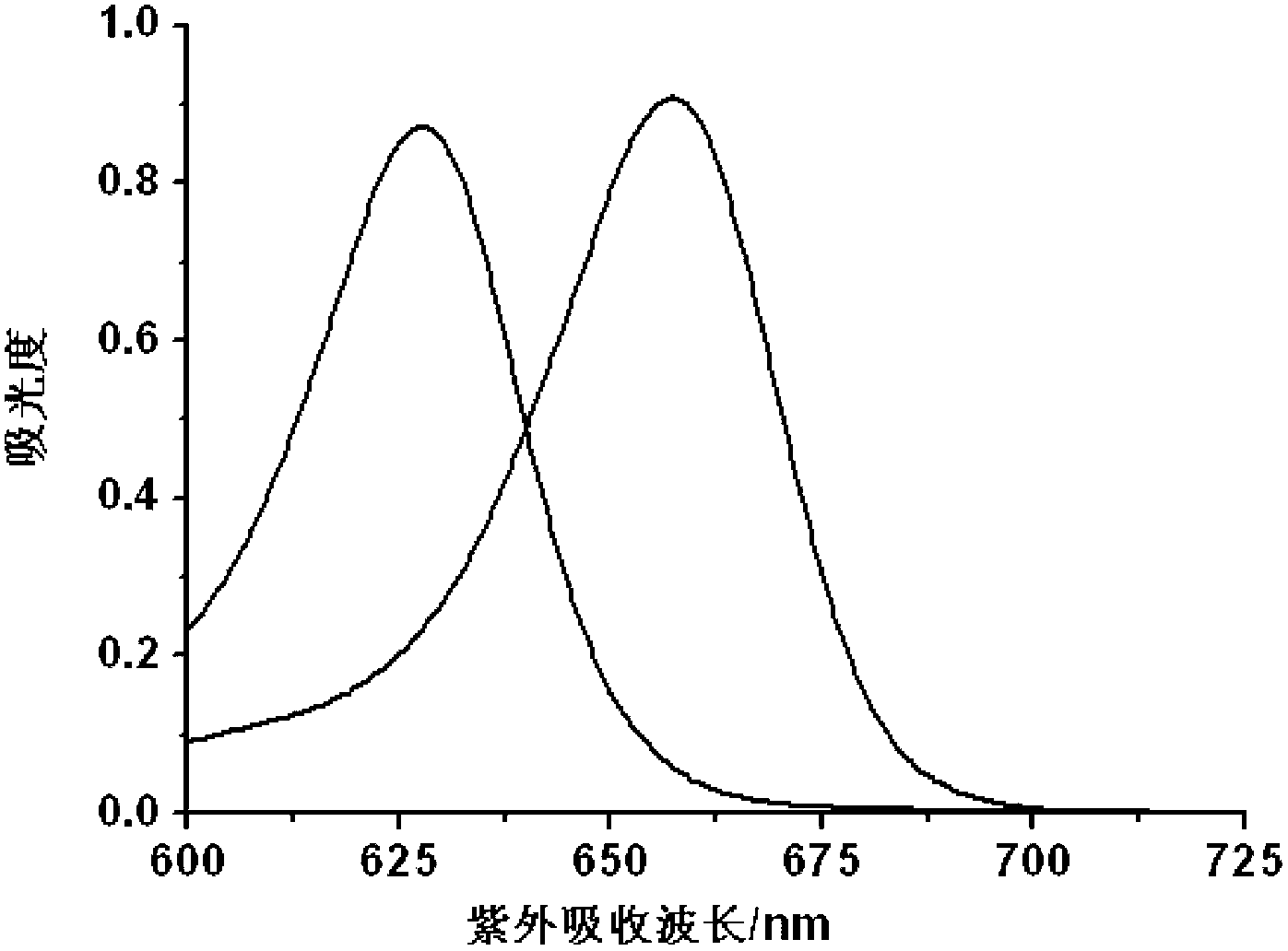
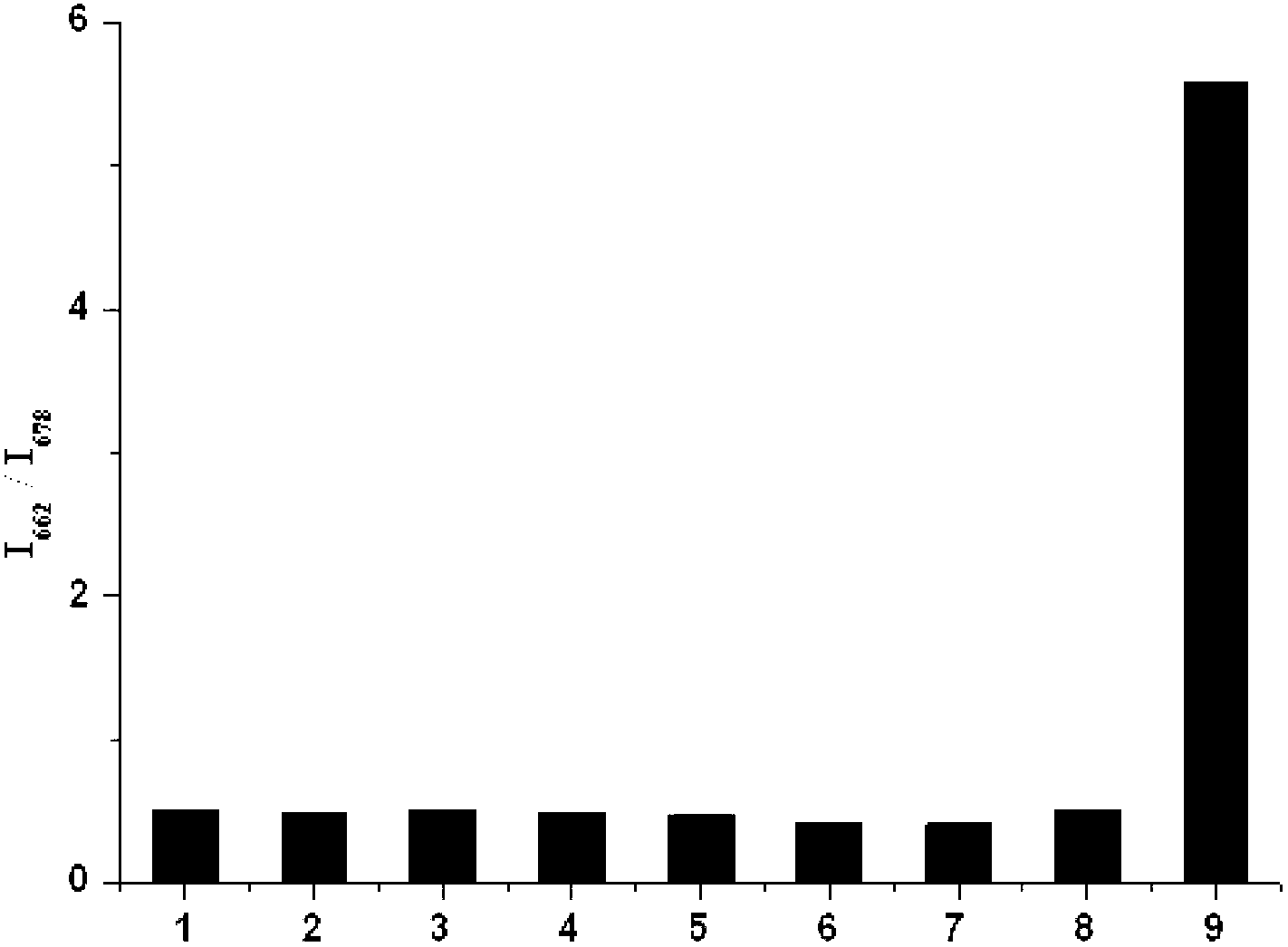
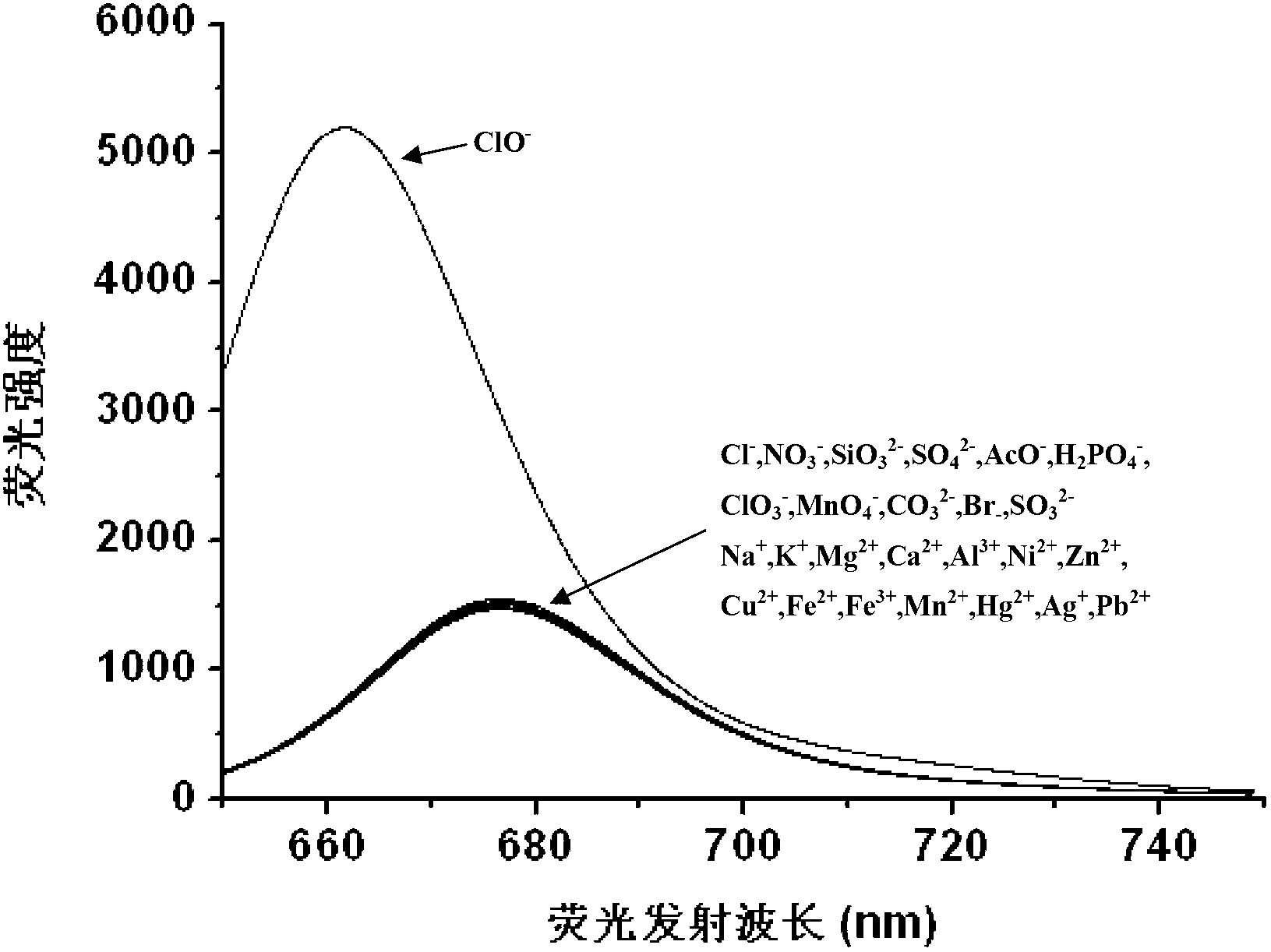
InactiveCN103173213AGood choiceLow detection limitThiol preparationFluorescence/phosphorescenceHypochloriteDistillation
The invention discloses a hypochlorite ion fluorescence probe, and a synthetic method and an application thereof. The synthetic method comprises the following steps: 1, adding 3,4-dihydroxy-3-cyclobutene-1,2-dione (squaric acid) and 3-N,N-diethylamino phenol to a solvent which is a mixed solution comprising toluene and n-butanol, carrying out a heating reflux reaction for 8-12h, filtering, washing the obtained reaction precipitate with methanol, and drying to obtain an intermediate squaric acid dye, wherein the molar ratio of 3,4-dihydroxy-3-cyclobutene-1,2-dione (squaric acid) to 3-N,N-diethylamino phenol is 1:1.8-2.2; and 2, carrying out the heating reflux reaction of the squaric acid dye and a Lawesson reagent in a solvent toluene under the protection of nitrogen for 6-24h, carrying out reduced pressure distillation to remove the solvent, and carrying out silica gel column chromatography to obtain the fluorescence probe. The probe has a simple synthetic process, has a very high selectivity and a very high sensitivity to hypochlorite ions, and has a good application prospect.
Owner:CENT SOUTH UNIV
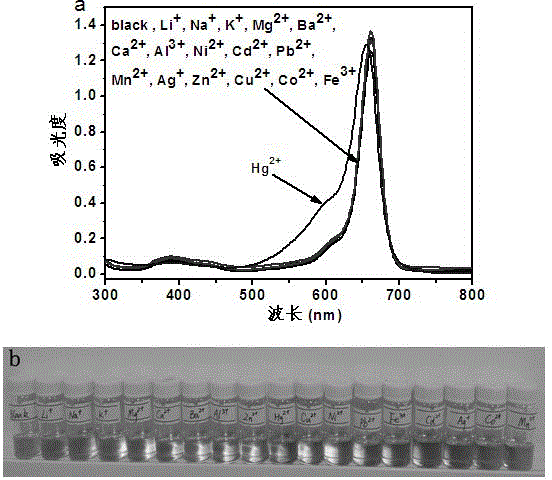
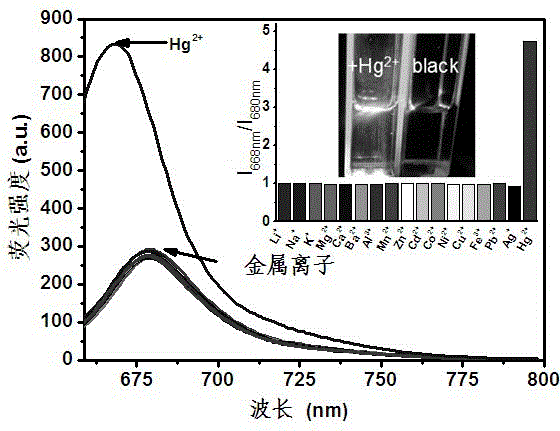
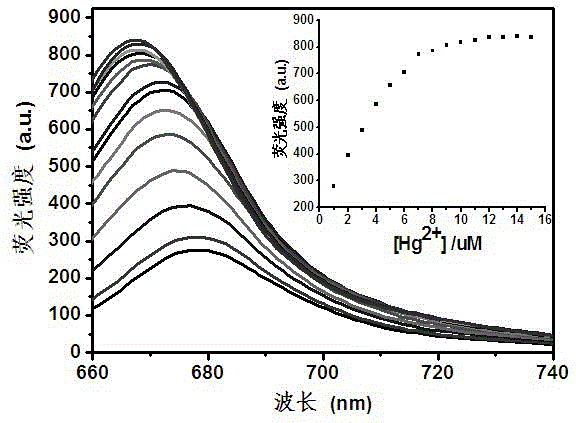
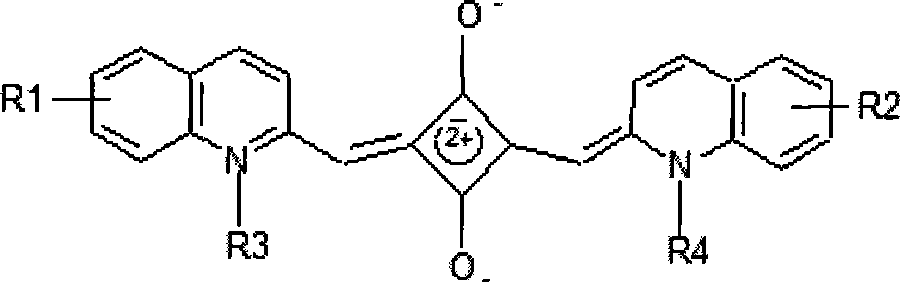
Mono-sulfo squarylium dye fluorescence probe for detecting mercury ions and preparation method thereof
InactiveCN103333677ARapid qualitative and quantitative detectionQuick checkAzo dyesFluorescence/phosphorescenceMercuric ionFluorescence
The invention discloses a mono-sulfo squarylium dye fluorescence probe for detecting mercury ions and a preparation method thereof as well as application thereof. The preparation method comprises the following steps of: proportionally mixing oxo squarylium essence and a Lawesson's reagent, dissolving the mixture in methylbenzene, performing backflow reaction for 1.5-4 hours under the protection of inert gas after the mixture is dissolved, cooling the reaction mixture to room temperature, decompressing and distilling the reaction mixture to remove a solvent and obtain a rough product; and performing silica gel column chromatography separation on the rough product to obtain the mono-sulfo squarylium dye fluorescence probe. The mono-sulfo squarylium dye fluorescence probe provided by the invention is high in stability and can quickly, sensitively and specially detect low-concentration mercury ions; a synthesis method is simple; and the production cost is low.
Owner:FUZHOU UNIV
Water-soluble near infrared luminescent quinoline squaraine dye and preparation and application thereof
InactiveCN101544844AImprove stabilityGood compatibilityAzo dyesBiological testingQuinolineWavelength
The invention relates to water-soluble near infrared luminescent quinoline squaraine dye, of which the molecular structural general formula is shown on the figure. The method for preparing the water-soluble near infrared luminescent quinoline squaraine dye comprises that: firstly, 2-methylquinoline is subjected to bromination, sulfonation, nitration and acylation and reacts with acetonitrile and iodo-acid or iodo-ester to generate quinoline quaternary ammonium salt; and secondly, the quinoline quaternary ammonium salt is mixed with squaric acid, and the mixture is subjected to azeotropic distillation and dehydration, vacuum distillation and silica gel column chromatography and recrystallization through ethanol to obtain the water-soluble quinoline squaraine dye. The dye is applied in the fields of development of novel medicines, fluorescence labeling, probes, biological immunoassay, biological immunodetection and the like. The fluorescence-emission wavelength of the water-soluble quinoline squaraine dye is near infrared, so that the water-soluble quinoline squaraine dye has superior penetrability on environments and biological tissues and reduces self absorption and background absorption, and the sensitivity of fluorescence analysis can reach 10<-10> mol / L. The preparation method is simple and easy, has low cost and good economic benefit and is suitable for industrialized production.
Owner:DONGHUA UNIV
Squarylium type chemical sensor for colorimetric identification of copper ion and preparation method thereof
InactiveCN102786459ADetection of copper ion contentThe synthesis method is simpleOrganic chemistryMaterial analysis by observing effect on chemical indicatorN-ButanolSolvent
The invention relates to a squarylium type chemical sensor for colorimetric identification of a copper ion and a preparation method thereof, belonging to the field of chemical analysis and testing. The squarylium type chemical sensor is provided with a symmetric 2, 3, 3-trimethyl-indole squarylium compound and is prepared by the following steps of: mixing a 2, 3, 3-trimethyl-indole ring compound and squaric acid with a molar ratio of 2:1, adding mixture into a three-necked flask, adding mixed solvent of toluene and n-butanol with a volume ratio of 1:1, performing heating, mixing and back-flowing for 2-24 hours, and separating water produced in reaction with a water separator; stopping heating, cooling to the room temperature, depressurizing and evaporating to remove part of the solvent, and filtering to obtain green solid; and repeatedly washing the obtained green solid with ethyl acetate, drying at 60 DEG C, and recrystallizing with ethanol solvent to obtain pure green crystals. When the squarylium compound is applied in identification of the copper ions, the color can change in addition to changes in the absorption spectrum, the characteristics are obvious, and detection of the copper ions can be facilitated.
Owner:CHANGZHOU UNIV
Organic Photosensitive Devices Comprising a Squaraine Containing Organoheterojunction and Methods of Making Same
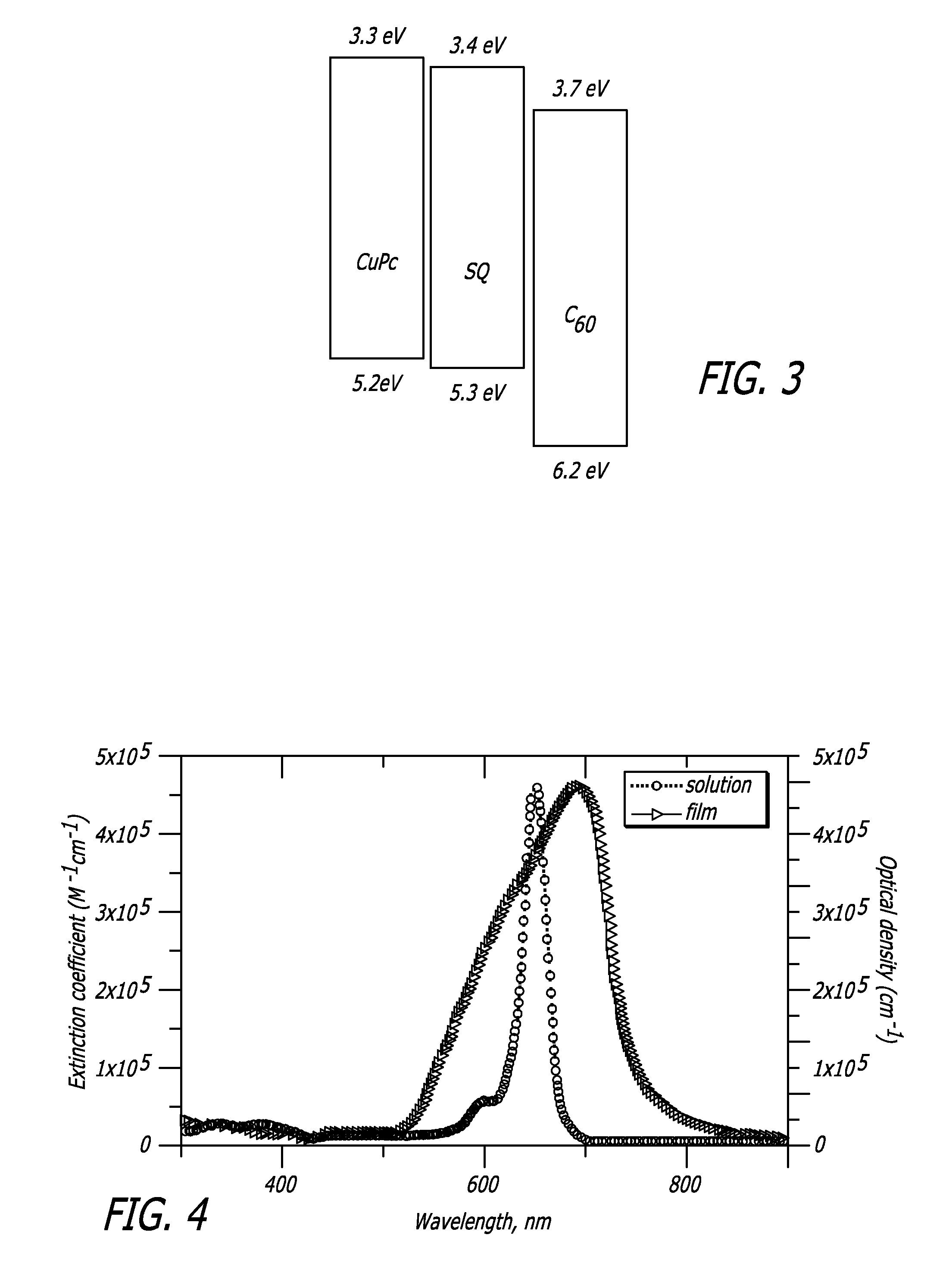
InactiveUS20100065112A1Improve performanceNanoinformaticsSolid-state devicesSquaric acidPhotochemistry
An organic photosensitive optoelectronic device comprising at least one Donor-Acceptor heterojunction formed from a squaraine compound of formula I:wherein each of Ar1 and Ar2 is independently chosen from an optionally substituted aromatic group. The organic optoelectronic device described herein may also comprise a Donor-Acceptor heterojunction that is formed from at least two different squaraine compounds. A method of making the disclosed device, which may include at least one sublimation step for depositing the squaraine compound, is also disclosed.
Owner:THOMPSON MARK E +3
Squarylium compound, method for producing the same and infrared absorbent
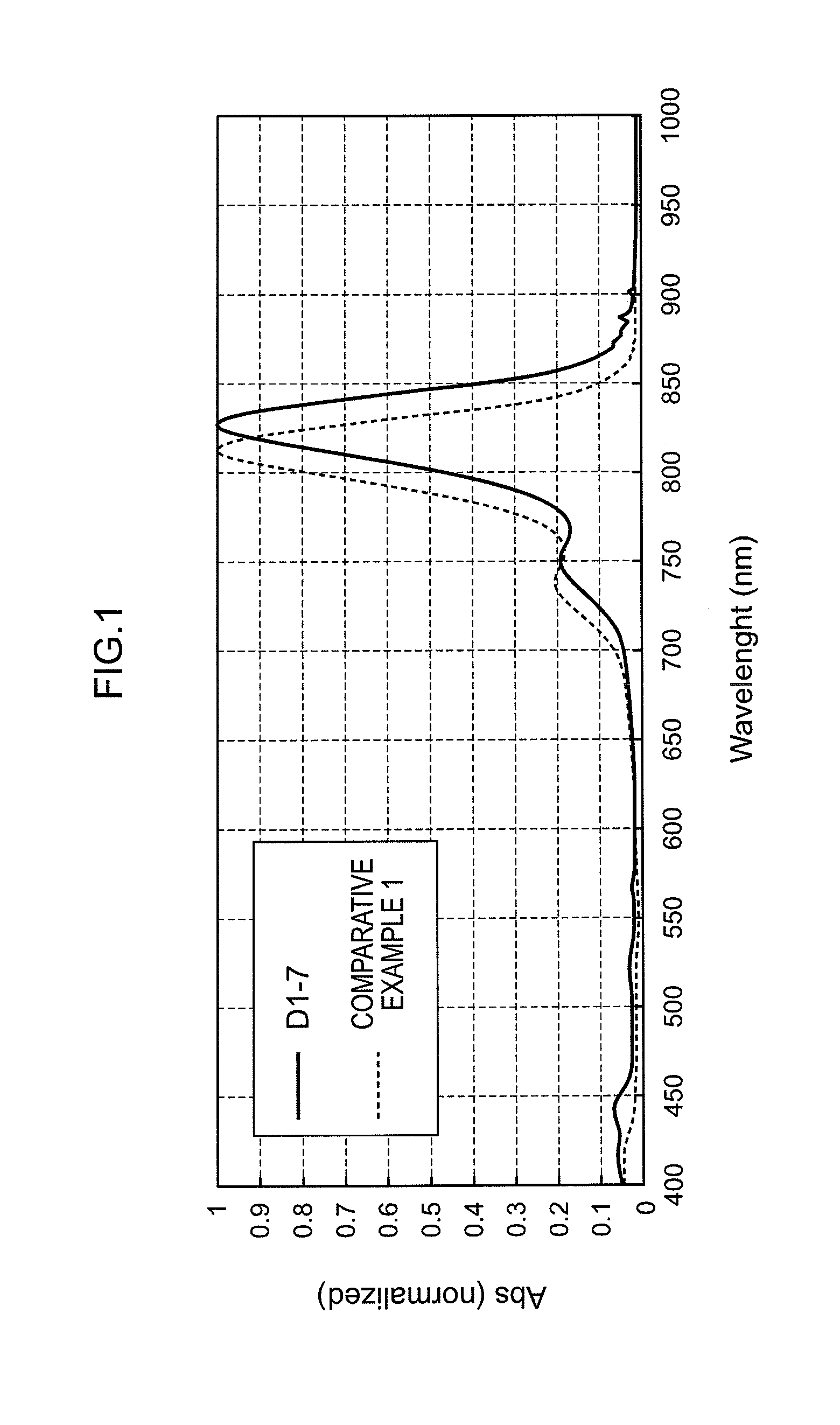
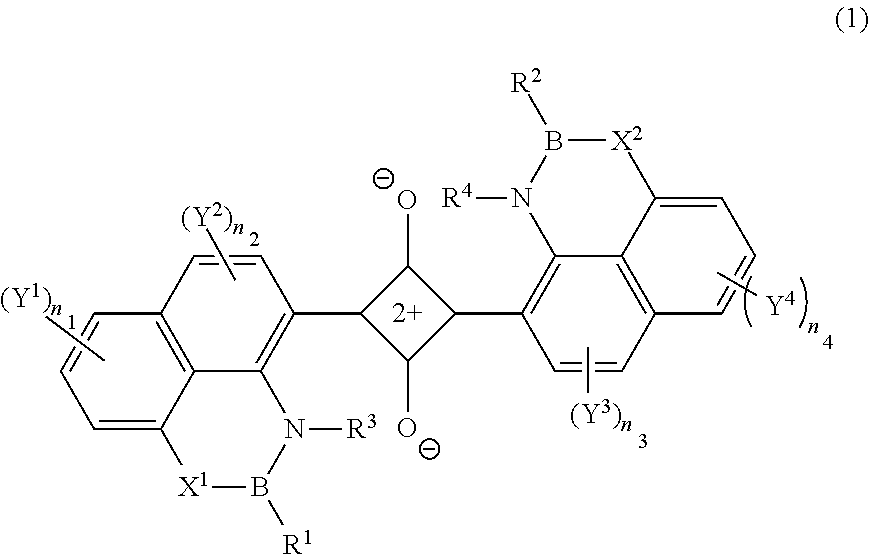
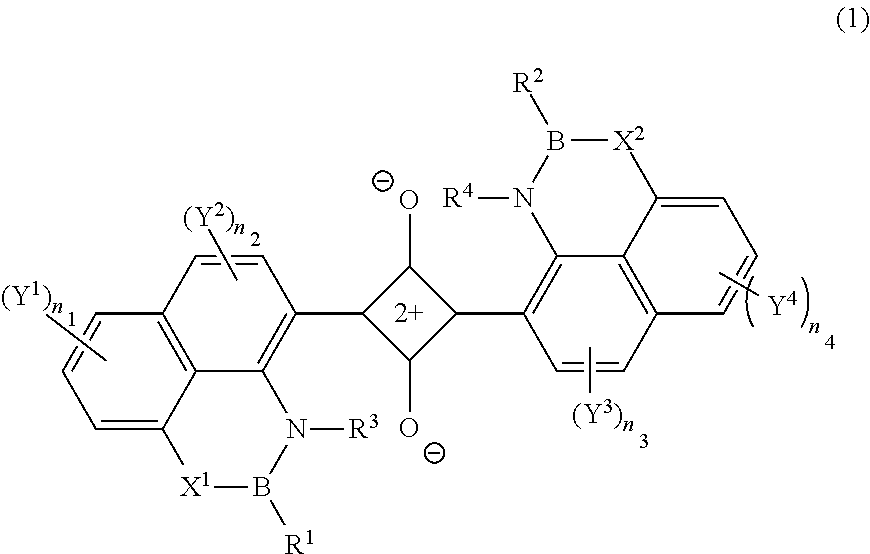
ActiveUS20110245538A1Superior invisibility and robustnessInfrared absorbabilityGroup 3/13 element organic compoundsHalogenPhotochemistry
Provide is a compound having absorbability in an infrared region, excellent invisibility and robustness. The compound is a squarylium compound represented Formula (1):wherein, R1 and R2 represent an alkyl group, cycloalkyl group, aryl group, or heteroaryl group, which may be substituted by a substituent; R3 and R4 represent a hydrogen atom or alkyl group; X1 and X2 represent an oxygen atom or —NR5—, in which R5 represents a hydrogen atom or alkyl group; Y1, Y2, Y3 and Y4 represent a halogen atom, alkyl group, cycloalkyl group, aryl group, heteroaryl group, arylcarbonyloxy group, or alkylcarbonyloxy group; a plurality of Y1's, Y2's, Y3's, or Y4's may be bonded to form a ring structure, respectively; Y1 and Y2, or Y3 and Y4 may be bonded to form a ring structure; n1 and n4 represent an integer of 0 to 3; and n2 and n3 represent an integer of 0 to 2.
Owner:FUJIFILM CORP
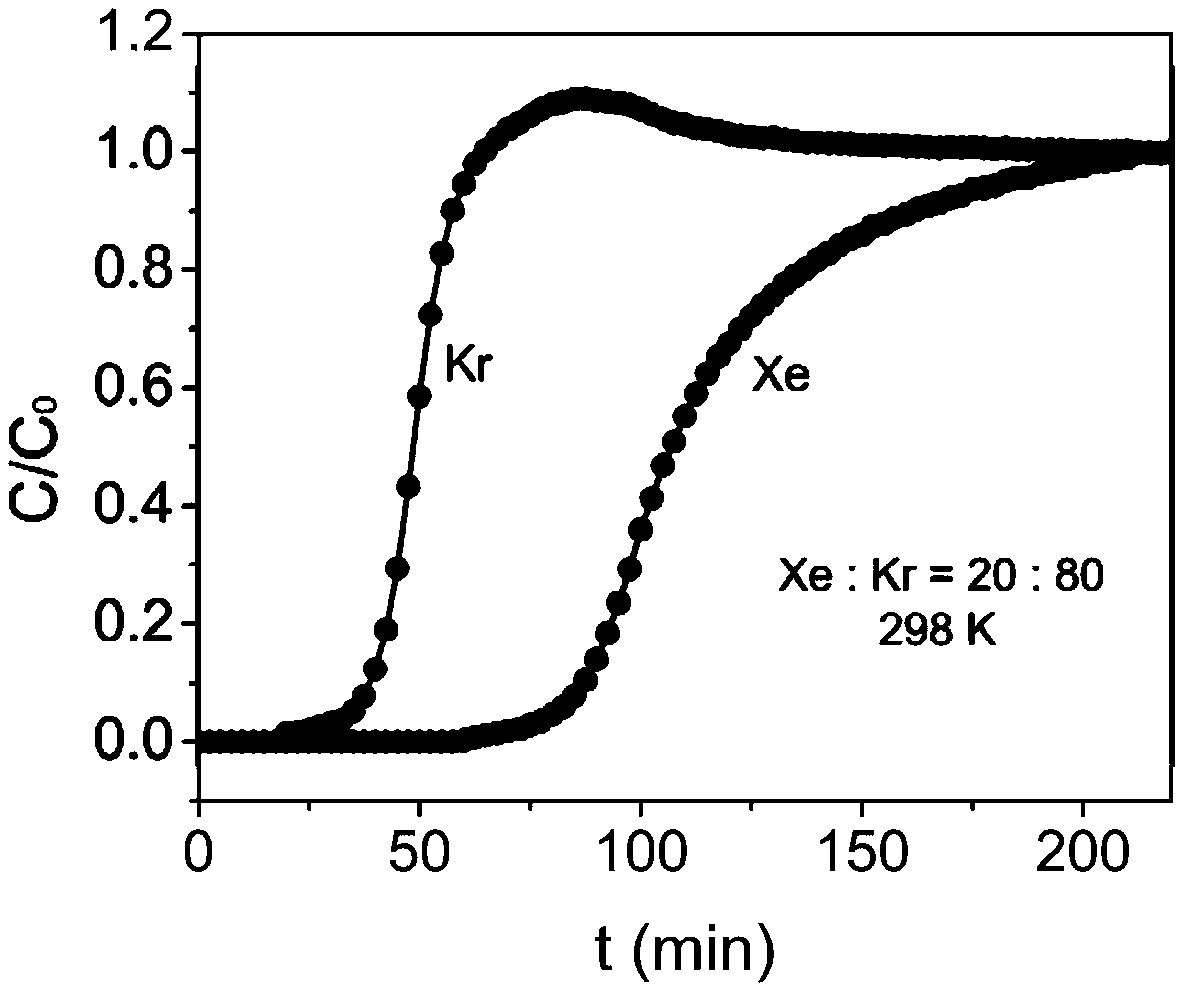
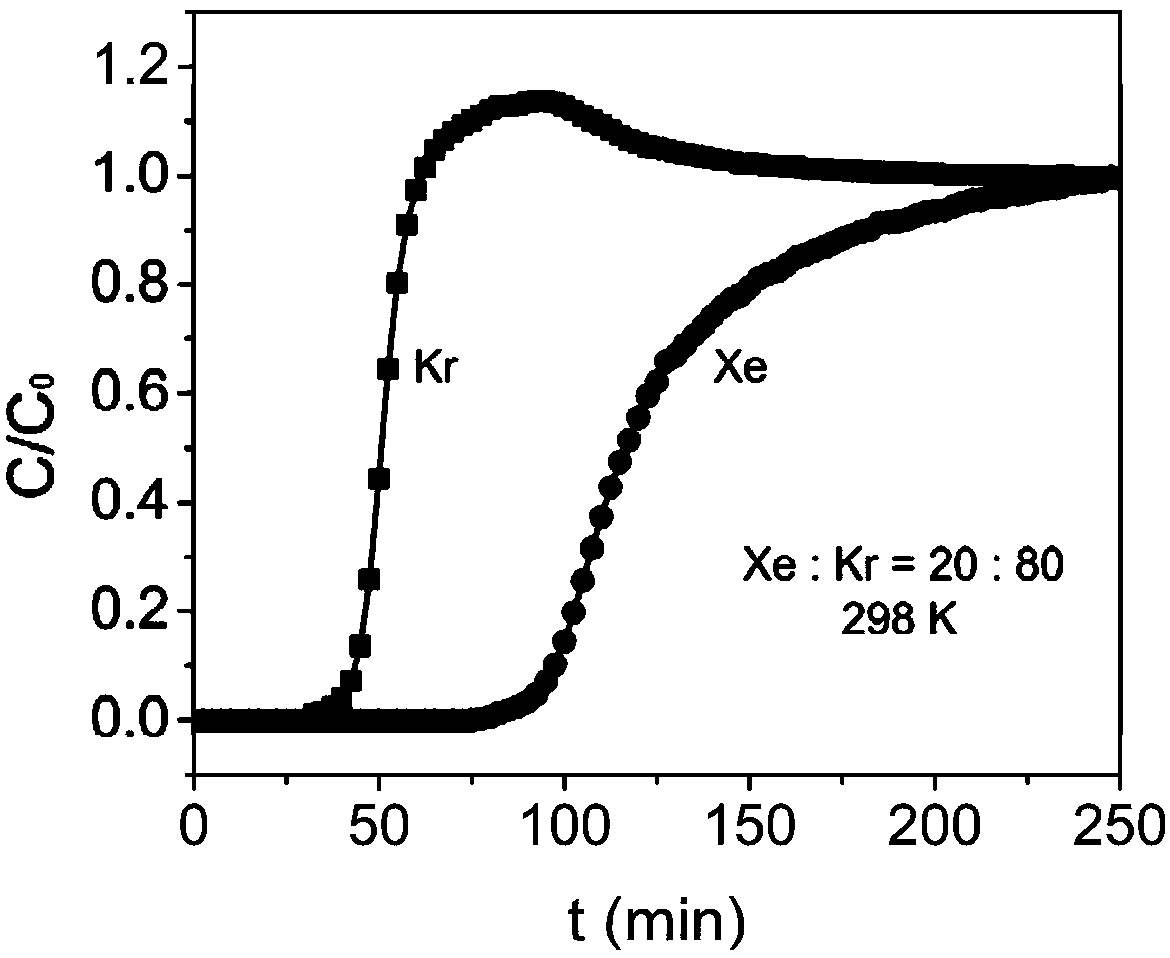
Metal-organic framework material for separating xenon and krypton and method for separating xenon and krypton
ActiveCN108727607ALower synthesis costStable structureOther chemical processesDispersed particle separationKryptonAlkaline earth metal
The invention discloses a metal-organic framework material for separating xenon and krypton and a method for separating xenon and krypton. The metal-organic framework material is good in stability andhigh in adsorptive separation selectivity, the preparation method is simple, and the preparation cost is low. The general structural formula of the metal-organic framework material is [M(C4O4(OH)2).3H2O or [M(C4O4)].2.5H2O, wherein M is metal ions, and transition metal ions or alkaline earth metal ions and squaric acid form a 3D network structure through coordinate bonds or intermolecular actingforce. A preparation method comprises the steps as follows: (1) inorganic salt, squaric acid, alkali and deionized water are mixed in proportion, and the obtained mixture is put in a reactor for a hydrothermal reaction after being stirred to be dissolved, wherein the inorganic salt comprises chlorate, nitrate, acetate, carbonate, sulfate or perchlorate of metal ions; (2) after the hydrothermal reaction, a product is washed with deionized water multiple times, vacuum drying is performed, and the metal-organic framework material is obtained. The metal-organic framework material is used as an adsorbent to perform adsorptive separation on mixed gas containing xenon and krypton.
Owner:ZHEJIANG UNIV
Novel indolenium squaraine cyanine dye containing quinazolinone structure
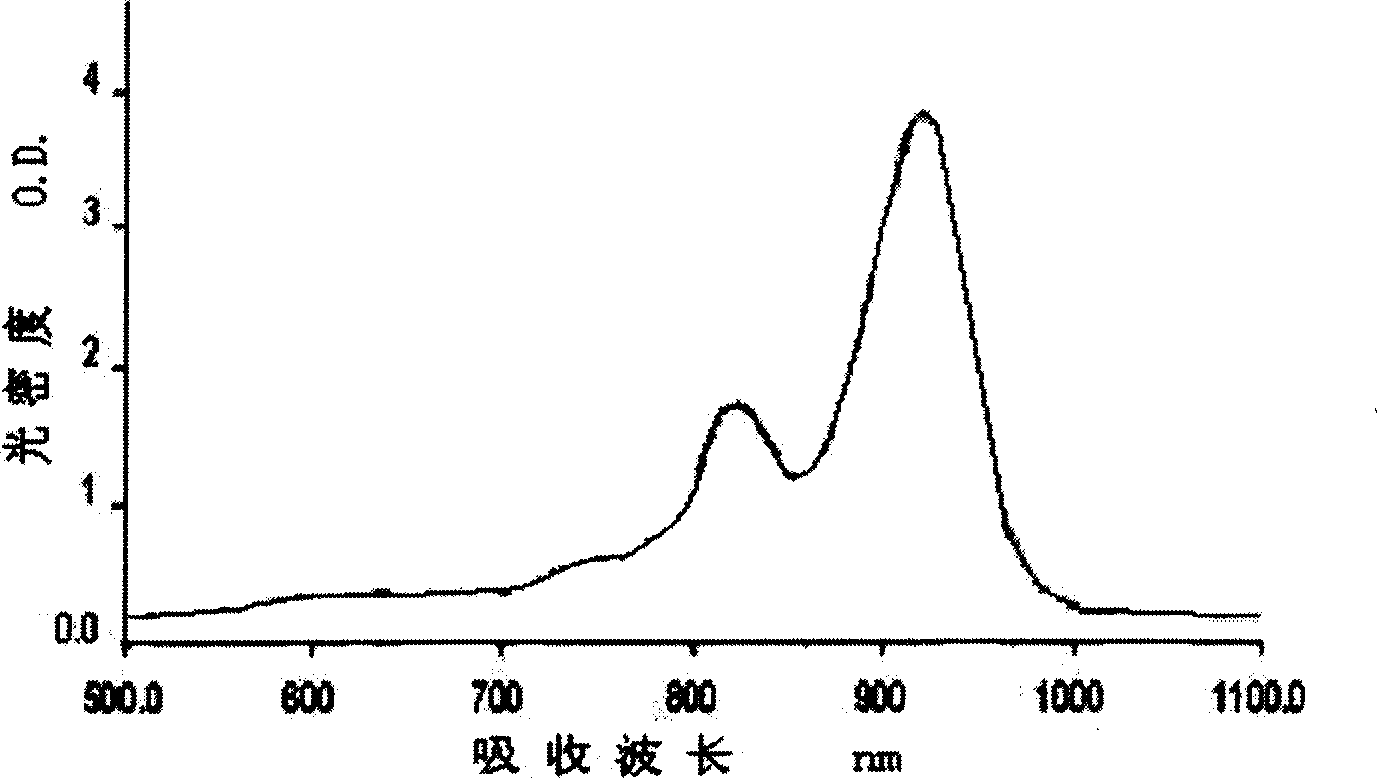
The present invention relates to a new kind of infrared absorbing squaraine cyanine dye as one new kind of compound containing quinoxalinone heterocycle in its structure. It has absorption wavelength of 700-1100 nm. It is prepared through condensation of squaric acid and quinoxalinone as precursor compound. It may be used in laser protection, fluorescent labeling, photoelectronic functional material and other fields.
Owner:NO 63971 TROOPS PLA
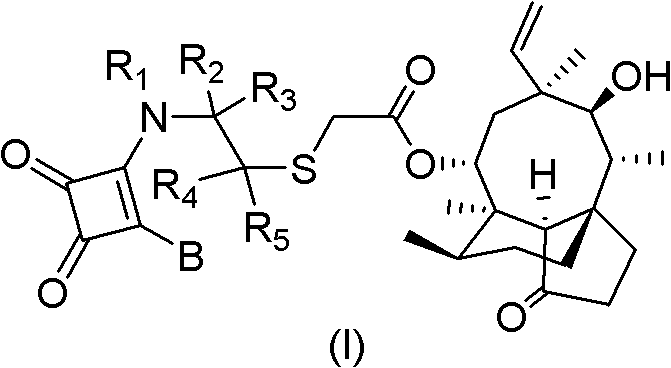
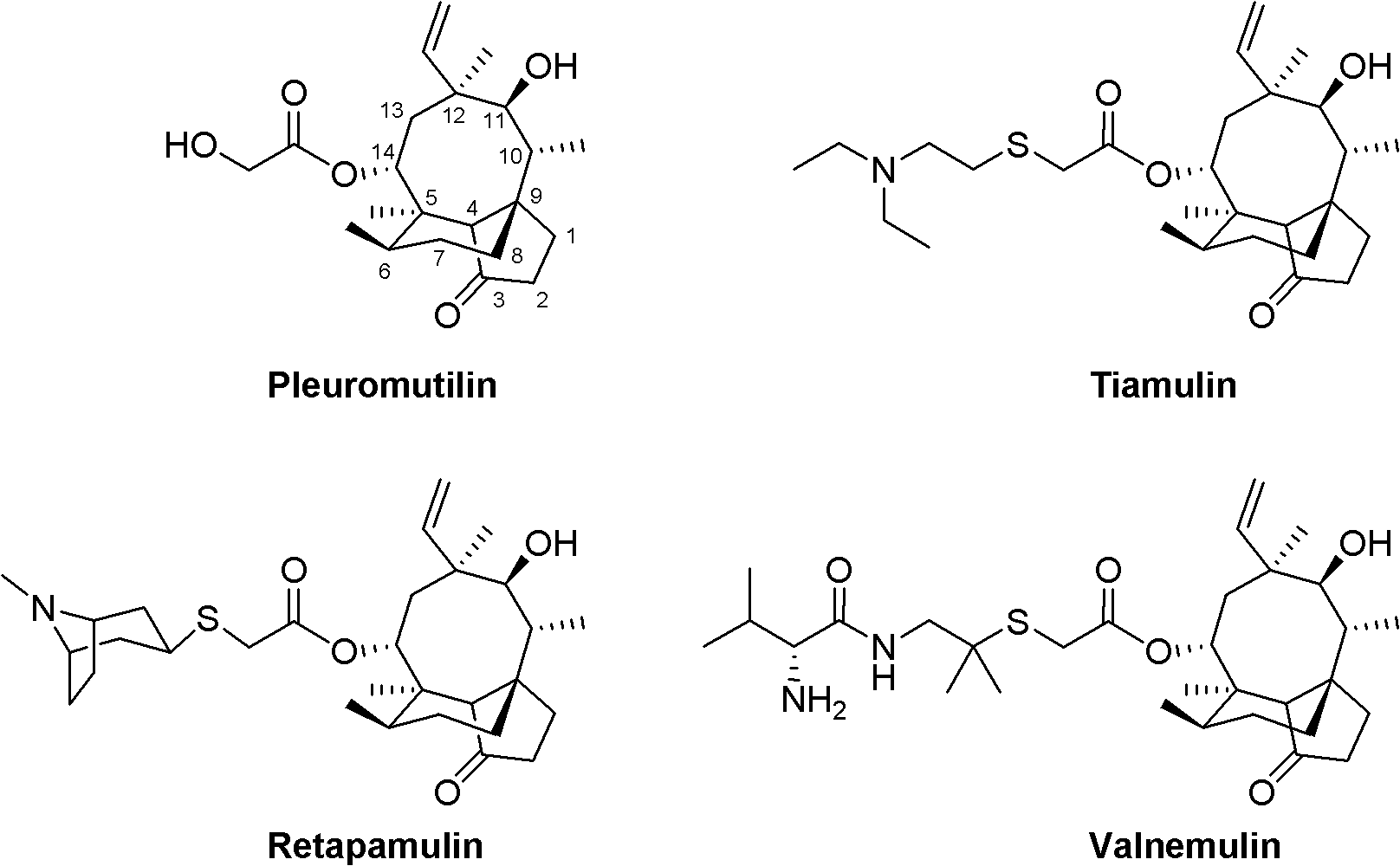
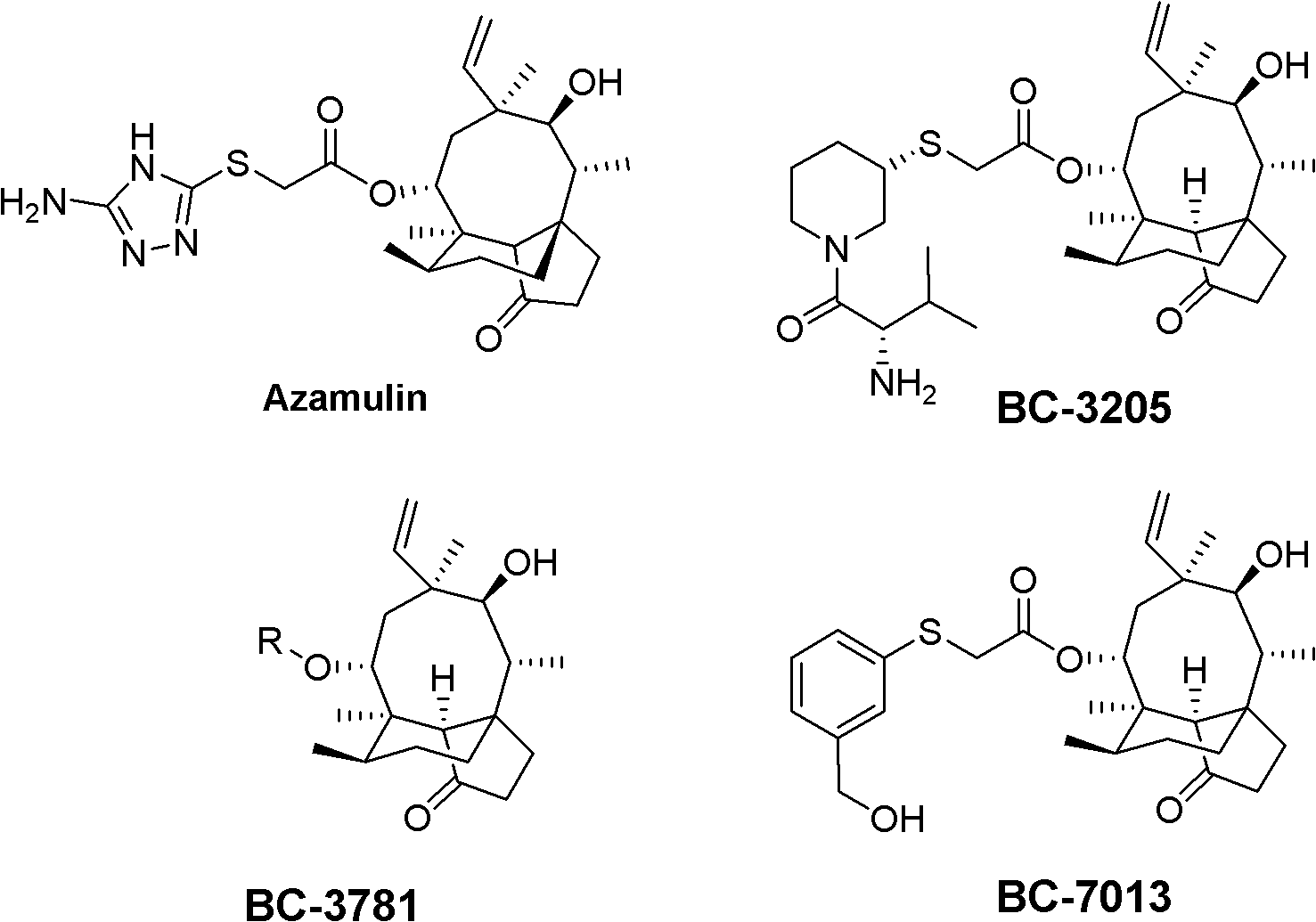
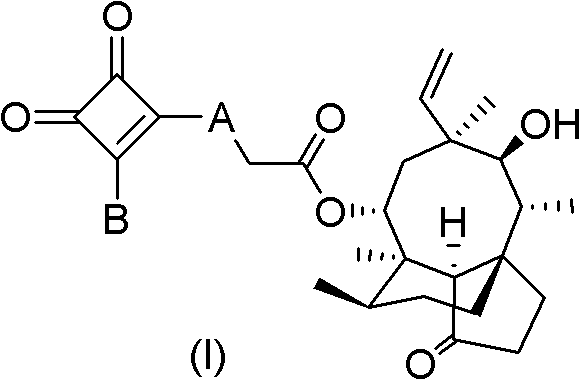
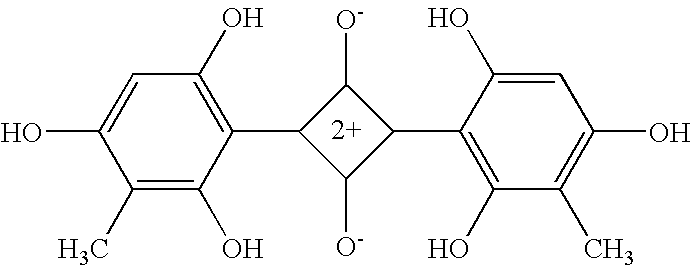
Mulin acetate containing substituted squaric acid and application thereof
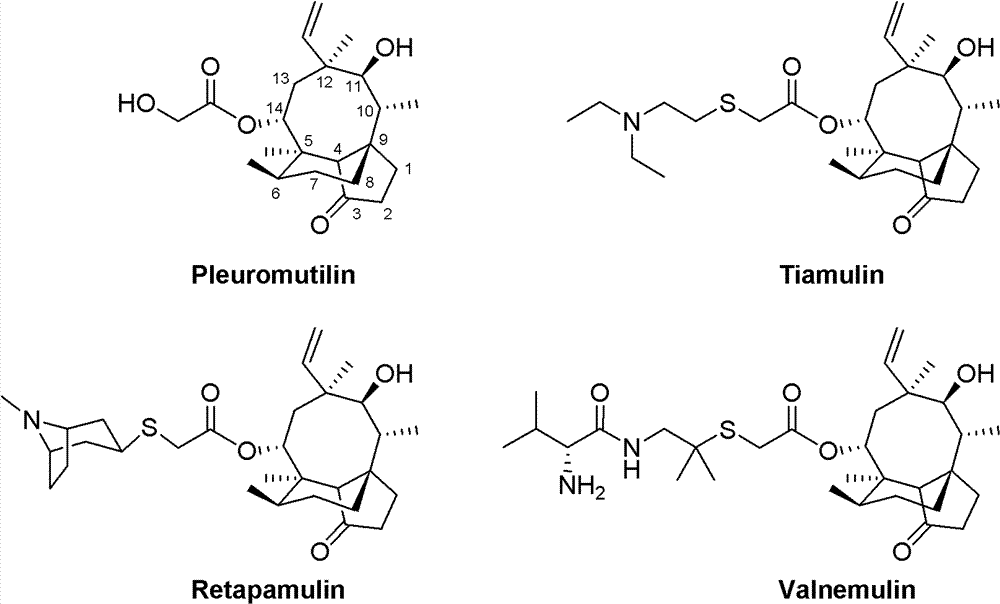
The invention relates to Mulin acetate containing substituted squaric acid and an application thereof which belong to the technical field of medicines, specifically to substituted squaric acid containing Mulin acetate as shown in a general formula (I), pharmaceutically acceptable salt, a hydrate and an isomer thereof, wherein R1, R2, R3, R4, R5 and B are defined in the specification. The invention also relates to a preparation method of these compounds, a pharmaceutical composition containing these compounds and an application of these compounds in the preparation of drugs for treating or preventing bacteria and virus. As for staphylococcus aureus and Streptococcus equin MIC value, the compounds have an antibacterial effect 15-20 times higher than a control commercially-available antibiotic tiamulin in the test. And the compounds provided by the invention are effective antimicrobial agents. And the compounds provided by the invention are effective antimicrobial agents.
Owner:BEIJING ABLEPHARMTECH CO LTD
Soluble fluorescent cyanogen dye
A soluble fluorescent dicyan dye is prepared by condensing compound (2) with compound (3), by squaric acid and inducing squaric acid bridged ring into linear conjugate polymethin chain. In the structural formula, X is S, CH2 or C(CH3)2; R1 and R2 are selected from any kind of C1-C6 alkyl or (CH2)kSO3H separately; k=1-6; R3 is H or C1-C6 alkyl; R4 is H or CH2COOH; n is 1,2 or 3. It has excellent light stability.
Owner:EAST CHINA UNIV OF SCI & TECH
Benzindole and aniline derivative based asymmetric squaraine colorimetric probe and preparation method and application thereof
InactiveCN105623648AGood optical performanceGood light stabilityOrganic chemistryMethine/polymethine dyesBenzeneAniline
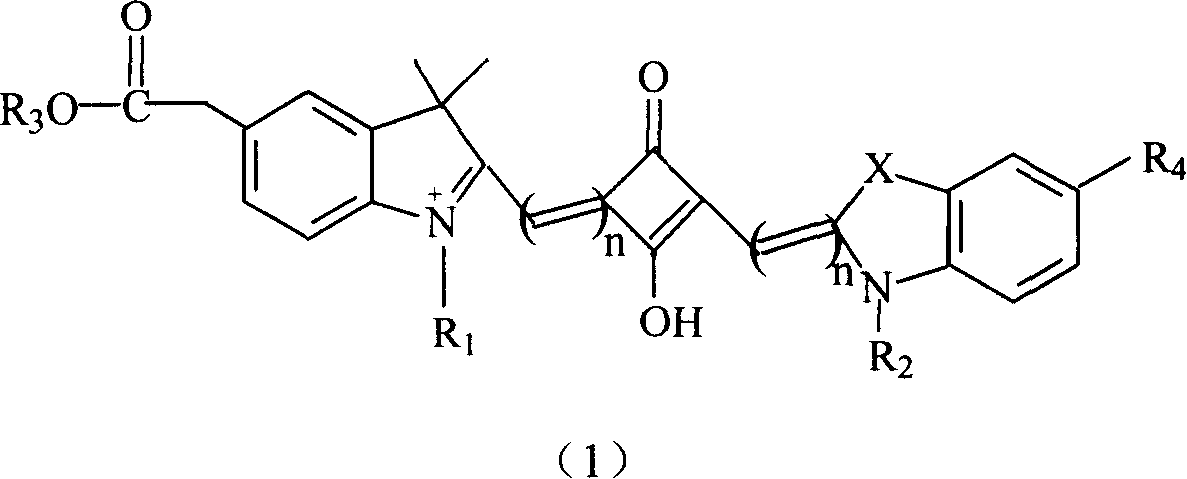
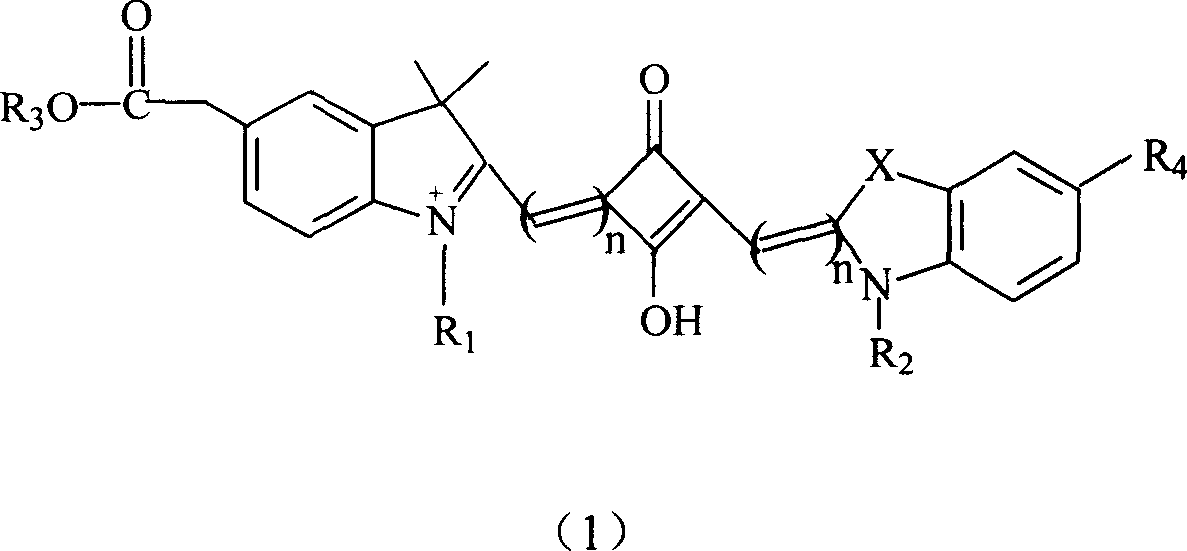
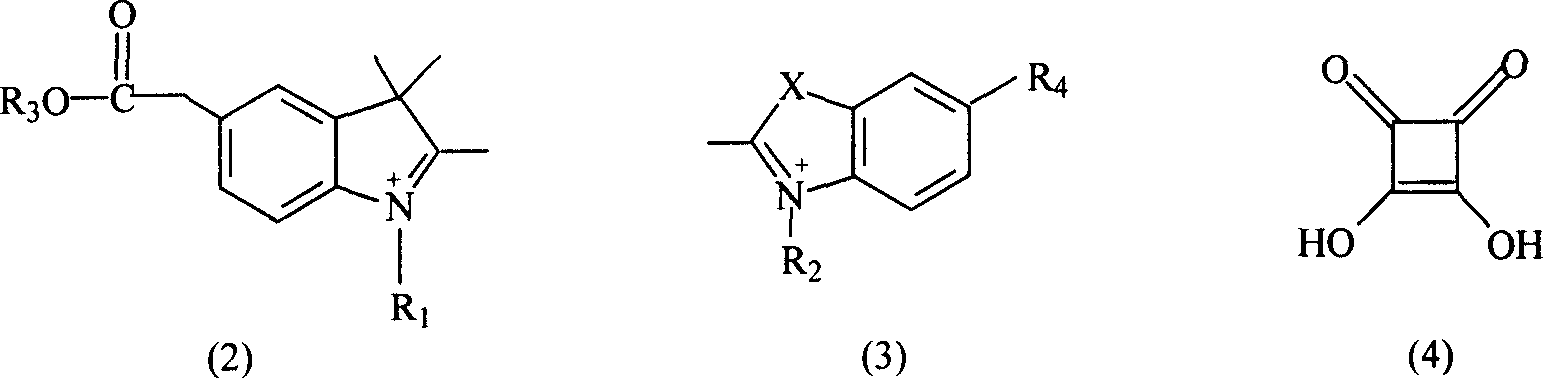
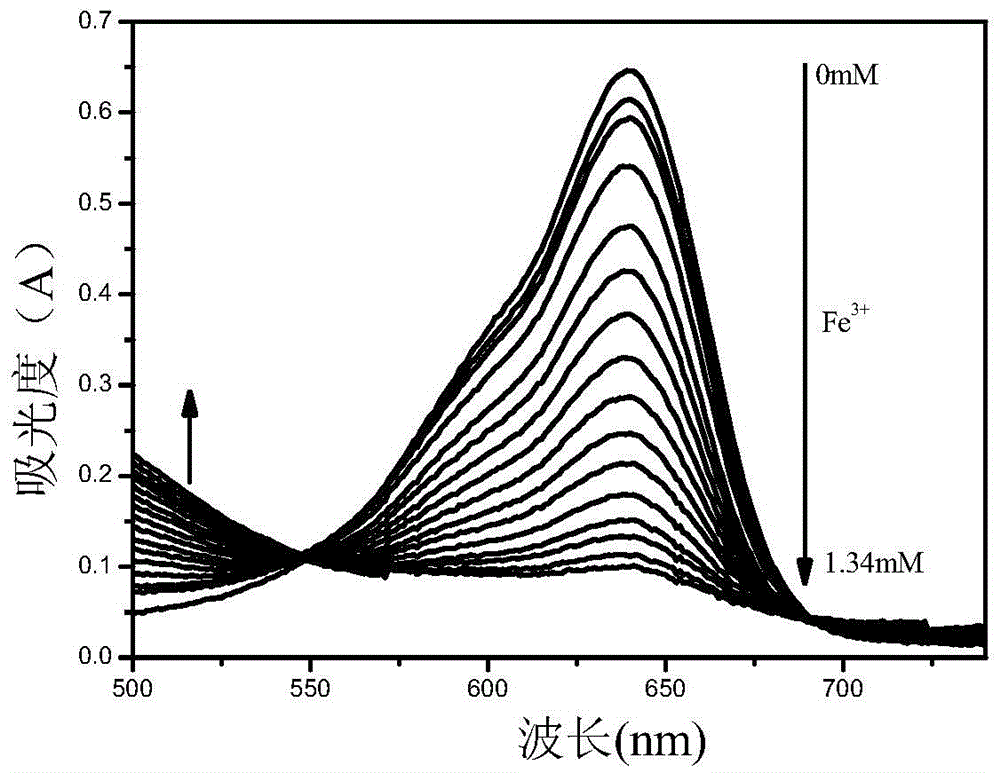
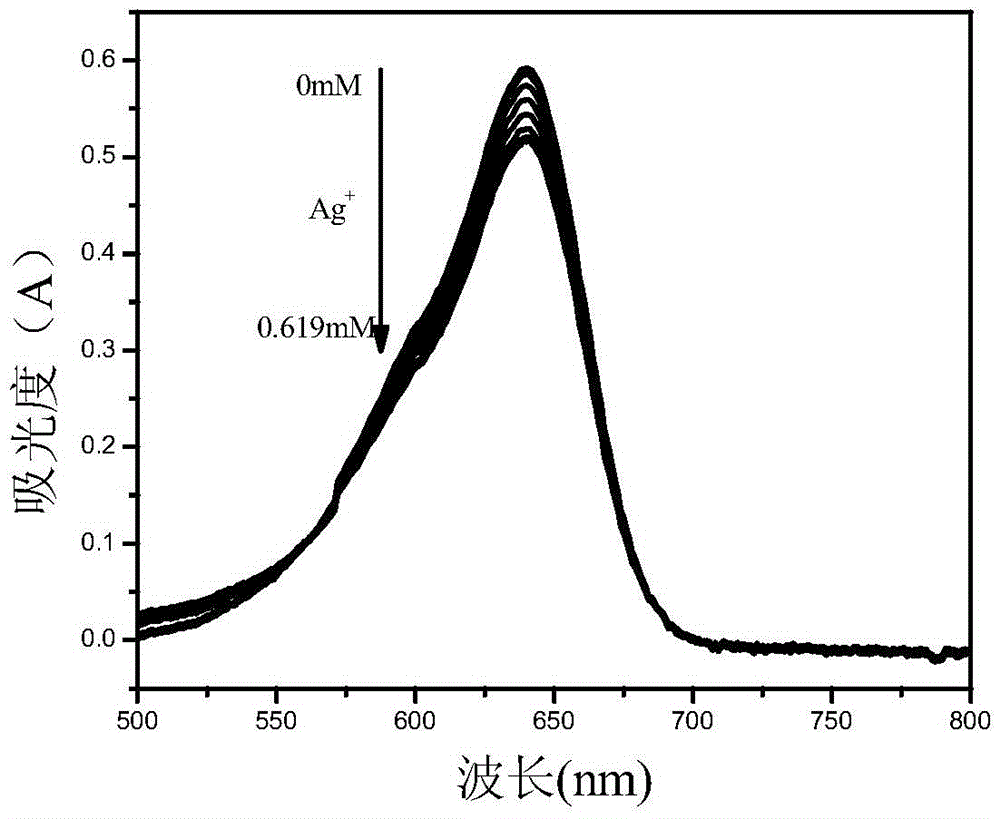
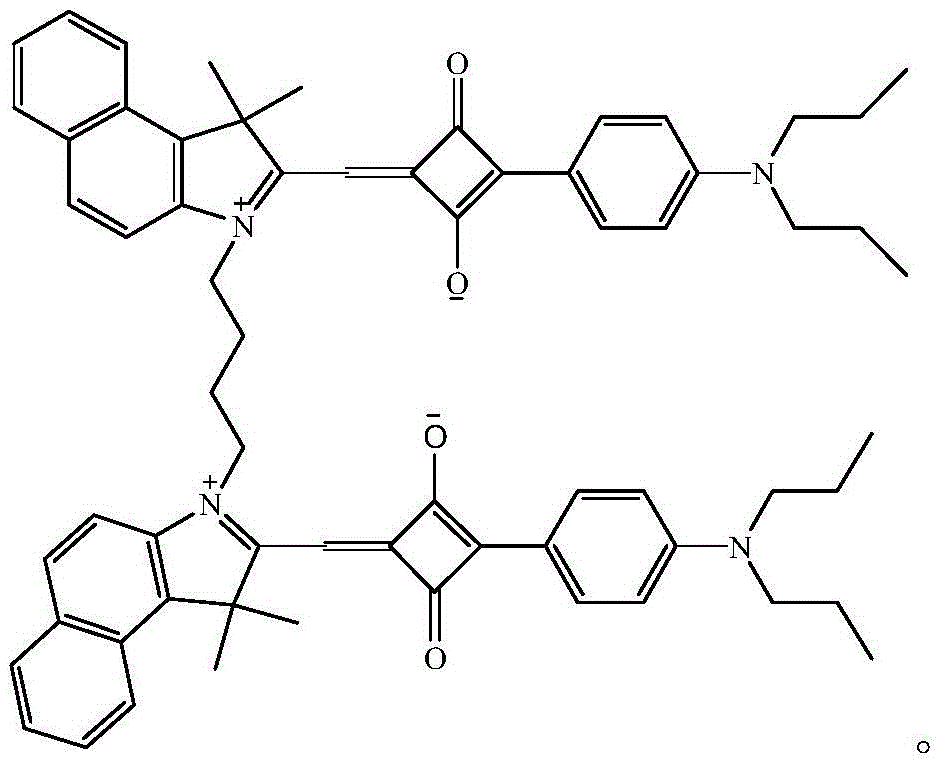
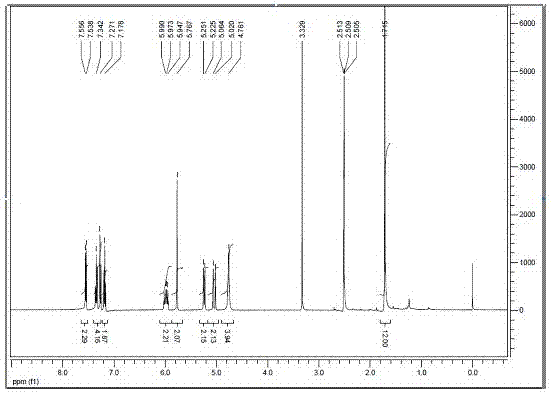
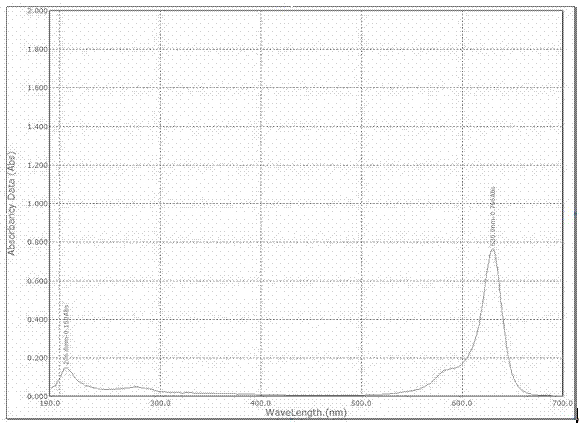
The invention relates to a benzindole and aniline derivative based asymmetric squaraine colorimetric probe. The colorimetric probe is N-butyl bis-substituted-2,3,3-trimethyl-4,5-benzindole-N,N-dipropylaniline asymmetric squaraine. A preparation method of the colorimetric probe includes the step of enabling 2,3,3-trimethyl-4,5-benzindole and 1,4-dibromobutane to synthesize N-butyl bis-substituted-2,3,3-trimethyl-4,5-benzindole quaternary ammonium salt and an N-butyl bis-substituted-2,3,3-trimethyl-4,5-benzindole quaternary ammonium salt and N,N-dipropyl squaric acid hemicyanine synthetic product. The benzindole and aniline derivative based asymmetric squaraine colorimetric probe and the preparation method and application thereof have the advantages that the obtained asymmetric squaraine colorimetric compound is excellent in optical performance and photostability, obvious in feature since color changes except that absorption spectrum changes during ferric ion and silver ion recognition, and beneficial to ferric ion and silver ion detection.
Owner:CHANGZHOU UNIV
Benzpyrole squaric acid cyanine dye and preparation method thereof
ActiveCN102952413AImprove stabilityMild and single reaction conditionsMethine/polymethine dyesCyanineSquaraine dye
The invention provides benzpyrole squaric acid cyanine dye. The general formula of the structure of the benzpyrole squaric acid cyanine dye is shown in the description, wherein X is one of Br, Cl and I, and R is one of the following structures shown in the description. The benzpyrole squaric acid cyanine dye provided by the invention has good stability and large absorption strength, can be applied in matching reagents of a 5-classified hematology analyzer to be used as dye of nucleated red blood cells, and can also be applied to fluorescent marks and other life science research fields.
Owner:SHENZHEN MAXCHEMTECH
Method for adsorption separation of propylene, propyne, propane and propadiene
ActiveCN109293467AStable structureImprove performanceGas treatmentOther chemical processesAlkaline earth metalSorbent
The invention discloses a method for separating propylene, propyne, propane and propadiene from a mixed gas. The method comprises: separating a mixed gas containing propylene, propyne, propane and propadiene by using a metal organic framework material as an adsorbent to obtain the gases of each single component, wherein the structural general formula of the metal organic framework material is [M(C4O4)(H2O)].1.5H2O, M is a metal ion, and transition metal ions or alkaline earth metal ions and squaric acid form a three-dimensional network structure through a coordination bond or intermolecular force. According to the present invention, the metal organic framework material has excellent adsorption separation selectivity to propylene, propyne, propane and propadiene, further has characteristicsof cheap and easily available raw materials, simple preparation process, low cost, good regeneration property and good repeatability, can maintain the original adsorption effect after vacuum or heating regeneration, and has broad industrial application prospects.
Owner:ZHEJIANG UNIV
Fluorescent dye having phenazine condensed structure and synthesis method of same
ActiveCN108690032AHigh fluorescence quantum yieldGood light stabilitySilicon organic compoundsAzo dyesQuantum yieldSalicylaldehyde
The invention discloses a fluorescent dye having phenazine condensed structure, of which the structural formula is represented as one of the formulas (I) to (IV). The fluorescent dye having phenazinecondensed structure is prepared through steps of: reactions of cyclohexanedione condensation, sodium borohydride reduction, Vilsmeier formylation and the like are carried out to obtain an intermediatecompound; and then reactions with 4-subsituent amino-2-nitrosophenol, benzophenone acid, trans-4-subsituent amino-salicylaldehyde and the like compounds are carried out. Compared with classical fluorescent dyes (e.g. rhodamine, oxazine, squaric acid, anthocyanin dyes), the fluorescent dye in the invention has high photo-stability, is near-infrared in fluorescence emission wavelength of 635-829 nm, and has large Stokes shift capable of reaching 122 nm and high fluorescence quantum yield capable of reaching 0.43. The fluorescent dye can be applied to fields such as luminescent materials, bio-fluorescence probes, multicolor fluorescence imaging, etc.
Owner:HUNAN UNIV
Polyalkyl-fluorene conjugated polymer and use
InactiveCN1438254AImprove quantum efficiencyImprove long-term stabilityLuminescent compositionsStructural unitPolymer chemistry
The invention refers to a polyalkyl fluorene conjugated polymer whose construction consists of 9,9' double substituted fluorene construction unit and diaryl substituted cubic acid or maleic amino one. The conjugated polymer is used as the luminescent material and can be applicable to make electroluminescent devices.
Owner:SICHUAN UNIV
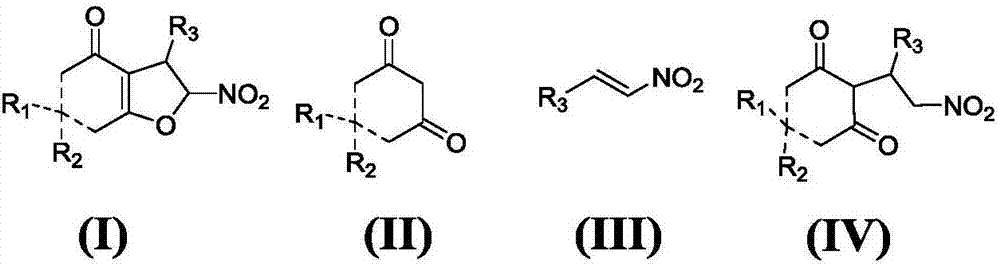
Asymmetric synthesis method for chiral dihydrofuran compound
The invention provides an asymmetric synthesis method for a chiral dihydrofuran compound shown as formula (I). The synthesis method is carried out according to the following steps: 1,3-cyclohexanedione compound shown as formula (II), nitroolefin compound shown as formula (III), chiral squaric acid catalyst and organic solvent A are mixed and thoroughly react under negative 20 DEG C to negative 60 DEG C, so that a compound shown as formula (IV) is obtained; iodine source additive, an alkaline substance and organic solvent B are added into the compound shown as formula (IV) and thoroughly react under negative 20 DEG C to negative 60 DEG C, and after reaction solution is post-treated, the chiral dihydrofuran compound shown as formula (I) is obtained. the asymmetric synthesis method has the advantages of mild reaction conditions, high product yield and excellent selectivity; the prepared chiral dihydrofuran compound is chiral, and the core framework structure is novel. (img file='DDA00012852165300000II.TIF' wi='1549' he='406' / ).
Owner:ZHEJIANG UNIV OF TECH
Amphiphilic squaraine dyes, process for preparation thereof and use thereof
InactiveUS20090068113A1Ultrasonic/sonic/infrasonic diagnosticsOrganic chemistrySquaraine dyeSquaric acid
The present invention relates to amphiphilic squaraine dyes of the general formula (1) as shown below Formula (1) wherein, R1=—(CH2—CH2—O)n—CH3, n=4-8, or —(CH2)n—CO2X, n=3-6, X=H, succinamide and R2=—CH3 or —(CH2—CH2—O)n—CH3, n=4-8 and pharmaceutically acceptable derivatives thereof, for use as near infrared fluorescence probes in photodynamic diagnostic and biological, biochemical and industrial applications.
Owner:COUNCIL OF SCI & IND RES
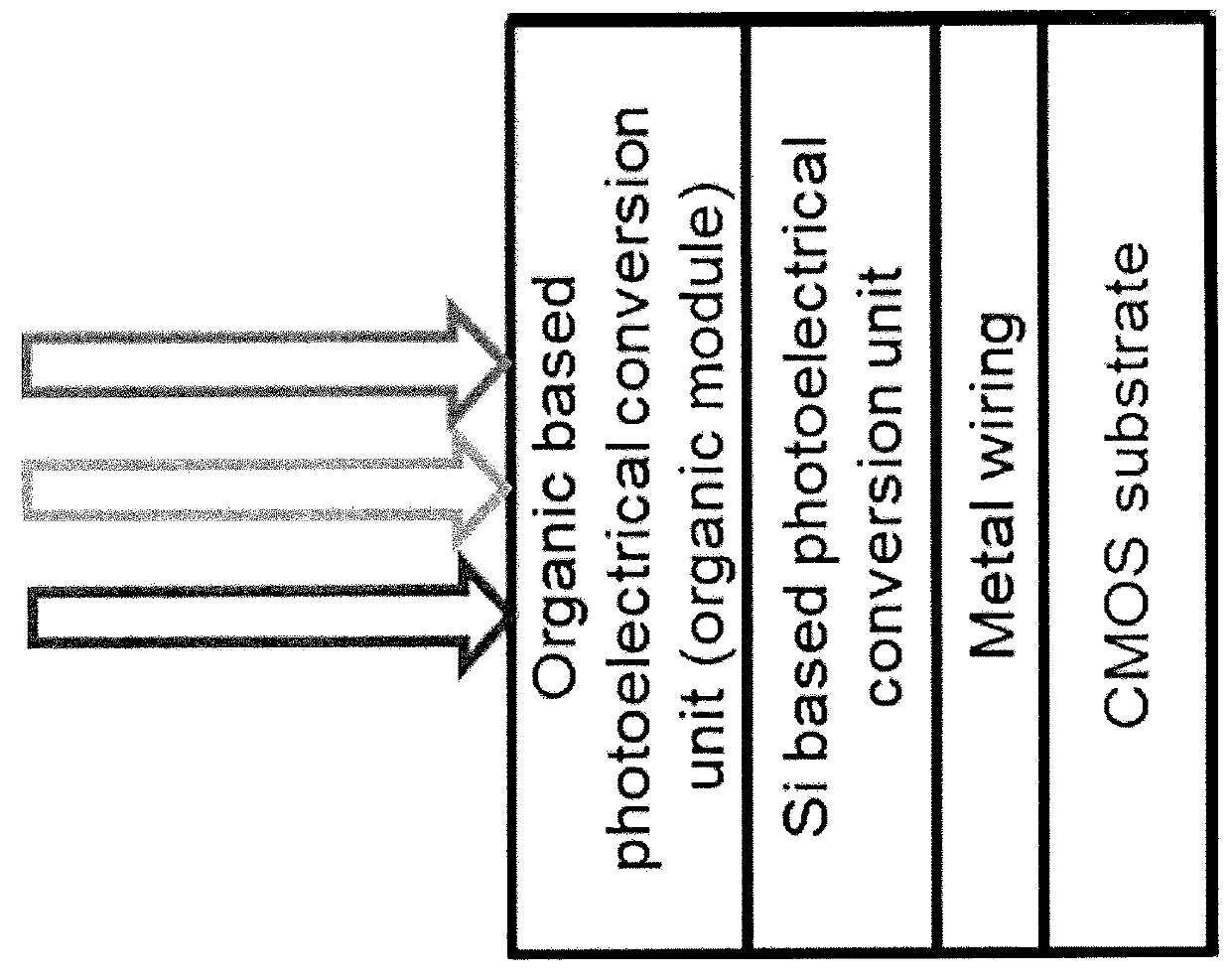
Squaraine-based molecules as material for organic photoelectric conversion layers in organic photodiodes
An active material for organic image sensors, where the active material is a squaraine-based active material or a thiophene-based active material. A photoelectric conversion layer containing the active material, which is a squaraine-based active material or a thiophene-based active material. An organic image sensor containing the photoelectric conversion layer containing the active material.
Owner:SONY CORP
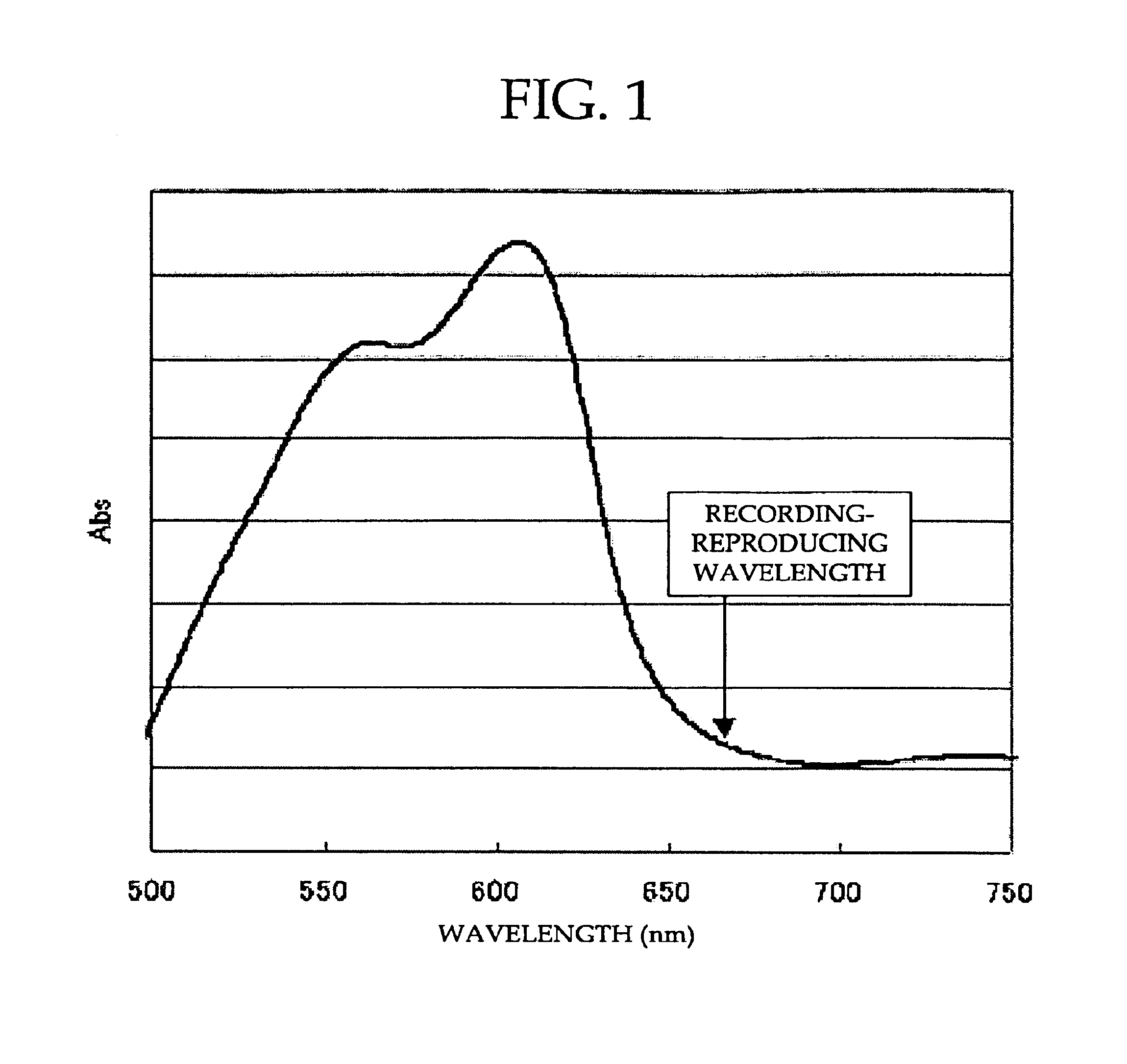
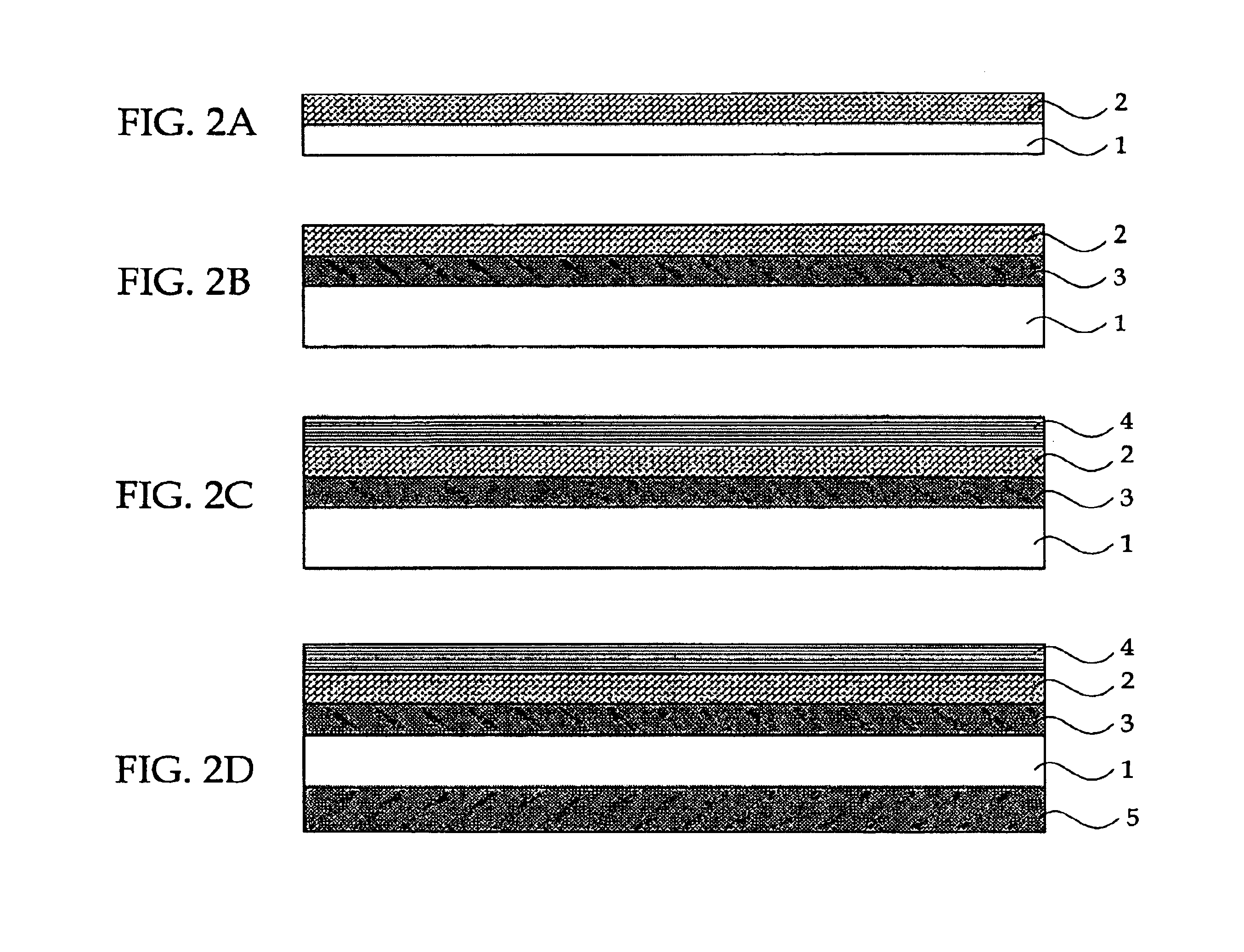
Optical recording medium, and method and device using the same
An optical recording medium includes a substrate and at least a recording layer deposited on or above the substrate, and the recording layer contains at least one formazan-metal chelate compound containing a formazan compound and a metal component; at least one squarylium-metal chelate compound containing a squarylium compound and a metal component; and at least one diarylamine compound.
Owner:RICOH KK +1
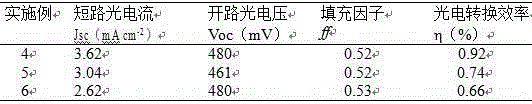
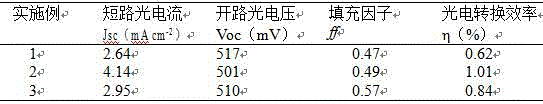
Preparation method for porous carbon material electrode for counter electrode of dye-sensitized solar cell
InactiveCN106531445AImprove photoelectric performanceSimple processLight-sensitive devicesPhotovoltaic energy generationOrganic dyePorous carbon
The invention discloses a preparation method for a porous carbon material electrode for a counter electrode of a dye-sensitized solar cell. A porous carbon counter electrode which is prepared from a metal organic framework coordination compound under different carbonization conditions, and a squaric acid organic dye-sensitized TiO<2> porous thin film are assembled together; and the middle part is poured with a redox electrolyte to obtain the dye-sensitized solar cell taking the porous carbon material as the counter electrode. Photoelectric conversion efficiency of different cells can be obtained from different carbonization conditions, so that the photoelectric performance of the dye-sensitized solar cell can be optimized consequently; and meanwhile, the preparation method has the advantages of simple process, low cost, large area, no pollution and the like, so that the application of the dye-sensitized solar cell can be facilitated.
Owner:TIANJIN NORMAL UNIVERSITY
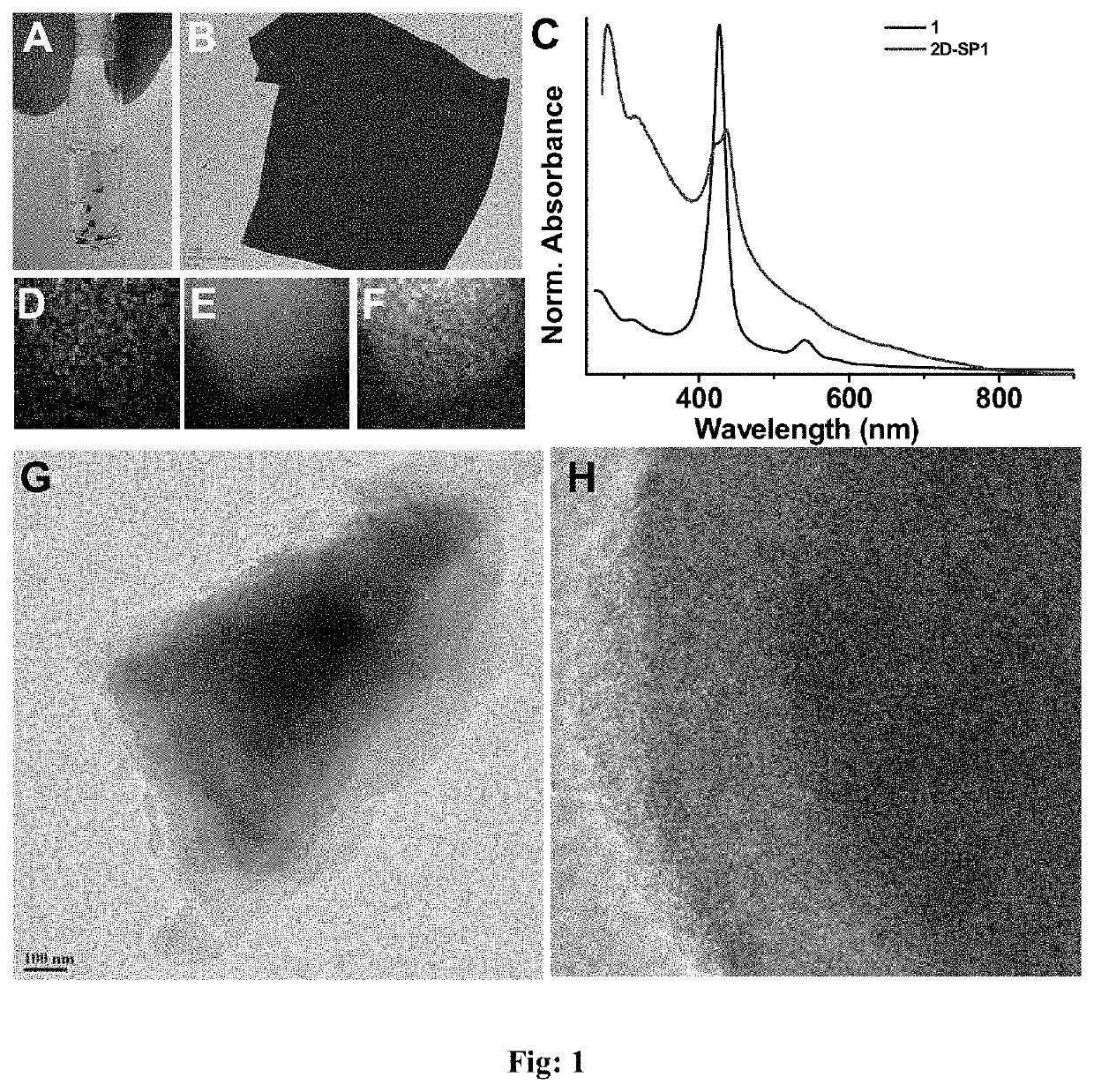
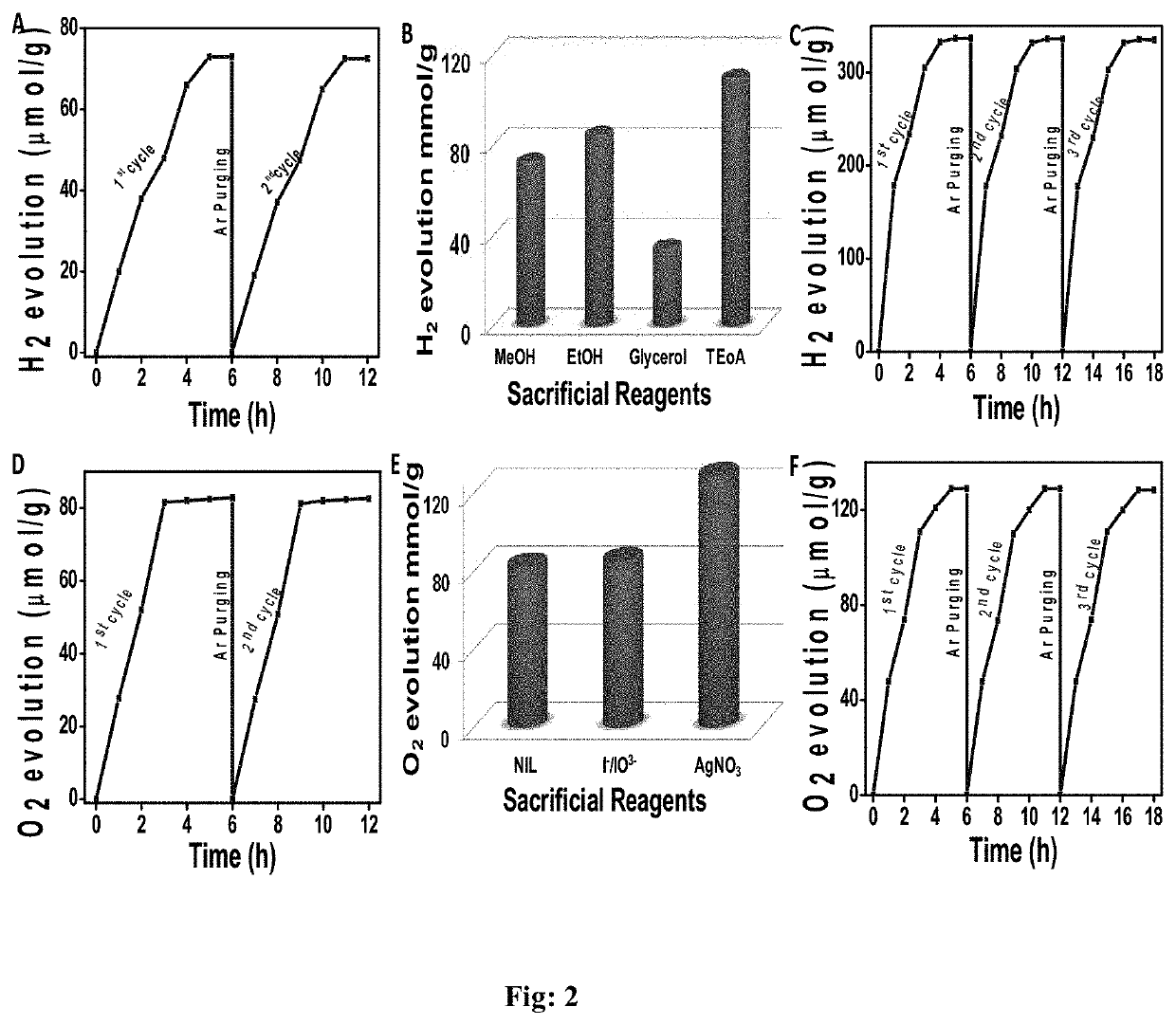
Metalloporphyrin 2d-sheets for efficient photo- and electro- catalytic splitting of water
The present invention disclosed a novel squaraine linked metalloporphyrin based 2D-sheet polymer catalyst of formula (I), process for preparation thereof and use of said catalyst for efficient photo- and electro-catalytic splitting of water.
Owner:COUNCIL OF SCI & IND RES
Mulin acetate comprising substituted squaric acid, and application thereof
ActiveCN103204787AAntibacterial agentsOrganic compound preparationReference productStaphylococcus aureus
The invention relates to mulin acetate comprising substituted squaric acid, and an application thereof. The invention belongs to the field of chemical medicine. A mulin acetate compound comprising substituted squaric acid, a pharmaceutically acceptable salt thereof, a solvate thereof, an isomer thereof, a prodrug thereof, or a prodrug of salt thereof have a structural general formula represented by the formula (I). The MIC values of the compound against staphylococcus aureus and strepococcus equi are higher by 20-30 times than the antibacterial effect of a reference product of tiamulin in market. The mulin acetate comprising substituted squaric acid is a high-efficiency antibacterial medicine.
Owner:BEIJING ABLEPHARMTECH CO LTD
Poly-squaric acid conjugated polymer preparation method and application thereof
InactiveCN102382284AImprove solubilityGood and stable solubilityFinal product manufactureSolid-state devicesPolymer scienceSide chain
The invention relates to a squaric acid polymer, and particularly relates to a poly-squaric acid conjugated polymer; the polymer has good dissolving stability, good matching between an absorption spectrum and a solar spectrum, and is applicable to polymer polymer solar cells. The invention also relates to a preparation method of the poly-squaric acid conjugated polymer and an application of the polymer. The technical solution schemes of the invention are that: 1) a large aromatic side chain, such as alkoxyphenyl, alkoxyphenyl methylene, alkoxybiphenyl methylene, and the like, is introduced onnitrogen atoms of pyrrole so as to reduce the accumulation between polymer molecular chains, to increase the dissolving stability of the polymer, and to improve the processing performance; 2) mesogenic fragments are introduced on nitrogen atoms of pyrrole so as to increase the local orderliness of the polymer.
Owner:SICHUAN UNIV
Method for synthesizing and forming UTSA-280 adsorbent materials on large scale
ActiveCN110639475AImprove the coordination effectEfficient and fast mass synthesisOther chemical processesSorbentCrystallinity
The invention relates to the field of preparation of a UTSA-280 adsorbent material, particularly relates a method for synthesizing and forming UTSA-280 adsorbent materials on a large scale, and particularly relates to a method for preparing UTSA-280 MOFs materials in batches by regulating reaction raw materials and regulators. The materials are subjected to molding granulation by additives. The method comprises the following steps: adding NH3*H2O into a squaric acid supersaturated aqueous solution to promote dissolution of squaric acid, adding a calcium nitrate aqueous solution into the formedsquaric acid aqueous solution, maintaining stirring in the process of adding the calcium nitrate aqueous solution, and obtaining a strip-shaped UTSA-280 crystal with high crystallization degree at room temperature. Compared with addition of the original NaOH, the rate of promoting dissolution of the squaric acid by the NH3*H2O is higher, the adding amount is convenient and accurate to control, and the method is a method capable of synthesizing the UTSA-280 with high crystallization degree efficiently and rapidly.
Owner:TAIYUAN UNIV OF TECH
Organic photosensitive devices comprising aryl squaraines and methods of making the same
ActiveUS20160005983A1Controlled diffusionNo net contributionMethine/polymethine dyesLight-sensitive devicesArylHeterojunction
There is disclosed squaraine compounds of formula I:wherein each of Y1 and Y2 is independently chosen from an optionally substituted amino group and an optionally substituted aryl group. Also described are organic optoelectronic devices comprising a Donor-Acceptor heterojunction that is formed from one or more of the squaraine compounds. A method of making the disclosed device, which may include one or more sublimation step for depositing said squaraine compound, is also disclosed.
Owner:UNIV OF SOUTHERN CALIFORNIA +1
Filters for electronic display device
InactiveUS20030165640A1Color purity is loweredClearLiquid crystal compositionsOrganic chemistrySulfurDisplay device
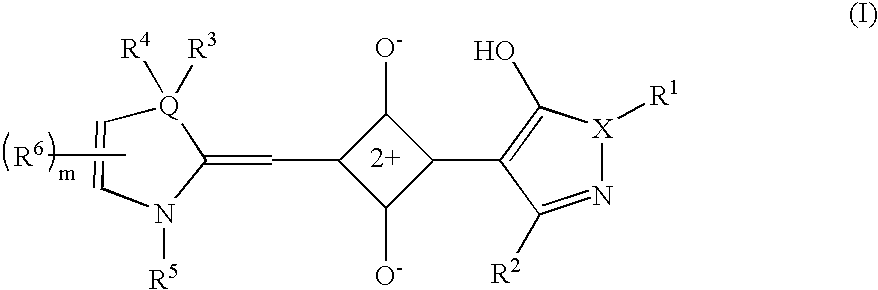
The present invention provides filters for an electronic display device comprising a squarylium compound represented by the general formula (I): wherein X represents a nitrogen atom or an oxygen atom; Q represents a carbon atom, a nitrogen atom, an oxygen atom or a sulfur atom; and R1, R2, R3, R4, R5, R6 and m are as defined in the specification. The filters for an electronic display device of the present invention can selectively shield the light having such a wavelength that reduces the color purity, and thereby can provide clear images.
Owner:KYOWA HAKKO CHEM CO LTD
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com